Abstract
Groundwater pollution from anthropogenic sources is a serious concern affecting several river basins and coastal aquifer environment. It is very important to acquire the impacts of the enhanced anthropogenic pressure and climatic changes on the evolution of groundwater characteristics in this study. The present study aims to concentrate on the hydrogeochemical characteristics by evaluating the groundwater quality through geographical information system (GIS) and multivariate statistical approach. Fifty groundwater samples were collected from the river basin and analyzed for major physicochemical parameters. The analytical results were interpreted in GIS and multivariate statistical techniques and demarcated the spatial variation of groundwater quality and their site-specific influencing factors over time. The presence of Na–Cl facies reflects the saline nature in groundwater in and around salt pan area. Factor analysis reveals the characteristics of four factors, and it accounted for 88.7% total variability. Significant correlation between TDS with major components of Na+, Mg2+, Cl− ions indicate the presence of saline influence on the groundwater. Cluster I and Cluster II represent fresh to slightly saline in nature, Cluster III mainly indicates the average concentration of EC (8615 µS/cm), chloride (2738 mg/l), and salinity (4.67 mg/l) is mainly due to small-scale industrial effluents, salt pan, agricultural activities, and rock–water interaction with related minerals in evaporite deposits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater lies at the heart of many water management issues faced in semiarid areas. India is one of the largest groundwater consumers in the globe, mainly dependent on 85% of drinking water supplies and 60% of irrigated agriculture. Different states of India are facing a number of problems in the groundwater quality and quantity. High levels of salinity, nitrate, and fluoride come across in several states, and microbial pollution affects the shallow aquifers throughout India (CGWB 2010). Since 2000, a drastic increase in groundwater demand in India has induced various groundwater degradation mostly groundwater depletion, land subsidence due to continuous extraction, and saline water intrusion. If current trends continue, in 20 years about 60% of all India’s aquifers will be in a critical condition (World Bank Report 2012). The groundwater quality contamination assessment requires monitoring and measuring of a wide range of physical, chemical, and biological parameters. The geochemical processes are responsible for the spatial and seasonal variation of groundwater chemistry (Krishna Kumar et al. 2011) and give vital information on suitability for drinking and irrigation, recharge area, lithological characters, and anthropogenic pressure. Numerous studies have been conducted to evaluate the coastal groundwater characteristics in southern parts of India (Purushothaman et al. 2013; Subba Rao et al. 2012; Elango et al. 2003; Gajendran and Thamarai 2008; Chidambaram et al. 2009; Selvam et al. 2013; Sivasubramanian et al. 2013; Sridhar et al. 2013; Srinivas et al. 2014; Srinivasamoorthy et al. 2014; Rajesh et al. 2015; Selvakumar et al. 2014, 2015). An intensive anthropogenic activity and lack of rainfall have influenced the coastal aquifer systems, leading to seawater intrusion. Coastal saline water intrusion creates a major concern for agricultural, industrial, and various other domestic purposes and its safe uses. The GIS-based kriging interpolation techniques and multivariate statistical analyses, such as Pearson’s correlation matrix, principal component analysis, and hierarchical cluster analysis (HCA), help to interpret the huge dataset for a better understanding of the ecological status and groundwater pollution sources in the coastal river basin.
In the Karamaniyar river basin, groundwater is the main source for the purposes of domestic, agricultural, and industries; however, water accessibility and quality is reduced due to many anthropogenic behaviors such as arid evaporation, development of salt pans, agricultural activities, coastal urbanization, and industrial development (power plant and coastal sand mining). These activities are associated with the use of water as an input material. The surface water resources in this basin are not much appreciative rainfall due to semiarid climatic conditions; therefore, the extraction of water for such needs depends on groundwater. Groundwater is a major source of water supply in most of the small-scale industries and irrigation. A large number of industrial and mining activities have changed the geomorphological and hydrological settings. Thus, preserving and conserving this natural resource have become an increasingly important issue in the area. Therefore, it is important to assess and monitor the aquifer system in the Karamaniyar river basin since its hydrogeochemistry is poorly understood and no significant work has been so far reported. In recent years, multivariate statistical techniques coupled with PHREEQC software and geographical information systems (GIS) have been applied to extract information from the hydrogeochemical data in complex systems. This multidisciplinary approach will be useful to identify the different physiochemical processes and pollution sources in the groundwater in complex aquifers. Salt pan activities and seawater intrusion, particularly in the coastal areas, create a major concern for agricultural, industrial, and various other domestic purposes and its safe uses. The application of various multivariate statistical techniques, such as principal component analysis, factor analysis, and cluster analysis, aids in the interpretation of complex data for a better understanding of water quality and ecological status of the study region (Abin et al. 2014). Factor analysis was found to be a helpful method for grouping the groundwater quality parameters according to their sources and provides information about their natural and anthropogenic origin. The present research aims to address the drinking and irrigation suitability assessment, hydrogeochemical characteristics with an evaluation to groundwater degradation through the help of GIS and multivariate statistical approach.
