Abstract
Spontaneous latex exudation is the main symptom of papaya sticky (meleira) disease caused by the Papaya meleira virus (PMeV), a double-stranded RNA (dsRNA) virus. This paper describes different effects of PMeV on papaya latex. Latex samples were subjected to different histochemical tests to evaluate their chemical composition. Additionally, the integrity of the latex particles was assessed by transmission and scanning electron microscopy analysis. Biochemical and micro- and macro-element measurements were performed. PMeV dsRNA extraction was performed to evaluate the interaction of the virus with the latex particles. Sticky diseased latex was positive for alkaloid biosynthesis and showed an accumulation of calcium oxalate crystals. PMeV also increased H2O2 synthesis within sticky diseased laticifers. The protein, sugar and water levels were altered, probably due to chemical changes. The morphology of the latex particles was further altered; PMeV particles seemed to be bound to the latex particles. The alkaloid and H2O2 biosynthesis in the papaya laticifers indicate a papaya defense response against PMeV. However, such efforts failed, as the virus affected the plant latex. The effects described here suggest some advantages of the infection process, including facilitating the movement of the virus within the papaya plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papaya (Carica papaya L.) laticifers are a series of ramified and interconnected cells forming an array of complex tubes throughout the papaya plant (Esaú 1976). When mature, they are mainly composed of large latex vesicles. Papaya latex is a fluid with a milky appearance that contains about 85% water. An insoluble particulate fraction, whose composition is still practically unknown, makes up 25% of the dry matter. The soluble fraction, on the other hand, contains both the usual ingredients such as carbohydrates (~10%), salts (~10%) and lipids (~5%), and representative biomolecules such as glutathione, cysteine proteinases (~30%) and several other proteins (~10%) (El Moussaoui et al. 2001). Latex from other species contains carbohydrates, organic acids, alkaloids, oils, terpenes, resins, rubber and other compounds (Calvin 1987; Hunter 1994). Until now, latex composition was suspected to inhibit microorganisms from colonizing the laticifers, as only a few examples of such an infection had been observed. Bacterial colonization in bunchy top-affected papaya laticifers has been demonstrated (Davis et al. 1996), and flagellate protozoan (Phytomonas sp.) are well-known microorganisms located in the Euphorbiaceae laticifers (Da Cunha et al. 2000). However, Papaya meleira virus (PMeV) is a unique plant virus among those observed in laticifers described so far.
First reported by Rodrigues et al. (1989), the ‘papaya sticky disease’ or ‘meleira’ was characterized by a spontaneous fluid and translucent latex exudation from fruits and leaves. After atmospheric exposure, the latex oxidizes, resulting in small necrotic lesions on the edges of young leaves and, subsequently, stickiness on the plant organs (Ventura et al. 2001). Using electron microscopy, spherical 50 nm virus-like particles were observed only in diseased papaya laticifers (Kitajima et al. 1993). Additionally, 12 kbp double-stranded RNA (dsRNA) molecules were extracted from infected latex in abundance, suggesting a viral disease etiology (Kitajima et al. 1993; Rodrigues et al. 2005). This hypothesis was confirmed by Zambolim et al. (2003), who purified the virus and demonstrated Koch’s postulates by the development of disease symptoms in healthy plants after inoculation with purified particles. Koch’s postulates demonstrated that the dsRNA originated from the virus and proved that it was associated with the disease agent (Ventura et al. 2001, 2004; Zambolim et al. 2003). Recently, specific PMeV primers were designed based on viral dsRNA nucleotide sequences. Through RT-PCR, a 669-nucleotide fragment was amplified and found to be very similar to other viral RNA-dependent RNA polymerases after sequencing (Araújo et al. 2007). Despite the reports presented on the papaya sticky disease, the effects of the virus on plant laticifers are not yet understood. Here, we provide evidence that the PMeV alters the latex structure and composition by influencing the physiology of the laticifers.
