Abstract
Salt playas are among the most important sources of groundwater salinity and contamination in arid and semi-arid regions. In this study, the quality of groundwater in the Kavir-e Daranjir district was investigated using hydrochemical and statistical methods. For this purpose, 52 samples were collected from the abstraction wells. These samples were analyzed using standard methods to determine major ions, trace elements, and coliform bacteria. Spatial distribution maps of physicochemical parameters, bivariate diagrams, and ion ratios were used to identify factors affecting the salinity and contamination of groundwater. Statistical methods, such as cluster analysis and factor analysis, were also used for this purpose. The results of this study showed that chloride and sodium are the dominant ions in all water samples. The electrical conductivity has a wide range of variations (1450–27,300 μS/cm) which indicates salinity and quality degradation of groundwater. Based on the ionic ratios such as Cl/HCO3 and Na/(Na + Cl), most groundwater samples are contaminated by saline water. Geochemical processes such as groundwater mixing, dissolution of halite and gypsum, and reverse ion exchange have affected groundwater quality. Groundwater nitrate concentration is consistent with fecal coliform bacteria, which indicates groundwater contamination by the sewage of residential areas. The trace elements ratios such as Li/Cl and Br/Cl, also indicates that the groundwater has been affected by domestic sewage and brines resulting from dissolution of halite. Multivariate statistical analysis such as cluster analysis and factor analysis also confirm these findings. According to the results of this research, the control of extraction from abstraction wells (to prevent further intrusion of saline water) and the collection and treatment of domestic sewage plays an important role in the sustainable management of groundwater resources in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In arid and semi-arid regions, because of the lack of water resources, low and periodic rainfall, high rate of evaporation, low groundwater recharge, and climate changes, the management of groundwater resources faces numerous challenges (Zare and Khaledian 2017). Salt flats and saline groundwater are important environmental challenges in arid climate countries. In these regions, preventing the degradation of natural water quality means finding a new source of fresh water (Zarei et al. 2013). Groundwater salinization is an irreversible process (Amiri et al. 2020). The correct and timely diagnosis of the origin of salinity plays an important role in preventing the degradation of groundwater quality and optimal management of water resources (Abdalla 2016).
One of the important factors in the salinity of freshwater aquifers is natural saline waters (Falgas et al. 2009). The removal of native vegetation, which consumes a large part of the rainfall in semi-arid regions, causes the water level to rise. This issue causes the salinity of the groundwater because of the combined effect of the mobility of the salts in the unsaturated zone, the leakage of saline water from the underlying aquifers, and direct evaporation from the groundwater, especially when the groundwater has a short distance to the land surface (Cartwright et al. 2004). Several factors cause groundwater salinization of arid regions. These factors include local cyclic salts (Cartwright et al. 2004), salts in wind deposits (Acworth and Jankowski 1993), salts in marine deposits (Dahlhaus et al. 2000), salt diapers (Bagheri et al. 2017), unsaturated zone salts (Cook et al. 1994), salt playas (Mirzavand and Ghazban 2022) and salts resulting from rock weathering (Chang et al. 2022). In numerous cases, groundwater salinization results from halite dissolution. Halite dissolution is controlled by four factors (Johnson 1997): (1) a water source, (2) a salt deposit, (3) a drainage point that receives the resulting brine and, (4) hydrostatic pressure that causes water flow in the system. If these four factors are met, the mixing of salt water and fresh water will occur.
