Abstract
Papaya sticky disease (PSD) is an emerging disease-causing significant crop loss in some of the major papaya-growing regions of the world. The vectors of the PSD associated viruses in Brazil are still unknown. The papaya meleira virus complex comprised of a fusagra-like virus, papaya meleira virus (PMeV), and a umbravirus-like associated RNA (ulaRNA), papaya meleira virus 2 (PMeV2) is found infecting diseased papaya plants in Brazil. PMeV capsid protein packages both PMeV and PMeV2 genomes separately resulting in virions with the same morphology. Epidemiological analyses attributed fruit thinning as a mechanical mechanism responsible for the spread of sticky disease, but an aerial vector was not ruled out. Hemipteran insects have been implicated as vectors but a definitive conclusion on the biologically relevant vector has not been reached. Cicadellids have a population peak a month before the peak of papaya sticky disease incidence in the field and their ability to acquire and transmit the Mexican isolate of PMeV has been demonstrated. Whitefly (Bemisia tabaci MEAM1) is not considered a papaya pest in Brazil but has been reported to occur in plants near papaya trees and they transmit an Ecuadorian virus similar to PMeV2. In Brazil, Trialeurodes variabilis which colonizes papaya trees can acquire, but not transmit the PMeV complex. In this review, we discuss transmission assays and epidemiological analysis conducted in the last 30 years; the similarity of the PMeV complex capsid protein with viruses that infect fungi; the challenges imposed by laticifers, a well-known plant defense structure, in the acquisition of viral particles; and the presence of PMeV2. Elucidation of the PMeV complex vector would contribute to the efficient management of papaya sticky disease and increase understanding of the transmission mechanisms of plant-infecting fusagra-like viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papaya sticky disease (PSD) is a significant threat to papaya orchards in various countries, including Brazil (Rodrigues et al. 1989), Mexico (Perez-Brito et al. 2012), Ecuador (Quito-Avila et al. 2015), and Australia (Pathania et al. 2019), with the potential to result in complete crop loss. The distribution of PSD under field conditions points to possible mechanical transmission and an aerial vector, but the PSD insect-vector is still unknown in Brazil (Abreu et al. 2015b; Antunes et al. 2020), even though several transmission assays and epidemiology analyses have been done in the last 30 years. In Mexico, the dispersion occurs through Empoasca papayae Oman, 1937 (Hemiptera: Cicadellidae) (García-Cámara et al. 2019) and through the infected seeds (Tapia-Tussell et al. 2015). The presence of a vector in Australian orchards has not yet been confirmed, but seeds play an important role in the spread of the disease (Campbell 2019b; 2019a). In Ecuador, potential aerial vectors have been proposed for the three identified viruses. Recent transmission tests have now indicated that whiteflies serve as vectors for both papaya virus Q (PpVQ) (Cornejo-Franco et al. 2018) and papaya virus E (PpVE) (Cornejo-Franco et al. 2022).
An intricate correlation regarding the biotic agent has been observed among papaya-producing countries. In Brazil, the presence of a biotic agent responsible for PSD is dated in a 1989 publication showing that inoculation of infected latex leads to symptom development in healthy plants (Rodrigues et al. 1989). The etiology of disease was initially documented in the latex of diseased plants (Kitajima et al. 1993) and confirmed in 2003 as being caused by a virus (Maciel-Zambolim et al. 2003), and later on tentatively classified in family Totiviridae (named as totivirus-like). It was only in 2016 that a second virus was identified in mixed infections with the totivirus-like virus in diseased plants (Fig. 1a) (Antunes et al. 2016), grouped in the umbravirus-like associated RNA (ulaRNA) class 2 clade (Liu et al. 2021). However, the relationship between PMeV phylogenetics and fungi and insect-infection allowed a new designation belonging to the fusagra-like virus, order Ghabrivirales (which includes Totiviridae) (Maurastoni et al. 2023). In Mexico and Ecuador, diseased plants are associated with an ulaRNA similar to the one found in Brazil (Perez-Brito et al. 2012; Quito-Avila et al. 2015; Liu et al. 2021). The auxiliary virus has yet to be found in papaya plants in both Mexico and Australia. On the other hand, it has been reported the presence of the PpVQ’s auxiliary virus (the umbra-like virus) in Ecuador, named as papaya stick fruit-associated virus (PSFaV), tentatively classified as a toti-like virus (Fig. 1b) (Quito-Avila et al. 2023). However, none of them has yet been taxonomically classified by the International Committee on Taxonomy of Viruses - ICTV.
Genomic organization of papaya meleira virus - PMeV (blue), papaya meleira virus 2 - PMeV2 (pink), papaya stick fruit-associated virus - PSFaV (yellow) and papaya virus Q - PpVQ (green) isolates showing their open reading frames and their putative encoded proteins. (a) PMeV-ES: ORF1 encodes the capsid protein (CP) (1,563 aa) and ORF2 encodes the RNA-dependent RNA polymerase (RdRp) (1,147 aa). PMeV has a potential slippery heptamer, (GGAAAAC) at nt 5,297–5,303, immediately before the ORF1 stop codon which is followed by a stable secondary structure, such as a pseudoknot or hairpin. This genomic organization, typically found in totiviruses, leads to translation of a CP-RdRp fusion protein by a -1 frameshift (FS). PMeV2: ORF1 encodes a hypothetical protein (270 aa) and ORF2 encodes the RdRp (473 aa); Hypothetical proteins are indicated with an asterisk. NCBI accession numbers: PMeV (KT921784) and PMeV2 (KT921785); (b) PSFaV: ORF1 encodes the capsid protein (CP) (1,592 aa) and ORF2 encodes the RNA-dependent RNA polymerase (1,088 aa). PSFaV has a fused protein with a molecular mass of 310 kDa. PSFaV has a potential slippery heptamer (GGAAAAC) at nt 5,509–5,515, positioned before the ORF1 stop codon that lead to the translation of CP-RdRp (fusion protein) for − 1 ribossomal FS. PpVQ: ORF1 encodes a hypothetical protein (33 kDa) and ORF2 encodes the RdRp (54 kDa). NCBI accession numbers: PSFaV (ON226840) and PpVQ (MT113179)
During these last 30 years, publications on transmission and epidemiology have been presented and discussed at international meetings and are available across different libraries and journals (Table 1). Recently, the number of publications and experiments carried out to identify PSD vectors has intensified since the disease has reached other countries. Thus, in this review, we have compiled these works and discussed their main findings given the molecular diagnostic techniques developed over the years and the new proposed etiology. We open an important discussion for directing new research to understand the vectors of this virus complex and the use of new management practices in papaya orchards.