2 Study area
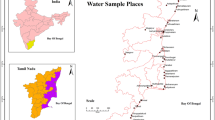
The Karamaniyar river basin is located in the southeast coast of India with the latitude extension of 8°31′00″E–8°54′00″E and longitude extension of 77°66′00″N–78°06′00″N which covers the parts of Tirunelveli and Tuticorin districts of Tamil Nadu state and (Fig. 1) bounded by the three sub-basins of different rivers, namely Tamiraparani, Pacchayar, and Nambiyar and the Bay of Bengal in the southeast. The river originates from the upland of western parts and flows through semiarid landforms toward southwest and confluences with Bay of Bengal (Fig. 2). The river basin is generally a plain terrain with a gentle slope toward south and east. There is a sand dune namely “Teri sand” in the south of Sattankulam having an elevation of 67 m. Similar formations can also be noticed in and around the villages of Kuttam and Uvari in the south of Thisaiyanvilai. The study area prevails subtropical climatic condition with poor precipitation throughout the year. The optimum temperature extends up to 32–39 °C. The annual average rainfall is 280 mm, and maximum rainfall receives during the NE monsoon (444 mm) than the SW monsoon (117.7 mm). The groundwater is an important source for drinking and irrigation purpose and influences the livelihood of the local people.
2.1 Geological and hydrological settings
Various rock types and the structural details of Karamaniyar river basin were collected from the Geological Survey of India (GSI 1995). The host rocks of coastal plains are mostly quaternary and recent age. It is generally hard, massive and shows a modular structure. The Migmatite complex consists of granite gneiss. The rocks of the Migmatite group are widely distributed and interlayered with Charnockite in the central and southern parts of the basin. Garnet–biotite gneiss occurs as bands and lenses and stands out as raised ridges. It is characterized by the presence of biotite foliation and concentration of garnet in layers. At places, the garnet–biotite gneiss also carries segregations of graphite flakes. The formation comprising of hard sandstone and calcareous shelly limestones is noticed across the north of Sattankulam. Small patches of limestone deposits are noticed in and around the river basin. Teri sands occur in Tisaiyanvilai (Ittamozi Teri) and mid of Sattankulam (Kudiramoli Teri) with a considerable thickness ranging from 20 to 35 m and wide portion of the river basin is dominated by water bodies, agricultural land, barren land, and salt pan/salt flat (Fig. 3).
The important aquifer systems are comprised of semi-consolidated and unconsolidated formations with fractured crystalline rocks. In hard rock areas, the weathered zone exists up to 25 m below ground level (mbgl) underlain by fractures up to 30 mbgl as per lithology of boreholes. In Nanguneri, Vadaku Valliyur, and Vijaynarayanapuram areas, bore well yields vary from 54.45 to 295 L per minute (lpm). The aquifer transmissivity in this region is between 10 and 20 m2/day, and the weathered zones exist up to 20 mbgl followed by fractures up to 40 mbgl. In the western part of the basin (Panagudi and Radhapuram areas), the weathered mantle persists from 30 to 45 mbgl and fractures continue up to 50 mbgl. The specific yield of the bore wells ranges from 15 to 80 lpm, and the aquifer transmissivity was found between 2 and 40 m2/day. In the sedimentary formations (Teri sands), the yield of bore wells ranges from 200 to 1950 lpm and in Tertiary sandstones of coastal alluvium area ranges from 75 to 1045 lpm. The groundwater level depth is varied between 1.35 and 14.80 mbgl at the pre-monsoon and 0.74 and 10.62 mbgl through post-monsoon. Most of the wells are experiencing seasonal groundwater level fluctuations when they are not exploited or are moderately used. Ground water quality of Tirunelveli is slightly alkaline in nature. The electrical conductance of ground water in phreatic zone (during May 2006 was in the range of 510–9320 µS/cm. And major parts are having the electrical conductivity below 2000 µS/cm. The quality of groundwater in porous formation shows variations with depth. The exploration at Puttataruvai revealed the presence of good-quality water in the depth range of 24–32 m while the saline water was noticed in the depth range of 49–54 and 75–78 mbgl (CGWB 2010).