Groundwater overexploitation in arid and semi-arid regions, successive droughts, and lack of sufficient recharge to aquifers has caused the quantitative and qualitative destruction of groundwater resources. Baghvand et al. (2010) studied the groundwater salinity near the central desert of Iran. According to the results, excessive pumping from abstraction wells has caused salt water intrusion into the fresh water aquifer. Zarei et al. (2013) investigated the salinity of groundwater in the Kanarsieh region of Iran using chemical and isotopic parameters. The results showed that the dissolution of halite in the salt diaper is the source of the groundwater salinity. Zaidi et al. (2015) studied the hydrochemistry of groundwater in arid regions of Saudi Arabia. They stated that intense evaporation and reverse ion exchange reactions are the most important processes influencing groundwater salinity. Based on the studies of Jahanshahi and Zare (2017), it was found that the origin of the deep saline groundwater of the Golgohar mine area is related to the intrusion of saline water from the salty playa of Sirjan. Marazuela et al. (2019) investigated the hydrodynamics of groundwater in the salt flat of Salar de Atacama of Chile. The results showed that the groundwater reaches the mixing zone from the mountains is largely evaporated in the mixing zone, lakes, and directly from the shallow water table. Shojaei Baghini et al. (2020) evaluated the hydrochemistry of groundwater and the risk of salt water intrusion next to Sirjan salt playa. Achieved results of this study indicated that overexploitation from abstraction wells has caused saline water intrusion into the aquifer. Based on the studies of Kumar et al. (2022), processes such as reverse ion exchange, silicate weathering, and seawater intrusion have controlled the groundwater evolution in Digha, India. Mirzavand and Ghazban (2022) investigated the origin of groundwater salinity in the Kashan plain using groundwater chemical quality and isotopic data. According to the results, high pumping from exploitation wells in the north of the plain has caused the intrusion of salt water from the salty playa into the aquifer. Nguyen and Huynh (2023a) studied the groundwater quality in the Mekong delta, Vietnam. This study indicated that groundwater was affected by anthropogenic, agricultural, and industrials contaminants. Studies by Kumar et al. (2023) indicated that processes such as reverse ion exchange, anthropogenic activities, and saltwater intrusion have affected groundwater quality in West Bengal, India. Nguyen and Huynh (2023b) investigated seasonal fluctuations of groundwater quality of Ben Tre region, Vietnam using a statistical approach. The results showed that factors such as geology, wastewater, agriculture, and saltwater bodies have affected the groundwater quality. A look at the above mentioned researches show that saline waters are one of the potential sources of groundwater quality degradation in arid regions.
Kavir-e Daranjir is one of the largest playas in the central part of Iran. Salt deposits accumulated because of strong evaporation rates sustained for several thousands of years. Groundwater is the most important source of agricultural water supply in the areas around the Daranjir playa. In recent years, the salinity of water in abstraction wells has increased. The exploitation of groundwater is limited due to water salinity, and some wells have become unusable. The groundwater level decline and the degradation of water quality are the most important environmental challenges in this region. A correct understanding of the groundwater quality situation and the causes of its salinity is an important step in the sustainable management of water resources. Determining the origin of groundwater salinity will play an important role in formulating management strategies for aquifer exploitation. For this reasons, this study was conducted to investigate the quality and salinity of groundwater. The most important purposes of this research are: (1) investigating the hydrochemical status of groundwater, (2) determining the origin of groundwater salinity, and (3) determining the processes affecting the concentration of groundwater solutes.
Material and methods
Study area
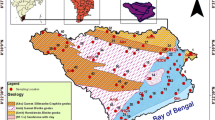
Kavir–e Daranjir district is between latitudes 55° 20ʹ and 58° 39ʹ E and longitudes 31° 20ʹ and 31° 50ʹ N, in the central part of Iran. The area of Kavir-e Daranjir district is 3080 km2 and its height above sea level varies between 970 and 1200 m. The overall slope of this basin is from south to north. The study area has an arid climate. Low rainfall and high evaporation are the main factors creating this arid climate. The average annual rainfall, evaporation, and temperature are 54 mm, 3200 mm, and 19.5 °C, respectively (Sarzamin Qanat 2021). Bafgh city is the most important city in the Kavir-e Daranjir district. As the warmest city in Yazd province, Bafgh city has rich iron ore mines and date palm gardens, and for this reason it is known as the land of palm, iron, and sunshine. 80% of agricultural products in the Daranjir area are horticultural crops such as dates, pistachios, citrus fruits, and olives.
General geology and hydrogeology
In terms of general morphology, the Daranjir desert is surrounded by mountains. The mountainous landscape that surrounds the Daranjir desert, like an uninterrupted ring, provides sediments and solutes to the Daranjir desert system. The transport of sediments is done by a network of drainages. The force of the wind can also cause the transfer of sediments from the mountainous part to the plain (Noujavan and Sadough 2008). Alluvial fans, sand dunes, salt and mud flats, and Nebka are common geomorphological features in the Kavir-e Daranjir district. From the geological point of view, different metamorphic, igneous, and sedimentary units are outcropped in the Daranjir district (Fig. 1). Marble and metamorphic rocks of green schist facies are the most important metamorphic units. These rocks are outcropped in the western margin of the study area. Igneous units include rocks such as granite, andesite and, basalt. These igneous rocks have a smaller area compared to other geological units. Sedimentary rocks and deposits are the most important geological units in the Kavir-e Daranjir district. Sedimentary rocks include a variety of clastic and chemical sedimentary rocks. Clastic rocks mainly include marl, shale, sandstone, and conglomerate. These rocks are found in the eastern part of the Daranjir district. Limestone and dolomite are the most important chemical sedimentary rocks that are exposed in the western part of the study area (Fig. 1). Among all geological units, Quaternary sediments are the most important because these sediments are reservoirs for groundwater accumulation. These sediments mainly include pediment fans and valley terrace deposits, alluvial plains and river alluviums, salt and clay flats, and sand dunes. Alluvial terraces and alluvial fans are widespread in the Daranjir plain. In terms of grain size, these sediments include rubble of different sizes with bad sorting. The alluviums next to the highlands are coarse-grained and have poor sorting. As get closer to the salt playa, the size of the sediments becomes smaller and their sorting improves. Aeolian deposits are also seen in as Barkhan and parallel dunes. These deposits comprise clay, silt, and evaporite minerals such as gypsum and halite. In the central part of the Kavir-e Daranjir district, groundwater has a short distance from the land surface. The groundwater gradually evaporates and its solutes are deposited on the ground surface. Over the years, a considerable thickness of gypsum and halite has covered the surface of the earth (Noujavan and Sadough 2008).