Could PMeV complex be transmitted by fungi?
Spontaneous latex exudation from green fruits and necrosis in the edge of young leaves are the main PSD symptoms (Ventura et al. 2004) which are associated with infection by the viral complex of PMeV and PMeV2, referred here as PMeV complex (Antunes et al. 2016; 2020).
The viral structural proteins protect the viral genome and play a role in several biological processes such as virus movement within the host, replication, translation, and specificity of transmission by a vector (Bol 2008). In mixed infections, capsid proteins (CPs) produced by PMeV are used for the package of PMeV2 (transcapsidation) resulting in hybrid virions with the same capsid but containing different RNAs (Antunes et al. 2016) which supports the idea that PMeV and PMeV2 could be transmitted by the same vectors. In plants, the transcapsidation phenomenon is also found between members of the Umbravirus genus and Polerovirus or Enamovirus. Umbraviruses lack the CP gene and, as a result, do not form conventional virus particles, even though they can systematically infect a plant when mechanically inoculated (Taliansky and Robinson 2003). Umbraviruses transmission between plants with the aid of insect vectors is only possible when the umbraviral genome is packaged by the helper virus capsid protein, which results in the same host range (Taliansky et al. 2000). Typically, viruses belonging to this family possess two open reading frames (ORFs). The first ORF is responsible for encoding an RNA-dependent RNA polymerase (RdRp) domain, which is thought to be expressed through ribosomal frameshifting, similar to what occurs in toti- and toti-like viruses (Salaipeth et al. 2014; Das et al. 2022). In comparison, members of the family Totiviridae, the CP is typically encoded by the 5’ ORF (ORF1), which generally have, sizes between 70–100 kDa (de Lima et al. 2019) and are predominantly α-helical (Luque et al. 2018). Totiviruses, primarily associated with fungi, yeast, and parasitic protozoa, have also been discovered infecting mollusks, arthropods (such as mosquitoes, ants, shrimps, and planthoppers), and even plants. Apart from vertical and horizontal transmission, no extracellular transmission route has been documented for totiviruses (Wickner et al. 2009). It is worth noting that viruses belonging to the family Fusagraviridae have been identified in fungi, plants, and insects, and they possess a dsRNA genome of approximately 8,500 base pairs. The viruses in this family have two ORFs separated by ribosomal frameshifting, coordinated by a heptameric nucleotide sequence XXXX (any nucleotide), YY (A or U) and Z (not G) (Lutz et al. 2023; Wang et al. 2019). There are few reports on the molecular characterization of fusagraviruses due to the lack of experimental methods to guarantee their classification. As a result, little is known about the viral etiology of many fusagravirus infections. Yet, Fusagraviridae show similarities with other dsRNA viruses from the orders Ghabrivirales (megatotivirus, megabirnarvirus, victorivirus and chrysovirus), Reovirales (mycorovirus) and Durnavirales (partitivirus) (Das et al. 2022; Aulia et al. 2019).
PMeV, maize-associated totivirus (MATV), panax notoginseng virus A (PnVA), and tea-oil camellia-associated totivirus 1 (TOCaTV1) are unclassified Fusagra or Totivirus-like viruses that infect plants (Guo et al. 2016; Akinyemi et al. 2018; Zhang et al. 2021). Although there are no reports of a CP coded by these viruses (except for PMeV), the PnVA, MATV, and TOCaTV1 ORF1 have a conserved region that includes the LA virus coat domain (pfam09220), present in CPs of totiviruses infecting fungi (Akinyemi et al. 2018). The fact that the CP of these plant viruses is more similar to totiviruses infecting fungi than totiviruses that infect insects supports the idea that fungi may act as vectors of the PMeV complex. Given the opportunities for transfer during fungal colonization, it is possible that PMeV, MATV, and PnVA can be transmitted to plants via a fungal host species (Andika et al. 2017; Roossinck 2019). Under controlled conditions, Rizoctania solani can acquire and transmit a plant virus, cucumber mosaic virus (CMV), during plant infection (Andika et al. 2017). Several fungi are found infecting papaya leaves and they are included in the genus Asperisporium, Stagonosporopsis (Syn.: Phoma), Colletotrichum, and Corynespora. Recently an (+) ssRNA virus was found in Phoma matteuccicola, the causal agent of leaf blight disease in Curcuma wenyujin (Zhou et al. 2020).
Experiments with Mexican (Tapia-Tussell et al. 2015), and Australian (Campbell 2019b) plants have shown that PSD can be spread through seeds at a high transmission rate. Seed-borne viruses can be transmitted through different routes: direct, indirect, or integrated within the plant genome, however, alternative unexplored pathways for plant virus transmission have been reported (Jones 2018), and need to be discussed for PMeV complex due to PMeV similarity with mycoviruses. Therefore, besides pathogenic fungi, beneficial fungi may also play a role in the spread of the PMeV complex. Endophyte fungi are plant-associated microorganisms that colonize and live part of their life cycle within a plant without inciting any obvious symptoms of disease in their host. Endophyte fungi are highly seed-transmissible, but can also be transmitted horizontally through vegetative propagules or spores (Bamisile et al. 2018). Viruses in the families Totiviridae, Partitivirdae, Chrysoviridae, Narnaviridae, Endornaviridae have been reported in several endophytic fungi, including species in the genera Alternaria and Phoma (Bao and Roossinck 2013), which are found associated with papaya plants (Rashmi et al. 2018; Eze et al. 2019). Thus, considering the high efficiency of endophytic transmission, it is reasonable to suggest that a high transmission rate would be found for PMeV when infecting a papaya endophyte. Also, the effects of disease dispersion in the field can be enhanced by further vector transmission and contribute to the introduction of the viruses into new areas triggering epidemic development (Jeger 2020). However, two independent experiments conducted in Brazil and Ecuador to test the transmission of the virus through seeds revealed that none of the seedlings tested positive for the PSD virus (Meissner Filho et al. 2021; Quito-Avila et al. 2023).
How can an insect acquire PMeV complex virions in a diseased plant?