Materials and methods
Plant material and latex collection
Leaves and unripe fruits were collected from a total of three healthy and three sticky diseased papaya (cv. Golden). Plants of 26 months old were collected from the INCAPER Experimental Field in Espírito Santo State, Brazil. Diseased plants were identified in the field by the typical sticky disease symptoms, which were later confirmed by PMeV molecular diagnosis (Rodrigues et al. 2005; Araújo et al. 2007). Papaya plants showed the most common symptom of meleira by exudation of fluid latex from fruits that oxidizes and becomes dark. Also, it was observed the exudation of latex from edges of young leaves in the top of the plant that provokes small light-brown necrotic lesions on the leaf tips (Ventura et al. 2004). Samples of plant latex were monitored for PCR detection of the viral dsRNA. The latex from fruits of the plants with meleira presented a clear watery aspect, due to its lower viscosity and lack of coagulation that darkens with greater facility than that of healthy fruits.
Latex was obtained by tapping the fruits using a steel razor blade. The samples were collected in 1.5 ml micro-tubes containing a 0.1 M citrate buffer at pH 5.0 1:1 (v:v) for molecular extraction of viral dsRNA. Crude latex aliquots were used for micro- and macro-elemental testing and biochemical measurements, as well as for histochemical tests. All latex samples were maintained at −20°C before use. For electron microscopy, the latex samples were kept at 25°C for 2 h in a solution containing 2.5% glutaraldehyde and 4.0% paraformaldehyde in a 0.05 M cacodylate buffer at pH 7.4 (1:1 v:v), followed by storage at 4°C.
Latex histochemical tests
Healthy latex (HL) and sticky diseased latex (SDL) (100 μl) were deposited on microscope slides separately. The following reagents were used at 25°C at a 1:1 (v:v) ratio. Reducing sugars were stained red after heating them together with Fheling’s reagent for 10 s (0.8 M cupric sulfate, 2.5 M potassium sodium tartrate; Purvis et al. 1964). Alkaloids were stained dark-green using Dragendorff’s reagent for 1 h (0.1 M bismuth nitrate, 5.5 M hydrochloric acid, 0.01 M potassium iodate; Kraus and Arduin 1997). Proteins were stained blue using a coomassie brilliant blue solution for 10 min (0.025% coomassie brilliant blue, 40% methanol, 7% acetic acid; Kraus and Arduin 1997). Starch grains were stained dark-brown using Lugol’s reagent for 5 min (0.09 M potassium iodate, 0.01 M iode; Jensen 1962). Lipids were stained yellow using a Sudan III solution for 20 min (0.01 M Sudan III, 80% ethanol; Jensen 1962). Phenols were stained dark-green using a ferric chloride solution for 2 min (0.37 M hexahydrate ferric chloride, 0.02 M sodium carbonate; Johansen 1940). The oxalate chemical nature of inclusions in papaya latex was investigated using a modification of a previously described method (Yasue 1969). The crystals were then separately subjected to 10% hydrochloric acid and 10% acetic acid for 20 min. According to Yasue’s method, the crystals are assumed to be composed of oxalate when they readily dissolve in hydrochloric acid without observable effervescence, while only partially dissolving in acetic acid. Unstained latex was used as a control. The microscope slides were recorded and analyzed using an Axiophoro ZEISS light microscope coupled with an Analysis Sis Link/Oxford Zeiss system. For each sample, 25 fields (1 mm2 each) were analyzed.
In situ detection of H2O2
Oxalate has been shown to be a substrate for oxidative enzymes during H2O2 production (Lane et al. 1993, 1994). In order to assess the correlation between calcium oxalate and H2O2 in papaya laticifers, H2O2 was investigated in papaya tissues. For this purpose, a DAB-uptake method based on the plant’s peroxidase activity was used. DAB is used as a substrate during the peroxidase reaction, and locally produces a reddish-brown precipitant (Orozco-Cárdenas and Ryan 1999; Ma et al. 2008). Laticifers were easily identified within leaf stalk tissues, recognized as articulated and anastomosed cells between the xylem and phloem tissues (Fig. 3). These anatomical features had already been fully described (Esaú 1976).