The aquifer of the study area is unconfined. Groundwater flows from the margins of the plain to its center. The thickness of the saturated part of Quaternary sediments varies between 10 and 450 m. The largest thickness of the aquifer is observed in the central part of the plain. The aquifer transmissivity varies from 100 to 990 m2/d. The highest aquifer transmissivity is observed in the central and southern parts of the Daranjir district (Sarzamin Qanat 2021). There are 217 abstraction wells in the Daranjir district. The wells discharge rate varies between 6 and 50 l/s. The groundwater level shows a downward trend. The average annual water level drop is 0.2 m (IWRMC 2021).
Study method
Groundwater samples were collected during January and February 2021 from 52 abstraction wells. The location of the sampling stations is shown in Fig. 1. Electrical conductivity (EC), temperature, and pH were measured at the sampling site using portable instruments. Water samples were collected in pre-washed polyethylene bottles. Two bottles were considered for each sample. One bottle for analysis of major ions and one bottle for measuring trace elements. Water samples were filtered through 0.2 μm filters. The samples were acidified with HNO3 for the analysis of trace elements.Water samples were analyzed at the water and wastewater laboratory of Azmoon Salamat Asa Company, Tehran, Iran. Major and minor ions concentrations including calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), chloride (Cl−), sulfate (SO42−), nitrate (NO3−), nitrite (NO2−), ammonium (NH4+), lithium (Li+), and bromide (Br−) were determined by ion chromatography method. The bicarbonate (HCO3−) and iodide (I−) were determined by the titration method. The concentration of trace elements such as boron (B), strontium (Sr), bromine (Br), lithium (Li), nickel (Ni), manganese (Mn), mercury (Hg), barium (Ba), arsenic (As), chromium (Cr), lead (Pb), molybdenum (Mo), and antimony (Sb) were determined by atomic absorption method. The number of coliforms was calculated using the most probable number (MPN) technique.
Investigating the spatial variation of groundwater quality parameters is the first step in studying the qualitative status of the aquifer. The results of the analysis of water samples were interpreted using spatial distribution maps of qualitative parameters. Spatial variations of qualitative parameters related to groundwater salinity such as electrical conductivity and ions such as sodium, chloride, and sulfate were investigated. The spatial distribution of nitrate ion and coliform bacteria were also considered to investigate the status of groundwater contamination. Several bivariate diagrams were used to investigate the geochemical processes governing the quality of groundwater. Bivariate diagrams are one of the most useful methods for determining the origin of groundwater solutes (Paul et al. 2019). Different ionic ratios such as Cl/HCO3, Na/Cl, Na/(Na + Cl), Na/(Na + Ca), Ca/(Ca + SO4), Li/Cl, and Cl/Br were also used in the bivariate diagrams. Pearson correlation analysis was used to determine the relationship between water quality parameters. Multivariate statistical methods such as cluster analysis and factor analysis were used to group the water samples and determine the factors affecting the qualitative degradation of the aquifer. Cluster analysis is a general title for a series of mathematical methods that are used to find similarities between individuals in a set (Farshadfar 2010). Factor analysis is a useful method for interpreting qualitative groundwater data and relating them to specific hydrogeological processes (Dragon 2006). Statistical analyses were performed using Minitab software ver. 21.4.2.