3 Materials and methods
3.1 Sampling methods
Fifty groundwater samples were taken at different sites representing open wells within the river basin. The groundwater level was measured in all the sampling wells, using a water-level recorder. The collected samples were filtered on-site using a fine-grade filter paper (Sartorius—0.45 µm) and collected in two separate 500-ml HDPE (high-density polyethylene) bottles. For anion analysis, filtered unacidified samples were used, and for cation analysis, samples were acidified with ultrapure HNO3 to pH < 2 to avoid precipitation and absorption. All the sample bottles were double-capped and sealed with parafilms and transported to the laboratory at 4 °C.
3.2 Analytical methods
Major physicochemical parameters of the groundwater samples were analyzed according to the standard methods described by APHA (1995). In situ parameters such as pH, EC, and TDS were measured using HANNA portable water quality probe (HI-9828, USA). For the chemical analysis, calcium (Ca2+) and magnesium (Mg2+) concentrations were determined by ethylene diamine tera-acetate (EDTA) titration. The sodium (Na+) and potassium (K+) ions were measured using digital flame photometer (DEEP VISION, Model-381)..Chloride (Cl−) concentration was estimated by titration method using silver nitrate (0.01 N) along with potassium chromate (5%) indicator. The bicarbonate (HCO3 −) content was estimated by H2SO4 titration (0.01 N). Sulfate (SO4 2−) estimation was done using UV/Visible spectrophotometer. Equipments is well maintained, cleaned, and fully calibrated and standardized before the analysis. To keep errors in the field sampling and laboratory analysis to a level acceptable to meet the objectives of the sampling campaign, a Quality Assurance (QA) program framework APHA (1995) was adopted. Quality control measures applied involved the use of blank samples—two blank samples were collected during the sampling. For analytical accuracy of each groundwater samples, the cation and anion concentrations were used to compute the ionic balance error, which was observed to be within the standard limit of ±10%.
The analytical results were compared to World Health Organization (WHO 2004) in accordance with the drinking suitability. The irrigation suitability of groundwater was estimated by certain indices. Hydrochemical data were exported into GIS platform and analyzed in ArcGIS software version 10.2. The Aquachem Scientific v4.0 software was used to construct piper trilinear diagram and Wilcox plot. The USGS (United State Geological Survey) software PHREEQC was used to simulate the aqueous speciation and mineral saturation indices. Multivariate statistical methods such as Pearson correlation matrix, principal component analysis, and cluster analysis were performed using IBM SPSS 20 statistical software.
4 Results and discussion
4.1 Geochemical and geospatial screening of groundwater quality data
The statistical summary of hydrochemical parameters is summarized in Table 1. The pH values in the study ranged between 6.72 and 8.04 with an average of 7.23 indicating mainly alkaline in nature. The EC values varied between 200 and 9840 µs/cm. TDS varies from 128 to 6297.6 mg/l with mean value of 1402.3 mg/l. Low amount of TDS (<1000 mg/l) is observed in northeast and western part of the river basin (Fig. 4a). It may be due to immense surface water recharge compare to a high concentration of TDS (slightly saline 1000–3000 mg/l). Relatively, a high concentration of TDS (moderately saline 3000–10,000 mg/l) is noticed in the groundwater samples (11, 12, 17) falling in the low topography region near to the coast, which is probable around the salt pan regions and neat the coast. It is the main reasons for such high concentration, where high saline water infiltrates into the groundwater and may be due to seawater intrusion. Moreover, the concentration of TDS in groundwater differs due to the degree of mineral solubility in heterogeneous geological regions. This is in agreement with the geochemical modeling-based saturation index of aragonite, calcite, and dolomite (Fig. 5). Drinking water with TDS concentration greater than 1000 mg/l (WHO 2004) is unpalatable to most consumers. 70% of the sample locations exceeds the tolerable limit (1000 mg/l) and becomes unfit for drinking.