A longstanding question in virus acquisition by an insect vector is how can they acquire PMeV virions from papaya laticifers, the only documented site of virus particle accumulation in papaya plants (Kitajima et al. 1993). PMeV virions were visualized, using transmission electron microscopy (TEM), in laticifers, a structure well known for its defense role against pathogens. C. papaya, laticifers are articulated, anastomosed (Hagel et al. 2008), and found in all organs (Fisher 1980; Rao et al. 2013). Mature papaya laticifers are living cells that store, under high pressure, vesicles containing carbohydrates, lipid salts, and proteins, mainly cysteine proteases (El Moussaoui et al. 2001). Upon tissue wounding, latex starts to exudate and cysteine proteases are activated resulting in the clotting of the wound (Silva et al. 1997). Whereas several studies report the different strategies adopted by mandibulate herbivores (Hohn 2007), little information is available on how sap-sucking insects can feed on latex-bearing plants. Any damage to laticifers could cause an overflow of harmful compounds (e.g proteolytic enzymes such as cysteine and serine proteases, organic acids, alkaloids, and terpenes) leading to the clogging or destruction of the insect’s mouthparts. However, it has been shown that when feeding in two different latex-bearing plants, Aphis nerii Boyer de Fonscolombe, 1841 can use its stylet to reach phloem cells avoiding the laticifers or completely circumscribing them during probing (Botha et al. 1975b; 1975a). It is not yet clear how the PMeV viral particles are acquired by an insect. It is possible that insects can acquire viral particles present in other cells, which due to not accumulating are not observed by TEM but could be detected with immunocytochemical techniques, hitherto unavailable. In another scenario, the physiological and biochemical changes present in laticifers of PSD plants could help viral particles to be acquired by an insect. Laticifers of PSD plants present a reduction of protease levels and activity, and an increase in its fluidity (Rodrigues et al. 2009b) which could minimize the damage and the clogging to an insect mouthpart when probing a laticifer.
Studies of PSD transmission by vectors
Insects are the most common vectors of plant viruses and are associated with more than 61% of virus species, and approximately 83% of insect-borne viruses are transmitted by hemipterans, e.g. aphids, whiteflies, leafhoppers, and planthoppers (Costa 2005; Hogenhout et al. 2008). Advancements in next-generation sequencing, particularly metagenomics (mNGS), have facilitated the discovery of novel plant-infecting viruses transmitted exclusively by arthropods. Viruses exhibit the capability to influence interactions within their insect vector hosts, thereby manipulating plant defenses and facilitating efficient viral transmission (Pan et al. 2021). Since the natural spread of viruses often depends on vectors, knowledge of the interrelationship between the virus and the vector is essential for establishing control strategies and mitigating the damage that the disease causes in plants. Actually, the development of new strategies for controlling and preventing plant diseases is hindered by the significant variability among existing insect vectors, as well as the limited understanding and inconsistent reporting of the transmissibility and biological mechanisms underlying the interaction between the virus and its vector (Lincheng 2023).
The possible involvement of insects as PSD vectors has been suggested based on early studies on the field spread pattern of this disease, especially with evidence of the existence of an associated aerial vector (Rodrigues et al. 1989; Maffia et al. 1993). Although epidemiological studies of PSD implicate the involvement of vectors in the transmission of PMeV and PMeV2 viruses, the identity of the vector has not been determined in Brazil and in the other regions where the disease is present. Insects of the order Hemiptera, suborder Sternorrhyncha and Auchenorrhyncha, have a large number of species that are reported as vectors of approximately 90% of the viruses transmitted by insects (Costa 2005). In addition to aphids (Family: Aphididae) reported as vectors of papaya ringspot virus (PRSV-P) and other hemipterans, such as cicadellids (Family: Cicadellidae) and whiteflies (Family: Aleyrodidae), are also reported as vectors of other diseases in papaya (Lima et al. 2003).
It is important to clarify that until 2007 most studies on PSD were based on virus detection through the visualization of the PMeV dsRNA. However, this technique requires that samples display a large amount of both viruses. The sequencing of both PMeV and PMeV2 (Araújo et al. 2007; Abreu et al. 2015a; Antunes et al. 2016) allowed the development of more sensitive techniques such as RT-PCR (Abreu et al. 2012; Antunes et al. 2016; Maurastoni et al. 2020) and qRT-PCR (Abreu et al. 2012) which have been applied to understand critical aspects of the PSD epidemiology. Using RT-PCR, it was possible to demonstrate that papaya plants infected with PMeV can remain asymptomatic in the field, serving as a viral reservoir for uninfected plants (Antunes et al. 2016).
Do cicadellids transmit PSD-associated viruses?
Cicadellids (leafhoppers) are threatening papaya pests because they cause significant damage and are potential vectors of viruses, phytoplasmas, and rickettsia (Wei et al. 2023; Acosta et al. 2017). Some dsRNA viruses, such as those belonging to the family Reoviridae, are also known to be transmitted by Cicadellids in rice plants. These viruses commonly circulate within the salivary glands of these insects, adopting a mechanism similar to exocytosis (where the virus is released into the extracellular fluid) (Pu et al. 2012). This process facilitates horizontal transmission when the insect feeds on plants. Cicadellids exhibit an intermittent transmission phase; varying from 2 to 14 days, associated with a phenotypic event that occurs after a latent phase of the virus within the insect, leading to a discontinuous viral transmission (Pu et al. 2012; Chen et al. 2021). More than twenty species of cicadellids had been associated with papaya plants but only one, Empoasca bordia Langlitz, 1964 (syn.: Solonasca bordia Langlitz), has been linked to papaya in Brazil (Culik et al. 2003). Cicadellidae emerged as potential vectors of the PMeV complex since their distribution in the field is related to the spread of the PSD (Lima et al. 2003; Ventura et al. 2003). Surveys of cicadellid populations in papaya orchards in Brazil were conducted using sticky traps and circular sweep nets. During the one-year sampling period, most cicadellids collected were identified as leafhopper of the species Empoasca bordia (syn.: Solanasca bordia) (Hemiptera: Cicadellidae: Typhlocybinae), accounting for 80% of the total (Gouvea et al. 2018). Studies on the involvement of leafhoppers, especially those of the genus Empoasca (syn.: Solanasca), as vectors of PMeV have shown a high correlation between the insect population and the incidence of diseased plants (Fig. 2). The population peak of leafhoppers precedes the highest peak of PSD incidence, which occurs about one month later (Gouvea et al. 2018). A delay of 45 days for symptom onset was also shown when papaya plants are mechanically inoculated with disease latex (Ventura et al. 2001). These results indicate that cicadellids can be potential vectors of PSD in Brazil and must be considered in further transmission assays (Lima et al. 2003; Ventura et al. 2003; Gouvea et al. 2018).

Source: (Gouvea et al. 2018).
Population fluctuation of cicadellids and incidence of plants with symptoms of papaya sticky disease in Northern Espírito Santo state, Brazil, with roguing management applied to control the PSD.