Result and discussion
General hydrochemistry
Table 1 indicates the results of chemical analysis of water samples. As seen in Table 1, the electrical conductivity of water samples varies from 1450 to 27,300 μS/cm. The lowest amount of electrical conductivity corresponds to sample no. 47 in the eastern alluvial fans (Fig. 2a). The highest amount of electrical conductivity was also recorded in sample no. 41 at the margin of the salt playa (EC = 27,300 μS/cm). According to Table 1, the dominant cation of groundwater is Na+. The concentration of this ion varies between 240 and 4299 mg/l. The sodium ion concentration increases from the margins of the plain towards its center (Fig. 2b). In all groundwater samples, sodium ion concentration is higher than calcium and magnesium ions. The dominant anion of groundwater is Cl−. The chloride ion concentration varies between 222 and 6345 mg/l. The spatial variation of chloride ion concentration is presented in Fig. 2c. The lowest concentration of chloride ion was recorded in the eastern alluvial fans (sample no. 47). The highest amount of chloride ion was also recorded at the margin of the salt playa (sample no. 41). The variation trend of sulphate ion is also similar to the spatial variation of chloride ion (Fig. 2d), which can indicate the identical origin of the mentioned parameters. The presence of evaporite sediments in the central parts of the plain is the most important reason for the increase in the electrical conductivity and dissolved ions. Jahanshahi and Zare (2017) and Shojaei Baghini et al. (2020) have also reported an increase in the electrical conductivity and groundwater solutes around the Sirjan playa. The Durov diagram of water samples is presented in Fig. 3. According to this figure, the type of all water samples is Na-Cl. The dominance of major ions is in the order of Na+ > Ca2+ > Mg2+ > K+ for cations and Cl− > SO42− > HCO3− > NO3− for anions. Baghvand et al. (2010), Zaidi et al. (2015), Marazuela et al. (2019), and Mirzavand and Ghazban (2022) have reported the Na–Cl type of groundwater in arid regions.
Nitrate concentration and the amount of coliform bacteria are indicators of groundwater contamination. Figure 2e shows the spatial distribution of nitrate concentration in the water samples. As seen in Fig. 2e, the concentration of nitrate ion varies between 5.5 and 54 mg/l. The maximum concentrations of nitrate ion were recorded in residential areas and cultivated lands. Figure 2f shows the spatial distribution of coliform bacteria. The maximum amount of coliform bacteria (especially fecal coliforms) is seen in samples with high nitrate concentrations. Therefore, it can be concluded that the source of nitrate ion is related to residential sewage. Cao et al. (2022) reported residential sewage as a source of groundwater pollution of Changle River watershed, China. Vasudevan et al. (2021) also reported high microbial activity and nitrate concentration in the residential areas of Tamil Nadu, India.
The ratio of chloride to bicarbonate can be used as an indicator of water contamination by saline water (Todd and Mays 2005). Figure 4 shows the ratio of chloride to bicarbonate versus electrical conductivity. The ratio of chloride to bicarbonate of groundwater samples varies between 2.12 and 112.9. According to Fig. 4, most of the groundwater samples are contaminated by saline water. The lowest level of contamination is observed in sample no. 47. This sample is for alluvial fans in the eastern part of the plain. Abdalla (2016) reported the ratio of chloride to bicarbonate of Jazan aquifer between 0.2 and 45.
Bivariate diagrams
Bivariate diagrams of qualitative parameters are one of the most common methods for determining the processes governing the quality of groundwater. Figure 5 indicates the bivariate diagrams of groundwater quality characteristics. As seen in Fig. 5a, b, c, and d, the concentration of sodium, calcium, magnesium, and sulfate ions increases linearly with the increase of chloride ion. This linear trend indicates the mixing of various water resources. A very good linear trend can be seen in the plot of sodium versus chloride (Fig. 5a). This pattern supports the dissolution of halite (NaCl). Calcium and magnesium ions also indicate a direct relationship (Fig. 5e). This relationship indicates their identical origin and hydrochemical behavior. The linear relationship between calcium and sulphate ions (Fig. 5f) indicates the dissolution of sulfate minerals containing calcium such as gypsum (CaSO4, 2H2O) and anhydrite (CaSO4). Magnesium and sodium ions also have a direct relationship with sulphate concentration (Fig. 5g, h). The lack of a strong correlation between nitrate with chloride and electrical conductivity (Fig. 5i, j) indicates that nitrate ion has a different origin compared to other ions. Anthropogenic sources such as agriculture and domestic sewage are important sources of nitrate in groundwater (Rahman et al. 2021).
Ion exchange is one of the main processes that affect groundwater hydrochemistry (Appelo and Postma 2005). Figure 6a shows the bivariate diagram of Ca + Mg versus HCO3 + SO4. According to Fig. 6a, the occurrence of the reverse ion exchange process is clear in most of the groundwater samples. The occurrence of the reverse ion exchange process is also concerning with respect to the plot of Na/Cl versus EC (Fig. 6b). As shown in Fig. 6b, most of the water samples are below the line of Na/Cl = 1. This process is shown in the following reaction (Hounslow 1995).