The production of H2O2 was detected by approximately 10 mm cuts in leaves, stalks and fruits. Transverse and longitudinal cuts were made by hand using a razor blade. The samples were then incubated for 12 h in 2.5 mM 3,3′-diaminobenzine (DAB)-HCl at pH 3.8 (Sigma, USA). Control samples were immersed in deionized water. Samples were decolorized in hot 96% ethanol for 20 min. They were then placed in 50% glycerol and observed using light microscopy as described elsewhere. H2O2 was seen as a reddish-brown coloration (Orozco-Cárdenas and Ryan 1999; Ma et al. 2008).
Protein and sugar assays
The protein content was determined according to the procedure described by Lowry et al. (1951), with modifications. Briefly, latex diluted in ultra-pure water (1:1 v:v) was homogenized using a vortex. An aliquot (1 ml) of the diluted latex was added to 5 ml of cupper reagent (48 ml of 3% sodium carbonate diluted in 0.1 M sodium hydroxide; 1 ml of 4% sodium and potassium tartrate; 1 ml of 2% copper sulphate). After 10 min, 500 μl of water-diluted Folin-Ciocalteu reagent (1:2 v:v) was added to the mixture. The samples remained at 25°C during 10 min and had the absorbance measured at 660 nm. The protein content was determined using a BSA standard curve.
The papaya latex was diluted in water and mixed using a vortex in order to make a more homogeneous latex suspension. Pure water (Moutim et al. 1999) or an aqueous solution (Azarkan et al. 2006) was previously used for the collection of papaya latex. This procedure allowed the disaggregation of the latex particles (instead of solubilize the latex particles) turning the samples more fluid and easy to process.
The total sugar concentration was determined by an anthrone reaction as previously described by Scott and Melvin (1953), with modifications. Shortly, the latex diluted in ultra-pure water (1:6 v:v) was centrifuged 11,000 g for 5 min and had the supernatant collected. A sample volume (500 μl) was added to 2.5 ml of 0.02% anthrone solution (1 g of anthrone in 50 ml of distilled water, making it to volume (500 ml) with concentrated sulfuric acid). The samples were incubated at 100°C during 10 min. They remained at 25°C in the dark and had their absorbance measured at 620 nm. The sugar content was determined using a glucose standard curve.
Quantification of micro- and macro-elements
A quantitative measurement of the nitrogen (N) content was carried out by Kjeldahl’s method (Stuart 1936). Phosphorous (P), potassium (K), calcium (Ca), iron (Fe), zinc (Zn), manganese (Mn) and magnesium (Mg) contents were determined by nitric–perchloric acid digestion (Miller 1997) followed by concentration measurements using an atomic-absorption spectrometer (AAS) (Varian AA 240 FS, Victoria, Australia). The P concentration was determined at 725 nm using a spectrophotometer (Celm E 225 D, São Paulo, Brazil).
Dry mass quantification and pH measurements
The mass of 5 ml fresh latex was measured before and after heating at 70°C for 5 h. The supernatant of latex, diluted in water (1:2 v:v) and centrifuged at 8,000 g for 10 min at 25°C, was used for pH measurement.
Electron microscopy analysis
Latex stored as previously described was centrifuged at 8,000 g for 5 min at 25°C, after which the solid phase was post-fixed in 1.0% osmium tetroxide (OsO4) for 1 h at 25°C and dehydrated in a graded series of 30, 50, 70, 90 and 100% (v:v) acetone, 30 min for each step. The dehydrated samples were then embedded with epoxy resin. Ultra-thin sections were investigated using a ZEISS 900 transmission electron microscope (TEM). For the scanning electron microscopy (SEM) studies, the latex was critical point dried in CO2 after fixation and dehydration, mounted onto carbon stubs and coated with 20 nm gold. The samples were then examined using a ZEISS 962 microscope. SEM and area-restricted X-ray analysis by energy dispersive spectrometry (EDS) were carried out using an SEM instrument with an attached energy-dispersive X-ray analytical system containing a lithium-drifted silicon detector. SEM analysis was carried out using an acceleration voltage of 20 kV.