The population fluctuation of cicadellids is compatible with the analysis of the temporal evolution of papaya sticky disease and provides subsidies to verify the dispersion and generate information about the influence of biological and environmental factors on the population dynamics of the pathogen/disease. Also, it seems that temperature has an important effect on disease development as symptoms are more visualized when it is cold and dry, while a wet and warm weather influences the mitigation of symptoms (Cosmi et al. 2017).
In Mexico, the ability of the E. papayae adults, but not nymphs, to transmit PMeV-Mx to C. papaya 'Maradol' has been proven. PMeV-Mx is an umbra-like virus found infecting papaya plants in Mexico. It is 71% and 79% identical at the nucleotide level to PMeV2 and the Ecuadorian virus, PpVQ respectively (Garcia-camara et al. 2019). Under controlled conditions, E. papayae can acquire PMeV-Mx six hours after exposure to infected plants, and viral titer increases if the exposure time is longer up to 5 days (Fig. 3). Little is known about the biology of E. papayae, and research is now focused on understanding the behavior of this insect in the field (García-Cámara et al. 2019). Despite the lower abundance among the collected species, insects from the family Cicadellidae (Agallia constricta, Agalliopsis novella, E. papayae, Draeculacephala soluta, Hortensia sp., and Xyphon sp.) and Aphididae (Aphis sp. and Uroleucon taraxaci) were also identified containing the PMeV-Mx but their potential as vectors has yet to be studied.
Schematic representation of the transmission assay of papaya meleira virus Mexican variant (PMeV-Mx) by Empoasca papayae. (a) A colony of E. papayae was established in the laboratory and periodically diagnosed for PMeV-Mx or phytoplasma infection. Two hundred adult insects were transferred to a cage containing PMeV-Mx-infected plants after a 20-hour starvation period; (b) The optimal acquisition access period was determined by qRT-PCR at different time points after exposure to infected plants. Five days after exposure (optimal AAP) insect-proof meshes that separate cage A from two other cages, named B and C, were removed allowing insects to fly from infected plants to healthy plants; (c) The plants in cages B and C were diagnosed at 7, 14, 35, and 60 days after exposure to insects that had fed on infected plants. After 14 days the plants were transferred to a greenhouse. Symptoms were observed within 3 to 4 months after insect exposure. Two controls were included in this experiment represented in cages D and E (a). In cage D six plants were exposed to 100 adult insects that had not fed on infected plants. In cage E, six plants were not exposed to insects. Plants in both cages remained healthy throughout the experiment (Garcia-camara et al. 2019).
Research teams in Brazil are currently conducting experiments to identify the virus vector and elucidate the transmission mechanism. Under field conditions, research on the papaya-producing region of the north of the State of Espírito Santo, the most frequent cicadellids families in papaya plants are Cercopydae, Cicadellidae, Membracidae e Delphacidae (Gouvea et al. 2018) and David dos Santos Martins (personal communication, Incaper).
Do whiteflies transmit PSD-associated viruses?
Whiteflies are considered secondary pests of papayas worldwide because they do not cause important damage to plants or fruits in field orchards (Habibe et al. 2001). Among the whitefly species reported worldwide two species, Bemisia tabaci MEAM1 and Trialeurodes variabilis, have been reported occurring in different areas of Brazilian papaya orchards (Martins et al. 2016b) (Fig. 4), and its ability to transmit the infectious agent of PSD in Brazil was evaluated in three different works through the visualization of PMeV dsRNA (Habibe et al. 2001; Vidal et al. 2003; Rodrigues et al. 2009a).
Whitefly species reported occurring in different areas of Brazilian papaya orchards. (a) Bemisia tabaci MEAM1 in a tomato leaf. The species is reported to occur in plants close to papaya trees Source: A. Nogueira-UFV; (b) High incidence of Trialeurodes variabilis population in the papaya leaf cv. Golden.
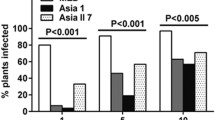
Bemisia tabaci MEAM1, despite being reported to cause damage to papaya in other biogeographic regions of the world, has limited occurrence in protected cultivation environments and is not considered a papaya pest in Brazil under field conditions (Ventura et al. 2004). As a polyphagous insect, the whitefly colonizes and multiplies on numerous cultivated, wild, and invasive plants. The ability of B. tabaci MEAM1 to acquire and transmit the PSD infectious agent was assessed by two different experiments. Habibe et al. (2001) inoculated macerated bodies of whiteflies collected from areas with PSD into healthy papaya plants. Ninety days after inoculation, healthy plants presented viral dsRNA of similar size to that detected in PSD plants, which suggested that B. tabaci MEAM1 is capable of acquiring the infectious form of the PMeV complex (Habibe et al. 2001). In another experiment, the ability of B. tabaci MEAM1 to transmit the PSD infectious agent was determined when the dsRNA of PMeV was detected in asymptomatic plants exposed for 24-72h to whiteflies that have previously forced feeding for 48h and 30min on diseased papaya plants (Fig. 5A) (Vidal et al. 2003). In this experiment, the authors do not mention any diagnostic test in asymptomatic plants. After the development of sensitive techniques for PSD-associated virus diagnosis (e.g RT-PCR), it is not uncommon to detect viral RNA in asymptomatic plants (Antunes et al. 2020; Maurastoni et al. 2020). This supports the idea that asymptomatic but infected plants were used for the experiment, instead of virus-free plants. Besides, the fact that few plants were exposed to the whiteflies raises the necessity to include a higher number of plants in this experiment. This group also verified the virus transmission by aphid species, Toxoptera citricidus Kirkaldy, 1907, and Myzus persicae Sulzer, 1776, but they were unable to transmit the PMeV dsRNA to healthy plants. Under field conditions Martins et al. (2016c) when studying aphid population species and their host plants in commercial papaya orchards, found no evidence that these insects were involved in the transmission of PSD.