The order of cation abundance is Na+ > Ca2+ > Mg2+ > K+, and the order of anion abundance is Cl− > HCO3 − > SO4 2−. The spatial distribution of Na+, Mg2+, HCO3 −, and SO4 2− is depicted in Fig. 6. The sodium values are varied between 4.03 and 930 mg/l. The increasing concentration of Na+ in majority of the samples suggests the process of cation exchange, mineral dissolution (Fig. 7), and salt pan activities. Sodium-rich montmorillonite clay reacts with calcium and magnesium and releases sodium into the groundwater. The higher amount of sodium in the groundwater indicates the reverse ion exchange process, where highly saline water is exposed to calcium-rich clays (Srinivasamoorthy et al. 2014). The calcium concentration in groundwater is varied from 15 to 821 mg/l, with a mean value of 134 mg/l and magnesium values ranges from 8 to 454 mg/l (average of 87.36 mg/l). The principal sources of Ca2+ and Mg2+ in the majority of the samples indicate the dissolution of carbonate rich minerals (Fig. 5), rock-forming minerals, a degree of silicate weathering, and anthropogenic activities.
The chloride values ranged from 25 to 3101 mg/l with an average value of 482 mg/l. Chloride is a major inorganic anion present in all natural waters. The spatial distribution of chloride (Fig. 4b) indicates high concentration (>2000 mg/l) in the sampling wells (11, 12, and 17) along the coastal region. This is due to saline infiltration from salt pans, liquid and solid domestic discharge, irrigation return flow, and percolation of saline residues into the soil. Further, the high concentration of chloride resulting from evaporation can be considered for understanding the degree of mineralization in groundwater. Extreme amount of chloride has led to the corrosion of metals in the water distribution system and it depends on alkalinity (Selvakumar et al. 2012). The sulfate concentrations varied between 9 and 335 mg/l with an average of 146 mg/l. The TDS and the concentrations of sodium, calcium, magnesium, chloride, and sulfate in the majority groundwater samples are beyond the permissible limits prescribed for drinking purposes (WHO 2004).
4.2 Groundwater quality analysis for irrigation purpose
The average EC value is 2191 μS/cm, attributing the dissolution of minerals, infiltration from salt pans and in situ salinity. The salinity hazard classes indicate that 10% of the groundwater samples fit in excellent to good category, and all the remaining samples indicate doubtful to unsuitable type for irrigation. The sources for salinity are mainly derived from nearby salt pans, irrigation return flow, municipal wastes, industrial activities, and through a natural source such as rock–water interaction. The SAR value in the groundwater varies from 0.40 to 8.43. Table 2 explains 82% of the samples falls under very low to low sodium hazards. 18% of the groundwater samples are found to be in a medium hazard. Sodium forms alkaline soil when combined with carbonates and saline soils are associated with chlorides, which do not support plant growth. The elevated concentration of sodium mainly affects the soil permeability (Chandrasekar et al. 2013). The permeability index values range from 28.5 to 73.6%. The computed concentration of %Na varied between 13.5 and 69. The relation between electrical conductivity (EC) and percentage of sodium (%Na) is plotted for assessing the groundwater quality for irrigation. The plot classifies the suitability of groundwater quality into five fields (Fig. 8). The Wilcox plot highlights 68% of the groundwater samples are noticed from the zone of excellent to permissible category, 10% belongs to doubtful to unsuitable, and remaining 22% falls under the unsuitable category for irrigation use. Magnesium hazard values ranged from 20.7 to 76.4. Twenty-three groundwater samples are exceeding the allowable limit (>50), which indicates unsuitable for irrigation because it harmfully affects the crop yield as the soils become more alkaline. 54% of the sampling sites were within the acceptable limit (<50), so it can be suitable for agriculture purpose.