Interaction between the PMeV and the latex polymers
Healthy and SDL samples (1.0 ml each), previously diluted in a citrate buffer at pH 5.0, were centrifuged at 11,000 g for 15 min at 4°C. The resulting pellet, composed of coagulated latex particles, was washed twice using 1.0 ml cold ultra-pure water followed by centrifugation at 11,000 g for 15 min at 4°C. The solid phase was resuspended in 100 μl ultra-pure water. Then, both the liquid and solid phases were subjected to nucleic acid extraction using 1 V 2:1 v:v phenol/chlorophorm. The nucleic acids were precipitated using 2 V cold-ethanol and 0.1 V 3 M sodium acetate at pH 5.2 in liquid nitrogen for 30 min. The pelleted nucleic acids were separated using 1% agarose gels stained with 10 ng ml−1 ethidium bromide (Sigma, USA), and were observed under UV light using the Eagle-Eye photo equipment (Rodrigues et al. 2005).
Statistical analysis
The plants in the field were arranged in a completely randomized design. The crop maintenance was similar to standard commercial production, and inoculated plants showed the most common symptom of meleira (Ventura et al. 2004). All experiments were performed with three biological samples and three technical replicates.
The data obtained from the chemical and biochemical measurements on the latex were analyzed by ANOVA using the SAEG software (Release 4.0, UFV, MG, Brazil). The Tukey test at P = 0.05 was used to determine statistical significance.
Results
Latex histochemical tests
Direct observation of the latex using bright-field microscopy revealed the occurrence of structures with irregular shape composed of latex particles (Fig. 1l, m). In order to determine their composition, latex samples were subjected to different reagents. Both sticky diseased papaya latex and healthy papaya latex were stained for phenols, proteins, reduced sugars (Fig. 1) and lipids (Fig. 2). SDL was stained for proteins at a lower intensity than HL (Fig. 1c, d). Reducing sugars showed an intense brown coloration uniformly distributed on HL particles (Fig. 1e). On the other hand, SDL had a diffuse light brown coloration, occasionally delimiting vesicle-like structures (Fig. 1f). Only SDL was stained for alkaloids, suggesting a papaya response to PMeV infection (Fig. 1g, h).
Histochemical tests of papaya latex. Latex samples collected from healthy (a, c, e, g, i, l) and diseased (b, d, f, h, j, m) plants were subjected to non-permanent coloration of different compounds such as phenols (a, b), proteins (c, d), reduced sugars (e, f) and alkaloids (g, h). Starch grains were not observed (i, j). Visualization by contrast of the gray tones background with that of unstained control latex (l, m)
Histochemical tests of papaya latex for lipids. Latex samples collected from healthy (a) and diseased (b) plants were stained using a Sudan III reagent. The dark background on latex particles compared with unstained latex (Fig. 1l, m) indicates the presence of lipids. After staining with Sudan III, some crystals could be observed (indicated by arrows). Agglomerates of crystals (a, b insets) were observed in both diseased and healthy latex. They were subjected to acetic (c) or hydrochloric (d) acids
Starch grains were not observed in any sample (Fig. 1i, j). When starch grains are present they can be identified as individualized structures instead of having a disperse coloration (as shown in Fig. 1i, j). The samples were analyzed in different microscopic magnification and such structures could not be observed. The occurrence of starch grains in papaya latex was somewhat expected. However, the negative result might be associated with the intrinsic latex amidase activity. It was reported that after tapping the papaya latex shows amidase activity that is maintained for about 15 min (Moutim et al. 1999). Perhaps, in our work, the period between tapping and the collection of the latex was long enough to cause the starch degradation that could explain for the observed result.