Transmission assays were conducted to test the ability of whiteflies (A and B) to transmit the PMeV dsRNA. (a) Plants inoculated with latex collected from disease fruits were kept under cages until the experimental assay. Aphids (Toxoptera citricidus and Myzus persicae) and whiteflies (Bemisia tabaci MEAM1) were kept in separate cages containing asymptomatic papaya plants. 10–20 nymphs and adults were kept for a starving period of 1 hour. Then, insects were transferred to a diseased plant for 48h and 30min (virus acquisition). Then 10–20 insects of each species were transferred to a cage containing an asymptomatic papaya plant (3 month-old) where they were fed for 24-72h. Insects obtained from the same colonies but submitted to feeding in healthy plants were used as negative controls. Infested plants were kept in a greenhouse for 30 days and subsequently transferred to field cages (two plants per cage) for 9 months or until fructification. Three and eight months after the virus acquisition, new emerging leaves of all plants were collected and submitted for diagnosis by detection of the viral dsRNA. Plants were monitored monthly until the visualization of symptoms; (Vidal et al. 2003) (b) This experiment was conducted in greenhouse conditions. A total of 32 plants were analyzed: 24 were inoculated with diseased latex and 8 were kept non-inoculated. One month after latex inoculation, plants were infested with a population of T. variabilis collected from fields with non-symptomatic papaya plants. 30 days after the infestation, three healthy plants of different cultivars (cv. Tainung, cv. Golden, and cv. Sunrise – outlined red, blue, and yellow rectangles, respectively) were added inside the greenhouse. 20 days after the exposition, latex from all plants was collected for detection of the viral dsRNA. Adults and nymphs exposed to healthy plants and inoculated plants were collected and submitted to molecular diagnosis by detection of the viral dsRNA (Rodrigues et al 2009a).
Trialeurodes variabilis initially infest papaya leaves on the top of the canopies and then move to newly developed leaves. Eggs and nymphs are found in all parts of the canopy, but insects preferentially feed and lay their eggs on new leaves. Also, it is common to see oviposition concentrated in the basal region, and nymphs more frequently in the central part of older leaves (Martins et al. 2016b). The ability of T. variabilis to transmit the PMeV dsRNA was assessed under greenhouse conditions (Fig. 5B). Twenty-four plants were inoculated with papaya diseased-latex, and one month later, they were infested with a population of T. variabilis collected from fields with non-symptomatic papaya plants. One month later, three healthy papaya plants of different cultivars (cvs. Tainung, Golden, and Sunrise Solo) were added inside the greenhouse to be infested by the whiteflies. Twenty days later, dsRNA was detected in plants used as initial inoculum, and in adults and nymphs exposed to latex-inoculated plants but not in healthy plants that were exposed to "viruliferous" whiteflies. The authors suggested with this result that T. variabilis can acquire the virus from infected plants and it is not able to transmit it to healthy plants under controlled conditions (Rodrigues et al. 2009a). The amount of virus inoculated through latex injection is higher than through vector transmission. This difference could result in a lower virus load in plants that were exposed to "viruliferous" whiteflies undetectable for dsRNA visualization.
Epidemiological analysis revealed that PSD spread does not follow the same pattern as the fluctuation of the whitefly population (Andrade et al. 2003b; Lima et al. 2003). PSD occurs initially scattered and randomly in the orchard, later evolving to aggregation. Clouds of whiteflies are regularly observed in papaya crops during peak periods of the insect population and low incidence of plants with PSD, which suggests that whiteflies could not be the major insect involved in PSD spreading (Andrade et al. 2003a; Martins et al. 2016a) (Fig. 6). Whiteflies have a preference for certain hosts and even though they acquire viruses they only transmit a few, for example, viruses belonging to the genera Begomovirus, Carlavirus, and Crinivirus, and family Luteoviridae (Ghosh et al. 2019).

Source: (Andrade et al. 2003b).
Population of whitefly (Trialeurodes variabilis) and incidence of plants with symptoms of PSD in Northern Espírito Santo, Brazil.
In Ecuador, the latest transmission tests pointed to whiteflies (B. tabaci) as vectors of the umbra-like virus PpVQ (Cornejo-Franco et al. 2018) and of PpVE (Cornejo-Franco et al. 2022). Epidemiology data suggests an aerial vector for PpVQ, which commonly occurs associated with papaya ringspot virus (PRSV). Efforts to transmit the virus from plants using aphids were only successful for PRSV but not for PpVQ (Quito-Avila et al. 2015). To understand the vector of PpVQ in Ecuador, a field survey identified whiteflies, red mites, and mealybugs as the main arthropods present in papaya-infected plants and detected PpVQ in all three groups. These arthropods were transferred to PpVQ virus-free papaya plants where they were fed for 7 days. Ninety days after exposure to whiteflies, the virus was detected in three out of ten plants that whiteflies had fed, but not in field-collected whiteflies 7 days after feeding in PpVQ virus-free plants. None of the plants exposed to field-collected red mites and mealybugs tested positive for PpVQ (Cornejo-Franco et al. 2018). Studies conducted in both greenhouse and field conditions to investigate seed or insect transmission revealed that none of the tested seedlings tested positive for the PSFaV virus. Instead, it is highly likely that an efficient aerial vector, but not yet identified, plays a significant role in the transmission (Quito-Avila et al. 2023).
Overall, the role of whiteflies as vectors of PSD needs to be carefully assessed since the experiments and analyses carried out so far reach different conclusions. T. variabilis does not have a field distribution correlated with the incidence of diseased plants and is not able to transmit the infectious form of the viral complex to healthy plants. B. tabaci MEAM1 is not found colonizing papaya plants in the field which does not support its role as a vector even though it can acquire the PMeV dsRNA from papaya plants under greenhouse conditions.