Figure 6c shows the ratio of Na/(Na + Cl) versus total dissolved solids. According to this figure, reverse ion exchange and saltwater intrusion are among the most important processes affecting groundwater quality. The variation of the Ca/(Ca + SO4) ratio versus pH (Fig. 6d) also indicates the occurrence of cation exchange and calcite precipitation in the aquifer.
Gibbs (1970) suggested the ratio of Na/(Na + Ca) and Cl/(HCO3 + Cl) to determine the processes governing groundwater quality. Gibbs diagrams of groundwater samples are presented in Fig. 7. According to this figure, the groundwater quality has been affected by the evaporation process and brines resulting from the dissolution of evaporite deposits.
Trace elements
Using trace elements is a useful method to investigate the origin of groundwater solutes (Saberinasr et al. 2019). Table 2 shows the statistical characteristics of trace elements in water samples. As seen in Table 2, the concentration of strontium, nickel, and arsenic in some water samples is higher than the World Health Organization standard (WHO 2022). 33% of the water samples have strontium concentration exceeding the WHO permissible limit. These samples are mostly in the salt flat of the central part of the Daranjir district. The concentration of nickel in 29% of the samples is higher than the permissible limit. These samples are also in the central part of the study area. The arsenic contamination is also observed in the samples related to the central salt flat (27% of total samples). Therefore, it can be concluded that the salt flat is the source of groundwater contamination with elements such as strontium, nickel, and arsenic. Kreitler (1993) has introduced salt playas as one of the potential sources of trace elements of groundwater.
As chloride and bromide ions behave conservatively in hydrological systems, these ions are often used as a good indicator of the origin of water salinity (Ahmed et al. 2013). Figure 8a shows the plot of Br concentration versus TDS. As seen in this figure, the mixing of groundwater and brine resulting from the dissolution of halite has affected the quality of groundwater. The ratio of Li/Cl versus Br/Cl (Fig. 8b) also indicates the effect of dissolution of evaporite deposits (especially halite) on groundwater quality. Panno et al. (2006) suggest the plot of Cl/Br versus Cl to investigate the evolution of groundwater and water salinization processes. Figure 8c shows the changes in the ratio of Cl/Br versus Cl. According to Fig. 8c, the groundwater of the study area is affected by domestic sewage and brines caused by the dissolution of halite.
Statistical investigations
Correlation matrix of water quality data
One of the most important steps to identify processes affecting groundwater quality is to examine the relationship between physicochemical parameters (Das et al. 2022). One of the best methods for this purpose is to prepare the correlation matrix of different water quality characteristics. Table 3 shows the correlation matrix of groundwater quality parameters. As seen in this table, there is a high correlation between the electrical conductivity and the concentration of calcium, magnesium, sodium, potassium, sulfate, and chloride ions (R > 0.82). The mentioned ions are the most important factors affecting groundwater quality. The concentration of sodium has a high correlation with the concentration of potassium (R = 0.81), sulfate (R = 0.9), and chloride (R = 0.99), which indicates the similar origin of these ions. Chloride ion also indicate a high correlation with calcium (R = 0.99), magnesium (R = 0.91), sodium (R = 0.99), potassium (R = 0.83), and sulfate (R = 0.87). The correlation of iodine with electrical conductivity, sodium, potassium, sulfate, and chloride is higher than other qualitative parameters (R > 0.5). The correlation between boron, magnesium, and sulfate ions is also significant (R > 0.5). Strontium concentration has a high correlation with electrical conductivity (R = 0.85), calcium (R = 0.9), magnesium (R = 0.9), sodium (R = 0.8), potassium (R = 0.65), sulfate (R = 0.77), and chloride (R = 0.84). The salt flats and their brines are an important source of magnesium, sodium, potassium, chloride, lithium, boron, and iodine in arid environments (Kesler et al. 2012). According to the above, the dissolution of evaporite minerals such as halite and gypsum can be an important source of groundwater solutes. Nitrate ion does not indicate high correlation with other qualitative parameters (R < 0.3). This issue indicates a different origin for nitrate. This finding is consistent with the research of Rojas Fabro et al (2015). These researchers reported the insignificant correlation between Nitrate, chloride, sulphate, and potassium ions in Merida, Mexico.