4.3 Hydrogeochemical facies
The term “hydrochemical facies” is used to differentiate the chemical character of the chemical interaction with rock and soil of the aquifer. The piper (1944) plot highlights the presence of four types of groundwater in the Karamaniyar river basin (Fig. 9). The more than half of the ground samples (66%) are mixed Ca–Mg–Cl-dominated fluids, followed by Ca–HCO3 type (16%), Ca–Cl type (8%), reflecting mixed sources of water drained from gneisses (garnet–biotite and hornblende–biotite) and fluvial–marine terrain at certain parts of the study area. These three facies indicate that the samples are related with alkaline earth ions like Ca2+, Mg2+, and a strong acidic anion of Cl−. The fourth group shows Na–Cl-type (10%) facies, and theses samples are found to be located near the coast as well as salt pan region and the northern part of the river basin. Jones et al. (1999) pointed out that Na–Cl type indicates anthropogenic contamination due to salt pan activities, disproportionate use of chemical fertilizers in the coastal land use, irrigation return flow and may be seawater intrusion. These relationships are well visualized from land-use map (Fig. 3). However, no comparison on hydrochemical changes is made due to the non-availability of previous studies in the study region.
4.4 Factors affecting the groundwater chemistry in Karamaniyar river basin
Mechanism of controlling factors in groundwater chemistry is evaluated by Gibbs (1970). He has recommended a diagram where the ratio of leading cations (Na+/(Na+ + Ca2+)) and anions (Cl−/(Cl− + HCO3 −) were separately plotted in opposition to the total dissolved solids. Figure 10 shows that the majority of the groundwater samples are falling under the evaporation dominance field. Evaporation is the dominant process in the area due to dry and semiarid condition with intensive salt pan activities. Evaporation of irrigation water will take out water and leave the salts behind. More salts can be dissolved from the soil as irrigation water percolates downward. Rock–water interaction is another important factor controlling groundwater chemistry in the aquifer system, which is due to ionic exchange of soil and rock, chemical weathering of calcareous limestone, carbonate-bearing minerals with lesser extend of gypsum and halite dissolution (Krishna Kumar et al. 2011). Geochemical modeling techniques using PHREEQC will aid in demarcating the main factors and mechanisms controlling the chemistry of groundwater (Srinivasamoorthy et al. 2014). The calculated values of saturation indices for gypsum, halite, and anhydrite are found within under saturation state. The carbonate group minerals are found in oversaturation state in dolomite, calcite, and aragonite, indicating that these carbonate-bearing minerals have influenced the chemical composition of the Karamaniyar river basin. The Gibb’s plot and SI indicate the cumulative effect of evaporation, and rock–water interaction is the prime source of salinity in the groundwater system.
5 Multivariate statistical analysis
The multivariate statistical methods such as correlation matrix, factor analysis, and cluster analysis are broadly used as impartial methods in the study of groundwater quality data for meaningful interpretation (Singh et al. 2005; Singh et al. 2013). Pearson correlation matrix is commonly used to evaluate the strength of a linear relationship between two variables. It helps in the identification of influencing factors, relationship among variables, and source apportionment. Correlation matrixes among various hydrochemical parameters are calculated, and the values of the correlation coefficient are given in Table 3. Most ions of the groundwater show very strong positive correlation with Cl− particularly TDS (0.973), Mg2+ (0.963), Na+ (0.925), and Ca2+ (0.844), which points out that these ions are largely derived from industrial, irrigational, and salt pan activities, in addition to an interaction between rock and water.