Conclusions
For the past 30 years, research groups studying PSD have made efforts to understand the vector of PSD causative agents in the main papaya producers in the world. It is still challenging to assign a specific vector but epidemiological analysis shows insects as potential spreaders, among them cicadellids and whiteflies. In Brazil, the results of the experiments conducted with whiteflies so far are contradictory, but we cannot rule out that these insects may play a role in the dispersion of viruses in the field, among invasive plants as sources of inoculum. Cicadellids need to be studied as potential vectors of the PMeV complex in Brazil since these insects already play a role in the disease spread in Mexico and their population fluctuation is related to the PSD occurrence in Brazil. Importantly, previous experiments need to be repeated and analyzed now that more sensitive molecular diagnostic techniques are available and with considerations of how the virus complex of a totivirus-like virus (PMeV) and the umbra-like virus (PMeV2) may impact disease physiology and vector transmission. Knowledge about the diversity of viruses tentatively classified in the family Fusagraviridae infecting plants is very limited, as well as their modes of transmission. Therefore, studies that elucidate the PMeV complex vector and its transmission mechanisms could contribute to the proper management of the disease in papaya fields.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abreu PMV, Piccin JG, Rodrigues SP, Buss DS, Ventura JA, Fernandes PMB (2012) Molecular diagnosis of papaya meleira virus (PMeV) from leaf samples of Carica papaya L. using conventional and real-time RT-PCR. J Virol Methods 180:11–17
Abreu EFM, Daltro CB, Nogueira EO, Andrade EC, Aragão FJ (2015) Sequence and genome organization of papaya meleira virus infecting papaya in Brazil. Arch Virol 160:3143–3147
Abreu PMV, Antunes TFS, Magaña-Álvarez A, Pérez-Brito D, Tapia-Tussell R, Ventura JA, Fernandes AAR, Fernandes PMB (2015) A current overview of the papaya meleira virus, an unusual plant virus. Viruses 7:1853–1870
Acosta KI, Zamora L, Piñol B, Quiñones ML, Ramos PL, Luis M, Leyva-López NE, Arocha Y (2017) Empoasca papayae Oman, 1937 (Hemiptera: Cicadellidae) the simultaneous vector of phytoplasmas and rickettsia associated with Bunchy Top Symptom in Cuba. Anales de Biología 39:35–42
Aulia A, Andika IB, Kondo H, Hillman BI, Suzuki N (2019) A symptomless hypovirus, CHV4, facilitates stable infection of the chestnut blight fungus by a coinfecting reovirus likely through suppression of antiviral RNA silencing. Virology 533:99–107
Akinyemi IA, Wang F, Chang Z-X, Wu Q (2018) Genome characterization of the newly identified maize-associated totivirus Anhui. Arch Virol 163:2929–2931
Andika IB, Wei S, Cao C, Salaipeth L, Kondo H, Sun L (2017) Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proceedings of the National Academy of Sciences 114:12267–12272
Andrade J, Ventura J, Rodrigues S, Fernandes P, Tatagiba J, Costa H (2003) Avaliação de diferentes métodos de inoculação da meleira em plantas jovens de mamão. Fitopatologia Brasileira 28:288
Andrade JDS, Ventura JA, Rodrigues SP, Couto ADOF, Lima RDCA, Tatagiba JDS, Fernandes PMB, Martins DDS (2003b) Evidência da não transmissão do vírus da meleira por mosca-branca Trialeurodes variabilis (Quaintance, 1900). Papaya Brasil: qualidade do mamão para o mercado interno. In: Martins DS (Eds). Vitória-ES, Brasil. pp. 605–608
Antunes TFS, Amaral RJV, Ventura JA, Godinho MT, Amaral JG, Souza FO, Zerbini PA, Zerbini FM, Fernandes PMB (2016) The dsRNA virus papaya meleira virus and an ssRNA virus are associated with papaya sticky disease. PLoS ONE 11:e0155240
Antunes TFS, Maurastoni M, Madroñero LJ, Fuentes G, Santamaría JM, Ventura JA, Abreu EF, Fernandes AAR, Fernandes PMB (2020) Battle of three: the curious case of papaya sticky disease. Plant Dis 104:2754–2763
Araújo MMMd, Tavares ÉT, Silva FRd, Marinho VLDA, Júnior MTS (2007) Molecular detection of papaya meleira virus in the latex of Carica papaya by RT-PCR. J Virol Methods 146:305–310
Bamisile BS, Dash CK, Akutse KS, Keppanan R, Wang L (2018) Fungal endophytes: beyond herbivore management. Front Microbiol 9:544
Bao X, Roossinck MJ (2013) Chapter two - multiplexed interactions: viruses of endophytic fungi. In: Ghabrial SA (ed) Advances in Virus Research, vol 86. Academic, pp 37–58
Bol JF (2008) Role of capsid proteins. Methods Mol Biol 451:21–31
Botha CEJ, Evert RF, Walmsley RD (1975) Observations of the penetration of the phloem in leaves of Nerium oleander (Linn.) by stylets of the aphid, Aphis nerii. Protoplasma 86:309–319 (B. de F.))
Botha CEJ, Evert RF, Walmsley RD (1975) Studies on Gomphocarpus physocarpus: Further evidence of preferential feeding by the aphid, Aphis nerii on the internal phloem. Protoplasma 84:345–356
Campbell P (2019a) Papaya Press. Papaya clean seed update G. Kath. Australia: 1
Campbell P (2019b) Papaya Press. Papaya Clean Seed Program Underway. G. Kath. Australia: 1
Chen Q, Liu Y, Long Z, Yang H, Wei T (2021) Viral release threshold in the salivary gland of leafhopper vector mediates the intermittent transmission of rice dwarf virus. Front Microbiol 12:639445
Cornejo-Franco JF, Alvarez-Quinto RA, Quito-Avila DF (2018) Transmission of the umbra-like papaya virus Q in Ecuador and its association with meleira-related viruses from Brazil. Crop Prot 110:99–102
Cornejo-Franco JF, Reyes-Proaño E, Mollov D, Mowery J, Quito-Avila DF (2022) Transmission and pathogenicity of papaya virus E: insights from an experimental papaya orchard. Plant Dis 106(2):685–690
Costa CL (2005) As inter-relações vírus-afídeos vetores e o controle da mancha anelar do mamoeiro causada pelo papaya ringspot virus-P. In: Martins DS (ed) Papaya Brasil: mercado e inovações tecnológicas para o mamão. Vitória-ES, Brasil, pp 183–191
Cosmi FC, Alves KDS, Moraes WB, Ventura JA, Moraes SDPCB, Moraes WB Jesus Júnior WCd (2017) Análise epidemiológica da evolução temporal da meleira do mamoeiro. Summa Phytopathologica 43:303–309