Cluster analysis
Cluster analysis can determine the spatial patterns of groundwater geochemical changes and reveal the processes affecting water quality (Yang et al. 2020). In this study, Ward's method (Ward 1963) was used to clustering the groundwater samples. The results of the cluster analysis of groundwater samples are presented in Fig. 9a. As seen in this figure, the groundwater samples are placed in three groups. The chemical parameters of these groups are shown in Table 4. The dominant type of groundwater in these three groups is chloride type, but their qualitative parameters are different. The lowest amount of total solutes is observed in the first group and the highest in the third group. The spatial distribution of different groups of water samples is presented in Fig. 10. According to this figure, the samples of the first group are mainly in the eastern part of the study area. The samples of the second and third groups are mainly located in the central areas. These samples are influenced by brines and have more soluble salts. Similarity of groundwater quality parameters was also investigated using the cluster analysis method. Figure 9b shows the dendrogram resulting from the cluster analysis of groundwater quality parameters. According to Fig. 9b, the parameters of electrical conductivity, sodium, potassium, chloride, sulfate, and calcium show the most similarities among the qualitative parameters. The reason for this is their same origin. As mentioned in the previous parts of this research, the main source of these parameters is the dissolution of evaporite sediments and the resulting brines. Bicarbonate and nitrate parameters are also placed in the same group, which can be a reason for the groundwater contamination by the sewage infiltrating the aquifer.
Factor analysis
Factor analysis is a multivariate statistical method which can be used to explore the hydrogeochemical processes influencing the groundwater system (Love et al. 2004). A scree plot was used to determine the number of factors affecting groundwater quality (Fig. 11). According to this diagram, two factors have eigenvalues greater than 1. Thus, these two factors are considered the main factors affecting groundwater quality. The factor analysis report is presented in Table 5. As shown in Table 5, the first and second factors express 71.1% and 14.8% of the total variance, respectively. Based on factor loadings (Table 5), the first factor can be attributed to the dissolution of evaporite minerals such as halite and gypsum, because sodium, chloride, calcium and, sulfate ions have a high factor loading. In the second factor, nitrate and bicarbonate ions have a high factor loading. Therefore, this factor can be attributed to the contamination of groundwater by sewage.
Conclusion
In this study, the factors affecting salinity and groundwater contamination in the Kavir-e Daranjir district in the central part of Iran were investigated. For this purpose, the major ions and trace elements of groundwater were analyzed. The spatial distribution of hydrochemical data and ionic ratios showed that the dissolution of halite and its resulting brines is the most important cause of groundwater salinity. Evaporation, reverse ion exchange, and domestic sewage have also affected the quality of groundwater. The examination of trace elements also confirms these cases. The results of the statistical analysis also indicate that two main factors have affected water quality. These two factors explain over 85% of the variance in water quality data. These factors are, in order of importance, the dissolution of evaporite deposits and the infiltration of sewage in residential areas. The achieved results showed Daranjir salt playa is the most important source of groundwater solutes except nitrate. Nitrate ion has an anthropogenic origin and is related to sewage infiltration. This research showed that the use of major ions, trace elements and multivariate statistical methods is a suitable method for evaluating the factors governing the quality of groundwater in arid and semi-arid regions. Reducing extraction from exploitation wells, spatial and temporal monitoring of groundwater quality, use of modern irrigation methods, implementation of artificial recharge projects, and collection, treatment, and reuse of wastewaters will play an essential role in preventing further degradation of groundwater quality. Mathematical modeling of groundwater flow and solute transport is suggested for the development of aquifer management strategies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdalla F (2016) Ionic ratios as tracers to assess seawater intrusion and to identify salinity sources in Jazan coastal aquifer, Saudi Arabia. Arab J Geosci. https://doi.org/10.1007/s12517-015-2065-3