Factor analysis using PCA method is carried out using a set of variables consisting of 50 groundwater samples with 11 physical and chemical parameters for characterizing the major variables affecting the groundwater quality. This method is used to deduce the source from a common origin. Major cations and anions, EC, TDS, salinity, and pH have been considered for the present analysis. Six principal components are extracted and rotated using the varimax normalization. The factor loadings >0.5 are considered as significant in the interpretation of the data (Table 4). The result explains four selected factors, which represent about 88.7% of the total variance and reflect different controlling factors of groundwater quality. PCA 1 is the main significant factor, representing large proportion (56.723%) of the total variance. The high positive loading on (>0.942) for TDS, salinity, Cl−, Mg2+, and Na+, shows sensible positive loading for Ca2+ (0.832) and SO4 2− (0.694). The high absolute loadings probably indicate the result of mineral water reactions, and it can be recognized to the groundwater salinization in the coastal region mainly SE to NW part of the river basin. The spatial distribution map of TDS and Cl− (Fig. 3) proved higher values for this region. The other positive loadings for Ca2+ and SO4 2− is mainly attributed from natural influences mainly dissolution of carbonates and sulfates, in addition using an excessive amount of synthetic fertilizers for plant growth. PCA 2 accounts for 13.1% of the total variance, which is mainly associated with high loading of pH with significant negative loading of HCO3 2−. PCA 3 accounts for 10.5% of the total variance and shows extreme negative scores that reflect areas unaffected by the natural and anthropogenic process.
Hierarchical cluster analysis was useful to detect similarities involving the different sampling sites. It joins the most similar observation first, followed by consecutive observations and the intensity of similarity is used to develop a dendrogram based on similar observations. 11 variables (pH, EC, TDS, salinity, Ca2+, Mg2+, Na+, K+, HCO3 −, Cl−, and SO4 2−) were first subjected to cluster analysis. It explains that the sampling sites are grouped into three clusters, and the mean parameter values of the three primary groundwater clusters (determined from HCA) are presented in Table 5. Group I represents 34 stations and accounts for 70% of the groundwater samples (Fig. 11). It mainly located in the NE and SW parts of the study area and relatively fresh with a mean TDS of 824.4 mg/l. This group is mainly HCO3 2− dominated; however, Ca2+ and Mg2+ are also present in a significant amount. Group II represents 13 sampling stations, and it occupies 24% of the groundwater samples. The mean TDS for this group is 2060 mg/l, which are the characteristics of slightly saline (mean value of salinity is 1.544 mg/l) and unsuitable for human consumption due to high salinity and higher ionic content which exceeds the drinking water guideline values (WHO 2004). Group III includes three groundwater samples (sampling stations 12, 17, and 32) and the mean concentrations of EC (8615 µs/cm), TDS (5513.6 mg/l), salinity (4.67 mg/l), chloride (2738 mg/l), and sodium (753.3 mg/l). The sampling sites 12 and 17 are near to salt pan and agricultural-rich areas. Sampling site 32 shows higher salinity (4.36 mg/l), mostly occupied by moisture land with the altitude of 96 m above MSL and enriched in rough stone and gravel. The higher salinity may be due to the effect of evaporation and dissolution of carbonate minerals (Belkhiri et al. 2010). The rough stone and gravel quarry will not create toxic effluence in the form of solid and liquid, but it can be possible to induce salinity level. However, the small quantity of municipal wastes, agricultural activities, and rate of evaporation will be the causative factor for salinization. Three distinct processes control the salinization in the groundwater (1) influence of salt pans, (2) seawater intrusion along the near coastal region, and (3) salinity through ion dominance. The seawater intrusion along the coastal aquifers is characterized by the dominance of sodium and chloride ratios. The salt pans also show similar characteristics, but they are clustered around the high salt pan-dominated areas. In the case of ion dominance, isolated patches through the spatial distribution of ions like calcium, magnesium, sulfate, and salinity apart from sodium and chloride are noticed in the study area. These three processes could have influenced the salinity in the study area, and it is evident from the factor analysis. The multivariate statistical analysis reveals that the groundwater is dominated by salinization along the coastal aquifers, salt pans also contribute to salinization around the salt pan-dominated areas. Evaporation controls the groundwater chemistry in the study area, as recharge by rainfall is scarce. Freshwater occurrence in the inner part of the study area is dominated by bicarbonate ions, and rock–water interaction regulates the dissolution of calcium and magnesium ions in the groundwater of the study area.