Interestingly enough, after the SDL and HL samples were treated with the Sudan III solution staining for lipids, raphyd-like crystals were observed (Fig. 2a, b). Moreover, no additional treatment was needed to observe these crystals, which were discernible even at the lowest microscopic magnification. The crystals were remarkably more pronounced in SDL (Fig. 2b) and were also seen forming clusters, which seemed to be associated with latex particles (Fig. 2). In order to reveal the chemical nature of the crystals, the samples were separately subjected to hydrochloric and acetic acid treatments. They completely dissolved in hydrochloric acid without any effervescence but only partially dissolved in acetic acid (Fig. 2c, d), indicating that they were composed of oxalate (Yasue 1969). An elemental analysis of the crystals by energy-dispersive X-ray spectroscopy confirmed their oxalate composition (data not shown).
In situ detection of H2O2
Papaya laticifers were brown after DAB treatment (Fig. 3), identifying the papaya laticifers as H2O2 production sites. Furthermore, the brown color was more intense in diseased samples (Fig. 3c, d), suggesting an H2O2 accumulation during PMeV infection. In addition, H2O2 was highly localized only in the phloem companion cells from diseased tissues (Fig. 3), suggesting a specific papaya response to PMeV. Similar results were obtained from fruit tissues (data not shown).
In situ detection of H2O2 in papaya. Petioles from healthy (a, c) and diseased (b, d) plants were subjected to a DAB reagent. The presence of H2O2 was seen in the laticifers (indicated by arrows) from both healthy and diseased samples. A more intense coloration was observed in the diseased laticifers (insets in c and d). The asterisk indicates the phloem tissues. Scale bars are 0.1 mm in a and b and 1.0 mm in c and d
Biochemical analysis
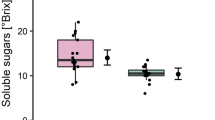
Latex from sticky diseased papaya is known to be more fluid and translucent than its healthy counterpart (Ventura et al. 2001, 2004). In order to understand what factor(s) could be related to this symptom, we measured the biochemical contents of the samples. As seen in Table 1, SDL contained a large amount of water (87.33%) when compared to HL (81.03%). Additionally, proteins and sugars were more concentrated in HL, with concentrations of 0.17 and 0.44 mg ml−1, respectively, compared to 0.13 and 0.18 mg ml−1 in SDL samples. There was no significant difference in pH, as similar values were obtained from diseased (pH 6.13) and healthy (pH 5.68) samples.
Measurements of chemical elements
Considering the SDL’s increased water content, we postulated that the infected laticifers might undergo some osmotic alteration, resulting in an uptake of water. To search for such osmotic constituents, micro- and macro-element measurements were performed by atomic-absorption spectroscopy. The percentages of potassium (K), phosphorus (P), iron (Fe), zinc (Zn), manganese (Mn), calcium (Ca), magnesium (Mg) and sulphur (S) were determined from HL and SDL dry mass samples. K and P constituted 0.77 and 0.33% of SDL dry mass, respectively, compared to 0.59 and 0.30% of HL dry mass. HL contained 0.71% Ca, compared to 0.35% in the SDL. None of the other tested elements varied significantly between HL and SDL (Table 1).
Scanning electron microscopy analysis
In addition to the chemical latex constituents, the coagulation process has also been associated with latex fluidity. It has been shown that the papaya latex protein profile is completely altered during coagulation, leading to an activation of several enzymes (Moutim et al. 1999). However, the exact coagulation mechanism is not yet known. Recently, Wititsuwannakul et al. (2008a, b, c) described the purification and characterization of three proteins (Hevea latex lectin-like protein, HLL; rubber-particle HLL biding protein, RP-HLLBP; C-serum lectin biding protein, CS-HLLBP) involved in the rubber tree (Hevea brasiliensis) latex coagulation process. According to the described model, the RP-HLLBP glycoprotein binds to HLL that is exposed on lutoid membranes when the laticifers are tapped. This causes the aggregation of adjacent rubber particles and the formation of the latex coagulum. The coagulation intensity is reduced by CS-HLLBP, that competes with RP-HLLBP by the HLL linkage (Wititsuwannakul et al. 2008a, b, c). Therefore, the surface of latex particles is important to the coagulation process.