Acworth I, Jankowski J (1993) Hydrogeochemical zonation of groundwater in the Botany Sands Aquifer, Sydney. Aus Geol Geophys 14:193–199
Ahmed MA, Abdel Samie SG, Badawy HA (2013) Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the eastern Nile Delta, Egypt. Environ Earth Sci 68:369–394. https://doi.org/10.1007/s12665-012-1744-6
Amiri V, Nakhaei M, Lak R, Kholghi M (2020) Investigating the salinization and freshening processes of coastal groundwater resources in Urmia aquifer, NW Iran. Environ Monit Assess. https://doi.org/10.1007/s10661-016-5231-5
Appelo C, Postma D (2005) Geochemistry, groundwater and pollution. Blackwell, London
Bagheri R, Bagheri F, Eggenkamp HGM (2017) Origin of groundwater salinity in the Fasa Plain, southern Iran, hydrogeochemical and isotopic approaches. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6998-6
Baghvand A, Nasrabadi T, Nabi Bidhendi Gh, Vosoogh A, Karbassi A, Mehrdadi N (2010) Groundwater quality degradation of an aquifer in Iran central desert. Desalination 260(1–3):264–275. https://doi.org/10.1016/j.desal.2010.02.038
Cao M, Hu A, Gad M, Adyari B, Qin D, Zhang L, Sun Q, Yu C-P (2022) Domestic wastewater causes nitrate pollution in an agricultural watershed, China. Sci Total Environ 1:2. https://doi.org/10.1016/j.scitotenv.2022.153680
Cartwright I, Weaver TR, Fulton S, Nichol C, Reid M, Cheng X (2004) Hydrogeochemical and isotopic constraints on the origins of dryland salinity, Murray Basin, Victoria, Australia. App Geochem 19(8):1233–1254. https://doi.org/10.1016/j.apgeochem.2003.12.006
Chang Y, Chen X, Guan Q, Tian C, Liu D, Xu D (2022) Study on the sources of salinity of groundwater in Holocene and Late Pleistocene sediments based on hydrochemical and isotopic methods in southern Laizhou bay. Water. https://doi.org/10.3390/w14172761
Cook PG, Jolly ID, Leaney FW, Walker GR, Allan GL, Fifield LK, Allison GB (1994) Unsaturated zone tritium and chlorine 36 profiles from southern Australia: their use as tracers of soil water movement. Water Resour Res 30:1709–1719. https://doi.org/10.1029/94WR00161
Dahlhaus PG, MacEwan RJ, Nathan EL, Morand VJ (2000) Salinity on the southeastern Dundas Tableland, Victoria. Aust J Earth Sci 47:3–11. https://doi.org/10.1046/j.1440-0952.2000.00759.x
Das CR, Das S, Panda S (2022) Groundwater quality monitoring by correlation, regression and hierarchical clustering analyses using WQI and PAST tools. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2021.100708
Dragon K (2006) Application of factor analysis to study contamination of a semi-confined aquifer (Wielkopolska Buried Valley aquifer, Poland). J Hydrol 331:272–279. https://doi.org/10.1016/j.jhydrol.2006.05.032
Falgas E, Ledo J, Marcuello A, Queralt P (2009) Monitoring freshwater-seawater interface dynamics with audiomagnetotelluric data. Near Surf Geophys 7(5–6):391–400. https://doi.org/10.3997/1873-0604.2009038
Farshadfar E (2010) Multivariate statistical methods. McGraw-Hill, Kermanshah
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hounslow A (1995) Water quality data, analysis and interpretation. CRC, London
IWRMC (2021) Groundwater situation in Yazd province, Iran Water Resources Management Company
Jahanshahi R, Zare M (2017) Delineating the origin of groundwater in the Golgohar mine area of Iran using stable isotopes of 2H and 18O and hydrochemistry. Mine Water Environ 36:550–563. https://doi.org/10.1007/s10230-017-0444-6
Johnson KS (1997) Evaporite karst in the United States. Carbonates Evaporates 12:2–14. https://doi.org/10.1007/BF03175797
Kesler SE, Gruber PW, Medina PA, Keoleian GA, Everson MP, Wallington TJ (2012) Global lithium resources: relative importance of pegmatite, brine and other deposits. Ore Geol Rev 48:55–69. https://doi.org/10.1016/j.oregeorev.2012.05.006
Kreitler CW (1993) Geochemical techniques for identifying sources of ground-water salinization. CRC, London
Kumar P, Biswas A, Banerjee S, Rathore S, Rana V, Ram K, Acharya T (2022) Integrating magnetic susceptibility, hydrogeochemical, and isotopic data to assess the seawater invasion in coastal aquifers of Digha, West Bengal, India. Environ Sci Pollut Res 29:23474–23503. https://doi.org/10.1007/s11356-021-16934-4
Kumar P, Biswas A, Banerjee S (2023) Spatio-temporal variability of seawater mixing in the coastal aquifers based on hydrogeochemical fingerprinting and statistical modeling. J Environ Manag. https://doi.org/10.1016/j.jenvman.2023.118937
Love D, Hallbauer D, Amos A, Hranova R (2004) Factor analysis as a tool in groundwater quality management: two southern African case studies. Phys Chem Earth 29(15–18):1135–1143. https://doi.org/10.1016/j.pce.2004.09.027