6 Conclusion
The concentrations of TDS, sodium, magnesium, and chloride have been observed in many groundwater samples, indicating moderate pollution and higher than the recommended limits with unsuitable for drinking and domestic usage. The high concentration of groundwater salinization is ascertained from following factors: (a) industrial activities, the discharge of wastewater from small-scale industries, and salt pans. It will not produce toxic effluence, but it influences the salinity through percolation into the soil profile; (b) irrigation activities, the majority of the land uses in Karamaniyar basin practices irrigated, and a minor amount of non-irrigated activities taking place in the alluvial and colluvium deposits of the coastal region. Extraction of groundwater, as well as river water to spread on land, accelerates the salts that are accumulated through evaporation. It has indirect effects on logging of water and soil salinization when groundwater levels rise nearer to the ground surface. The capillary action has permitted to accumulate the salt in the subsurface water, besides an excessive use of chemical fertilizer is causing degradation of water quality in the shallow aquifer system and over extraction of groundwater from basins near the coast induces seawater intrusion into the freshwater aquifer where the over withdrawal occurred and leads to the expansion of areas of degraded water quality, as pumpers rearrange wells to take advantage of better quality water in deeper aquifers or in aquifers farther inland. (c) Natural occurrence of fluviomarine deposits, evaporites, and rock–water interaction through dissolution, precipitation, decomposition of calcareous limestone in the bedrock. This study demonstrated the GIS coupled with multivariate statistical analysis to evaluate the groundwater quality in the study region is well described and can also address to solve the problems with various parameters. It also helps to understand the nature of salinization and degradation of groundwater quality in the river basin.
References
Abin, A., Sibin, R., Aghil, T. B., Magesh, N. S., & Sridhar, S. G. D. (2014). Statistical evaluation of groundwater geochemistry: a case study between Chinnakuppam and Kulathur, South Chennai, Tamil Nadu, India. Journal of Coastal Sciences, 1(1), 58–62.
APHA. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington, DC: APHA.
Belkhiri, L., Boudoukha, A., Mouni, L., & Baouz, T. (2010). Multivariate statistical characterization of groundwater quality in Ain Azel plain, Algeria. African Journal of Environmental Science and Technology, 4(8), 526–534.
CGWB. (2010). Ground water quality in shallow aquifers of India. Faridabad: Central Ground Water Board Ministry of Water Resources.
Chandrasekar, N., Selvakumar, S., Srinivas, Y., John Wilson, J. S., Simon Peter, T., & Magesh, N. S. (2013). Hydrogeochemical assessment of groundwater quality along the coastal aquifers of southern Tamil Nadu, India. Environmental Earth Sciences, 71, 4739–4750.
Chidambaram, S., Senthil Kumar, G., Prasanna, M. V., Peter, A. J., Ramanthan, A. L., & Srinivasamoorthy, K. (2009). A study on the hydrogeology and hydrogeochemistry of groundwater from different depths in a coastal aquifer: Annamalai Nagar, Tamilnadu India. Environmental Geology, 57(1), 59–73.
Elango, L., Kannan, R., & Senthilkumar, M. (2003). Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram District, Tamil Nadu, India. Journal of Environmental Geosciences, 10(4), 157–166.
Gajendran, C., & Thamarai, P. (2008). Study on statistical relationship between ground water quality parameters in Nambiyar River basin, Tamil Nadu, India. International Journal on Pollution Research, 27(4), 679–683.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 17, 1088–1090.
GSI. (1995). Geological and mineral map of Tamil Nadu, India. Geological Survey of India. Scale 1:500,000, 1 sheet.
Jones, B. F., Vengosh, A., Rosenthal, E., & Yechieli, Y. (1999). Seawater intrusion in a coastal aquifers-concepts, methods and practices (pp. 51–71). Dordrecht, The Netherlands: Kluwer Academic Publishers.
Krishna Kumar, S., Chandrasekar, N., Seralathan, P., Godson, P. S., & Magesh, N. S. (2011). Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island, India. Environmental Monitoring and Assessment, 184, 4127–4138.
Piper, A. M. (1944). A graphic procedure in geochemical interpretation of water analysis, Transactions. American Geophysical Union, 25(6), 914–928.
Purushothaman, P., Someshwar Rao, M., Rawat, Y. S., Kumar, C. P., Krishan, G., & Parveen, T. (2013). Evaluation of hydrogeochemistry and water quality in Bist-Doab region, Punjab, India. Environmental Earth Sciences. doi:10.1007/s12665-013-2992-9.