Culik MP, Martins DDS, Ventura JA (2003) Índice de artrópodes pragas do mamoeiro (Carica papaya L.).1th Ed. DCM – INCAPER, Vitória
Das S, Hisano S, Eusebio-Cope A, Kondo H, Suzuki N (2022) A transfectable fusagravirus from a japanese strain of Cryphonectria carpinicola with spherical particles. Viruses 414(8):1722
de Lima JGS, Teixeira DG, Freitas TT, Lima JPMS, Lanza DCF (2019) Evolutionary origin of 2A-like sequences in Totiviridae genomes. Virus Res 259:1–9
El Moussaoui A, Nijs M, Paul C, Wintjens R, Vincentelli J, Azarkan M, Looze Y (2001) Revisiting the enzymes stored in the laticifers of Carica papaya in the context of their possible participation in the plant defence mechanism. Cell Mol Life Sci 58:556–570
Eze PM, Abonyi DO, Abba CC, Proksch P, Okoye FBC, Esimone CO (2019) Toxic, but beneficial compounds from endophytic fungi of. EuroBiotech J 3:105–111
Fisher JB (1980) The vegetative and reproductive structure of papaya (Carica papaya). Lyonia 1:191–208
García-Cámara I, Tapia-Tussell R, Magaña-Álvarez A, Cortés Velázquez A, Martín-Mex R, Moreno-Valenzuela O, Pérez-Brito D (2019) Empoasca papayae (Hemiptera: Cicadellidae) -mediated transmission of papaya meleira virus-mexican variant in Mexico. Plant Dis 103:2015–2023
Ghosh S, Kanakala S, Lebedev G, Kontsedalov S, Silverman D, Alon T, Mor N, Sela N, Luria N, Dombrovsky A, Mawassi M, Haviv S, Czosnek H, Ghanim M (2019) Transmission of a new polerovirus infecting pepper by the whitefly Bemisia tabaci. J Virol 93:e00488–e00419
Gouvea R, da Vitória R, Rosa R, Alves WDS, Giuriatto N, Calatroni D, Fanton C, Martins DDS, Queiroz R (2018) Flutuação populacional de cigarrinhas (Hemiptera: Cicadellidae) e ocorrência do vírus da meleira do mamoeiro. In: Martins DS (ed) VII Simpósio do Papaya Brasileiro. Produção e Sustentabilidade. Vitória-Espírito Santo, Brasil, pp 1–6
Guo L, Yang X, Wu W, Tan G, Fang S, Zhang S, Li F (2016) Identification and molecular characterization of panax notoginseng virus A, which may represent an undescribed novel species of the genus Totivirus, family Totiviridae. Arch Virol 161:731–734
Habibe TC, Vidal CA, Nascimento AS (2001) Transmissão da meleira para mamoeiros inoculados com macerados de moscas-brancas Bemisia tabaci Genn. biótipo B. Fitopatologia Brasileira 26(supl.):526–526
Hagel JM, Yeung EC, Facchini PJ (2008) Got milk? The secret life of laticifers. Trends Plant Sci 13:631–639
Hogenhout SA, Ammar E-D, Whitfield AE, Redinbaugh MG (2008) Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327–359
Hohn T (2007) Plant virus transmission from the insect point of view. PNAS 104(46):7905–17906
Jeger MJ (2020) The epidemiology of plant virus disease: towards a new synthesis. Plants 149(12):1768
Jones RAC (2018) Plant and insect viruses in managed and natural environments: novel and neglected transmission pathways. In: Malmstrom CM (ed) Advances in Virus Research. Academic, Amsterdam, pp 149–187
Kitajima EW, Rodrigues CH, Silveira JS, Alves F, Ventura JA, Aragão FJL, Oliveira LHR (1993) Association of isometric viruslike particles, restricted to lacticifers, with meleira (sticky disease) of papaya (Carica papaya). Fitopatologia Brasileira 18:118
Liu J, Carino E, Bera S, Gao F, May JP, Simon AE (2021) Structural analysis and whole genome mapping of a new type of plant virus subviral RNA: umbravirus-like associated RNAs. Viruses 13:646
Lima RCA, Couto AOF, Andrade JS, Martins DDS, Ventura J, Tatagiba J, Costa H (2003) Flutuação populacional de insetos vetores de doenças do mamoeiro e sua relação com a ocorrência de doenças viróticas. In: Martins DS (ed) Papaya Brasil: qualidade do mamão para o mercado interno. Vitória-ES, Brasil, pp 539–541
Lincheng D (2023) Research on a plant virus vector insects transmission model based on system dynamics. In: Proceedings of the 2023 3rd International Conference on Bioinformatics and Intelligent Computing Bic 23:122–125
Luque D, Mata CP, Suzuki N, Ghabrial AS, Castón JR (2018) Capsid structure of dsRNA fungal viruses. Viruses 10:481
Lutz T, Bien S, Langer GJ, Heinze C (2023) Transcapsidation and polysomal encapsulation as putative strategies for the genome protection of the novel Diplodia fraxini Fusagravirus 1 (DfFV1). Preprints at https://www.preprints.org/manuscript/202307.0901/v1
Martins D, Fornazier M, Fanton C, Queiroz R, Zanuncio Junior J (2016) Pragas do mamoeiro (Pests of Papaya). Informe Agropecuário 37:30–43
Martins DS, Lima AF, Fornazier MJ, Barcellos BD, Queiroz RB, Fanton CJ, Junior JSZ, Fornazier DL (2016) Whiteflies (Hemiptera: Aleyrodidae) associated with papaya (Carica papaya L). Revista Científica Intelletto 2:78–86
Martins DDS, Ventura JA, Paula RDCAL, Fornazier MJ, Rezende JAM, Culik MP, Ferreira PSF, Peronti ALBG, Zonta de Carvalho RC, Sousa-Silva CR (2016) Aphid vectors of papaya ringspot virus and their weed hosts in orchards in the major papaya producing and exporting region of Brazil. Crop Prot 90:191–196
Maurastoni M, Antunes TFS, Oliveira SA, Santos AMC, Ventura JA, Fernandes PMB (2020) A multiplex RT-PCR method to detect papaya meleira virus complex in adult pre-flowering plants. Arch Virol 165:1211–1214
Maurastoni M, Sá Antunes TF, Abreu EFM, Ribeiro SG, Mehta A, Sanches MM, Fontes W, Kitajima EW, Cruz FT, Santos AMC, Ventura JA, Gomes ACMM, Zerbini FM, Sosa-Acosta P, Nogueira FCS, Rodrigues SP, Aragão FJL, Whitfield AE, Fernandes PMB (2023) A capsid protein fragment of a fusagra-like virus found in Carica papaya latex interacts with the 50S ribosomal protein L17. Viruses 15:541
Maciel-Zambolim E, Kunieda-Alonso S, Matsuoka K, De Carvalho MG, Zerbini FM (2003) Purification and some properties of papaya meleira virus, a novel virus infecting papayas in Brazil. Plant Pathol 52:389–394
Maffia LA, Rodrigues CH, Ventura JA (1993) Significância epidemiológica do conhecimento do arranjo espacial de plantas doentes em campo. I - Meleira do mamoeiro. Fitopatologia Brasileira 18:315