Marazuela MA, Vázquez-Suñé E, Ayora C, García-Gil A, Palma T (2019) Hydrodynamics of salt flat basins: The Salar de Atacama example. Sci Total Environ 651(1):668–683. https://doi.org/10.1016/j.scitotenv.2018.09.190
Mirzavand M, Ghazban F (2022) Isotopic and hydrochemical evidence for the source and mechanism of groundwater salinization in Kashan Plain aquifer in Iran. Environ Sci Pollut Res 29:34575–34593. https://doi.org/10.1007/s11356-021-17457-8
Nadri A, Bagheri R, Raeisi E, Ayatollahi SSh, Bolandparvaz-Jahromi K (2014) Hydrodynamic behavior of Kangan gas-capped deep confined aquifer in Iran. Environ Earth Sci 71:1925–1936. https://doi.org/10.1007/s12665-013-2596-4
Nguyen GT, Huynh NTH (2023a) Characterization of groundwater quality and human health risk assessment. Civ Eng J 9(3):497–510. https://doi.org/10.28991/CEJ-2023-09-03-09
Nguyen GT, Huynh NTH (2023b) Seasonal variations in groundwater quality under different impacts using statistical approaches. Civ Eng J 9(3):497–510. https://doi.org/10.28991/CEJ-2023-09-03-01
Noujavan R, Sadough H (2008) The geomorphology of Darangir playa. Territory 5(18):79–97
Panno SV, Hackley KC, Hwang HH, Greenberg SE, Krapac IG, Landsberger S, O’Kelly DJO (2006) Characterization and identification of Na–Cl sources in ground water. Ground Water 44(2):176–187. https://doi.org/10.1111/j.1745-6584.2005.00127.x
Paul R, Brindha K, Gowrisankar G, Tan LM, Singh MK (2019) Identification of hydrogeochemical processes controlling groundwater quality in Tripura, Northeast India using evaluation indices, GIS, and multivariate statistical methods. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8479-6
Rahman A, Mondal NC, Tiwari KK (2021) Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi-arid area of Rajasthan, India. Sci Rep. https://doi.org/10.1038/s41598-021-88600-1
Rittenhouse G (1967) Bromine in oil-field waters and its use in determining possibilities of origin of these waters. Am Assoc Pet Geol Bull 51:2430–2440
Rojas Fabro AY, Pacheco Avila JG, Esteller Alberich MV, Cabrera Sansores SA, Camargo-Valero MA (2015) Spatial distribution of nitrate health risk associated with groundwater use as drinking water in Merida, Mexico. Appl Geogr 65:49–57. https://doi.org/10.1016/j.apgeog.2015.10.004
Saberinasr A, Morsali M, Hashemnejad A, Hassanpour J (2019) Determining the origin of groundwater elements using hydrochemical data (case study: Kerman water conveyance tunnel). Environ Earth Sci. https://doi.org/10.1007/s12665-019-8182-7
Sarzamin Qanat (2021) Report of hydrolog of Kavire Daranjir district, Sarzamin Qanat Consulting Engineers
Shojaei Baghini S, Jahanshahi R, Mali S, Nasiri MA (2020) Destruction of groundwater quality and the risk of saltwater intrusion in the aquifers nearby Sirjan salt playa, Iran. Int J Environ Anal Chem 100(6):647–661. https://doi.org/10.1080/03067319.2019.1638373
Todd DK, Mays LW (2005) Groundwater hydrology. Wiley, New York
Vasudevan U, Gantayat RR, Chidambaram S, Prasanna MV, Venkatramanan S, Devaraj N, Nepolian M, Ganesh N (2021) Microbial contamination and its associations with major ions in shallow groundwater along coastal Tamil Nadu. Environ Geochem Health 43:1069–1088. https://doi.org/10.1007/s10653-020-00712-1
Ward JH Jr (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244. https://doi.org/10.1080/01621459.1963.10500845
WHO (2022) Guidelines for drinking-water quality. World Health Organization
Yang J, Ye M, Tang Z, Jiao T, Song X, Pei Y, Liu H (2020) Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J Hydrol. https://doi.org/10.1016/j.jhydrol.2020.124594
Zaidi FK, Nazzal Y, Jafri MK, Naeem M, Ahmed I (2015) Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4828-4
Zare HJ, Khaledian MR (2017) Study of spatial and temporal variations in groundwater salinity of Kerman province to use in drip irrigation of pistachio. Iran J Irrig Drain 11(5):810–821
Zarei M, Raeisi E, Merkel BJ, Kummer NA (2013) Identifying sources of salinization using hydrochemical and isotopic techniques, Konarsiah, Iran. Environ Earth Sci 70:587–604. https://doi.org/10.1007/s12665-012-2143-8
Funding
This research was financially supported by the Regional Water Company of Yazd, Yazd, Iran.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mohamad Hosein Rahimi. The first draft of the manuscript was written by Mohammad Faryabi. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faryabi, M., Rahimi, M.H. Factors controlling groundwater quality and salinization near the salt playa of Kavir-e Daranjir, central part of Iran. Sustain. Water Resour. Manag. 10, 99 (2024). https://doi.org/10.1007/s40899-024-01078-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01078-3