Rajesh, R., Brindha, K., & Elango, L. (2015). Groundwater quality and its suitability and its hydrochemical characteristic in a shallow weathered rock aquifer of Southern India. Water Quality Exposure and Health, 7(4), 515–524.
Selvakumar, S., Chandrasekar, N., & Magesh, N. S. (2012). Preliminary investigation of groundwater quality along the coastal aquifers of southern Tamil Nadu using GIS techniques. Bonfring International Journal of Industrial Engineering and Management Science, 2(1), 46–52.
Selvakumar, S., Chandrasekar, N., Srinivas, Y., Simon peter, T., & Magesh, N. S. (2014). Evaluation of the groundwater quality along coastal stretch between Vembar and Taruvaikulam, Tamil Nadu, India; a statistical approach. Journal of Coastal Sciences, 1, 22–26.
Selvakumar, S., Ramkumar, K., Chandrasekar, N., Magesh, N. S., & Kaliraj, S. (2015). Groundwater quality and its suitability for drinking and irrigational use in the southern Tiruchirappalli district, Tamil Nadu, India. Applied Water Science. doi:10.1007/s13201-014-0256-9.
Selvam, S., Manimaran, G., & Sivasubramanian, P. (2013). Hydrochemical characteristics and GIS-based assessment of groundwater quality in the coastal aquifers of Tuticorin corporation, Tamilnadu, India. Applied Water Science, 3, 145–159.
Singh, E. J. K., Gupta, A., & Singh, N. R. (2013). Groundwater quality in Imphal west District, Manipur, India, with multivariate statistical analysis of data. Environmental Science and Pollution Research, 20, 2421–2434.
Singh, K., Malik, A., & Sinha, S. (2005). Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques—A case study. Analytica Chimica Acta, 538, 355–374.
Sivasubramanian, P., Balasubramanian, N., Soundranayagam, J. P., & Chandrasekar, N. (2013). Hydrochemical characteristics of coastal aquifers of Kadaladi, Ramanathapuram District, Tamilnadu, India. Applied Water Science, 3, 603–612.
Sridhar, S. G. D., Kanagaraj, G., Mahalingam, S., & Amaladas, P. (2013). Hydrochemical analysis of Groundwater between Sadras and Chinnakuppam, Kancheepuram District, Tamil Nadu, India. Journal of Academia and Industrial Research, 2(3), 160–166.
Srinivas, Y., Hudson Oliver, D., Stanley Raj, A., & Chandrasekar, N. (2014). Quality assessment and hydrogeochemical characteristics of groundwater in Agastheeswaram taluk, Kanyakumari district, Tamil Nadu, India. Chinese Journal of Geochemistry, 33, 221–235.
Srinivasamoorthy, K., Gopinath, M., Chidambaram, S., Vasanthavigar, M., & Sarma, V. S. (2014). Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. Journal of King Saud University-Science, 37(1), 51–52.
Subba Rao, N., Surya Rao, P., Venktram Reddy, G., Nagamani, M., Vidyasagar, G., & Satyanarayana, N. L. V. (2012). Chemical characteristics of groundwater and assessment of groundwater quality in Varaha River Basin, Visakhapatnam District, Andhra Pradesh, India. Environmental Monitoring and Assessment, 184, 5189–5214.
Wilcox, L. V. (1955). Classification and use of irrigation waters. US Department of Agriculture. Arc 969, Washington, DC.
World Bank Report. (2012). Report for understanding and improving groundwater Governance. 71742. http://www.worldbank.org/water.
WHO. (2004). Guidelines for drinking water quality (3rd ed.). Geneva: World Health Organization.
Acknowledgments
The work was carried out as part of the research project supported by Department of Science and Technology, Government of India, with No. DST/WAR-W/WSI/10/2010 to the author NC. The author thanks the Central Groundwater Board (CGWB), Government of India, for providing baseline data. The authors would like to thank the anonymous reviewers for their valuable constructive comments and suggestions to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvakumar, S., Chandrasekar, N., Kaliraj, S. et al. Salinization of shallow aquifer in the Karamaniyar river basin, Southern India. Environ Dev Sustain 20, 1255–1273 (2018). https://doi.org/10.1007/s10668-017-9937-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-017-9937-5