Meissner Fiilho PE, Martins MVV, de Andrade EC, Lima JS, Dantas ACVL (2021) Avaliação da transmissão do vírus da meleira do mamoeiro através da semente. Magistra 31:727–735
Pan LL, Miao H, Wang Q, Walling LL, Liu SS (2021) Virus-induced phytohormone dynamics and their effects on plant-insect interactions. New Phytol 230(4):1305–1320
Pathania N, Justo V, Magdalita P, Cueva F, Herradura L, Waje A, Lobres A, Cueto A, Dillon N, Vawdrey L, Hucks L, Chambers D, Sun G, Cheesman J (2019) Integrated disease management strategies for the productive, profitable and sustainable production of high quality papaya fruit in the southern Philippines and Australia (FR2019-89). ACIAR. Australia, Australian Centre for International Agricultural Research (ACIAR) 2019: 64
Perez-Brito D, Tapia-Tussell R, Cortes-Velazquez A, Quijano-Ramayo A, Nexticapan-Garcez A, Martín-Mex R (2012) First report of papaya meleira virus (PMeV) in Mexico. Afr J Biotechnol 11:13564–13570
Pu L, Xie G, Ji C, Ling B, Zhang M, Xu D, Zhou G (2012) Transmission characteristics of southern rice black-streaked dwarf virus by rice planthoppers. Crop Prot 41:71–76
Quito-Avila DF, Alvarez RA, Ibarra MA, Martin RR (2015) Detection and partial genome sequence of a new umbra-like virus of papaya discovered in Ecuador. Eur J Plant Pathol 143:199–204
Quito-Avila DF, Reyes-Proaño E, Cañada G, Cornejo-Franco JF, Alvarez-Quinto R, Moreira L, Grinstead S, Mollov D, Karasev AV (2023) Papaya Sticky Disease Caused by Virus Couples: A Challenge for Disease Detection and Management. Plant Dis 107(6):1649–1663
Rao KS, Rajput KS, Kim YS (2013) Secondary growth and occurrence of laticifers in the root of papaya (Carica papaya L). Acta Bot Gallica 160:255–260
Rashmi M, Meena H, Meena C, Kushveer JS, Busi S, Murali A, Sarma VV (2018) Anti-quorum sensing and antibiofilm potential of Alternaria alternata, a foliar endophyte of Carica papaya, evidenced by QS assays and in-silico analysis. Fungal Biology 122:998–1012
Rodrigues C, Ventura J, Maffia L (1989) Distribuição e transmissão da meleira em pomares de mamão no Espírito Santo. Fitopatologia Brasileira 14:118
Rodrigues S, Andrade J, Ventura J, Lindsey G, Fernandes P (2009) Papaya meleira virus is neither transmitted by infection at wound sites nor by the whitefly Trialeurodes variabilis. J Plant Pathol 91:87–91
Rodrigues SP, Da Cunha M, Ventura JÁ, Fernandes PMB (2009) Effects of the papaya meleira virus on papaya latex structure and composition. Plant Cell Rep 28:861–871
Roossinck MJ (2019) Evolutionary and ecological links between plant and fungal viruses. New Phytol 221:86–92
Salaipeth L, Chiba S, Eusebio-Cope A, Kanematsu S, Suzuki N (2014) Biological properties and expression strategy of rosellinia necatrix megabirnavirus 1 analyzed in an experimental host, Cryphonectria parasitica. J Gen Virol 95:740–750
Silva L, Garcia O, Lopes M, Salas C (1997) Changes in protein profile during coagulation of latex from Carica papaya. Braz J Med Biol Res 30:615–616
Taliansky ME, Robinson D, Murant A (2000) Groundnut rosette disease virus complex: biology and molecular biology. Adv Virus Res 55:357–408
Taliansky ME, Robinson DJ (2003) Molecular biology of umbraviruses: phantom warriors. J Gen Virol 84:1951–1960
Tapia-Tussell R, Magaña‐Alvarez A, Cortes‐Velazquez A, Itza‐Kuk G, Nexticapan‐Garcez A, Quijano‐Ramayo A, Martin‐Mex R, Perez‐Brito D (2015) Seed transmission of papaya meleira virus in papaya (Carica papaya) cv. Maradol. Plant Pathol 64:272–275
Ventura J, Costa H, Tatagiba JDS (2001) Sintomatologia da meleira do mamoeiro e sua importância para o roguing. Fitopatologia Brasileira 26:536–536
Ventura JA, Costa H, Tatagiba JDS, Andrade JDS (2003). In: Martins DS, Eds (eds) Meleira do mamoeiro: Etiologia, sintomas e epidemiologia. Papaya Brasil: Qualidade do mamão para o mercado interno. Vitória-Espírito Santo, Brasil, pp 267–276
Ventura JA, Costa H, Tatagiba JDS (2004) Papaya Diseases and Integrated Control. In: Naqvi SAMH (ed) Diseases of Fruits and Vegetables: Volume II: Diagnosis and Management. Springer Netherlands, Dordrecht, pp 201–268
Vidal C, Nascimento A, Habibe T (2003) Transmissão do vírus da meleira do mamoeiro por insetos. In: Martins DS (ed) Papaya Brasil: Qualidade do Mamão Para o Mercado Interno. Vitória, Espírito Santo, Brasil, pp 612–615
Wang J, Xiao Y, Zhao H, Ni Y, Liu X, Zhao X, Wang G, Xiao X, Liu H (2019) A novel double-stranded RNA mycovirus that infects Macrophomina phaseolina. Arch Virol 164:2411–2416
Wei X, Xu D, Zhuo Z (2023) Predicting the impact of climate change on the geographical distribution of leafhopper, Cicadella viridis in China through the MaxEnt model. Insects 14:586
Wickner RB, Ghabrial SA, Nibert ML, Patterson JL, Wang CC (2009) International Committee on Taxonomy of Viruses. Family: Totiviridae, Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release, https://ictv.global/report_9th/dsRNA/Totiviridae Accesed on November 09, 2023
Zhang ZY, Huang H, Han XX, Li R, Wu LP, Wu L (2021) Identification and molecular characterization of tea-oil camellia-associated totivirus 1. Arch Virol 166(8):2347–2351
Zhou J, Wang Y, Liang X, Xie C, Liu W, Miao W, Kang Z, Zheng L (2020) Molecular characterization of a novel ourmia-like virus infecting Phoma matteucciicola. Viruses 12:231
Acknowledgments
We thank A. M. Nogueira for the whitefly photo in Fig. 4, panel A. We also thank Dr. D. S. Martins (INCAPER) for the permission to reuse the material in Figs. 2 and 6. We also like to thank Dr. C. A. Xavier for his time and expertise in helpful discussions. Thanks are due to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and to Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES) for the research productivity grants (JAV # 307905/2020-9, and PMBF # 308306/2021-0 and 269, respectively).
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida, J.M., Maurastoni, M., Sá-Antunes, T.F. et al. Efforts to understand transmission of the papaya meleira virus complex by insects. Trop. plant pathol. 49, 467–479 (2024). https://doi.org/10.1007/s40858-024-00661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-024-00661-5