In order to analyze the morphology of the papaya latex particles, SEM was used. Analysis of SEM micrographs showed that PMeV infection notably reduced the amount of papaya latex particles (Fig. 4a, c). Comparing Fig. 4b (SDL) and d (HL) we may also assume that the latex particles from diseased plants had their morphology altered and present a more loose structure. A similar pattern was observed by Da Cunha et al. (2000) analyzing the latex of Euphorbiaceae plants infected by Phytomonas.
Interaction between the PMeV and the latex particles
TEM was conduced in order to verify the interaction between the virus and the latex particles. The micrograph presented on Fig. 5 clearly shows the virus particles preferentially gathering at the grain boundaries. The PMeV was localized on and linked to the latex polymers. To confirm this hypothesis, we tested different treatments (pH 1.0, 8.0, 2–8 M urea, 50–300 MPa high hydrostatic pressure, 1–10% SDS detergent solution and ultrasonic treatment) for their ability to release the PMeV dsRNA from the latex solid phase (latex particles) to the latex liquid phase. However, none of the treatments was able to release the PMeV dsRNA from the latex particles (unpublished results). The inability of the detergent treatment to liberate the PMeV dsRNA suggests that such interaction can not involve the particle membrane fatty acids. By contrast, it is important to note that during the TEM sample preparation, the latex liquid phase was completely eliminated through successive washes. Moreover, the resistance to other severe treatments indicates that only strongly attached elements could remain on the latex particles. Taken together, these data suggest that the PMeV is entrapped in the papaya latex particles and the infection provoked by the PMeV alters the laticifer’s physiology compromising the perfect latex particles formation.
To confirm the close association of PMeV particles with the latex particles, both the SDL liquid and solid phases were subjected to nucleic acid extraction, and the approximately 12-kbp nucleic acid band seen in the agarose gel was correlated with the presence of the PMeV as previously published (Ventura et al. 2004; Zambolim et al. 2003; Rodrigues et al. 2005). In this work, the dsRNA was clearly observed only on the solid phase, indicating that the virus is linked to the papaya latex particles (Fig. 6). Similar results were obtained from plants infected for different periods of time (data not shown). Therefore, the results suggest that the PMeV is linked to the latex particles during different viral life cycle stages.
Nucleic acid extraction from the solid and liquid latex phases. Latex from diseased papaya was centrifuged, and both liquid (LP) and solid (SP) phases were subjected to nucleic acid extraction. The total latex (T) with nucleic acids extracted was used as a control. The samples were separated by a 1% agarose gel using a 1 kbp DNA molecular marker (M)
Discussion
After the papaya sticky disease was described (Rodrigues et al. 1989), studies were published investigating the interaction between the PMeV and the papaya plant. Initially, it was described a spontaneous latex exudation from fruits and leaves in the field (Rodrigues et al. 1989; Ventura et al. 2001, 2004). After the PMeV particles were found only in papaya laticifers and not in other cell types (Kitajima et al. 1993), the sticky disease symptoms were supposed to be a direct effect of the virus. However, the details of these effects have remained unknown until now.
Effects of the PMeV on latex composition and polymers structures
This work subjected papaya latex to different biochemical tests aiming to detect possible PMeV-induced changes. The HL was observed to have a sugar concentration about twice as high as that of the SDL (Table 1). This data was confirmed by latex histochemical tests, in which reducing sugars were stained at a lower level in diseased samples (Fig. 1e, f). It is noteworthy that the concentration difference is not directly correlated to an increase of water, as shown in Table 1. Similar effects were also observed in pumpkin (Cucurbita pepo cv. Eskandarani) leaves infected with Zucchini yellow mosaic virus (ZYMV), in which a decrease of carbohydrate, protein and pigment levels was observed (Radwan et al. 2007). Plant viruses have been shown to affect the host’s carbohydrate metabolism, altering, for example, photosynthesis and sugar transport (Sajnani et al. 2007; Kronberg et al. 2007). The flow of sugar sap from photosynthesizing leaves to sink tissues is compromised, as the plant reduces the plasmodesmata pore diameter in order to limit the mobility of the virus. As a result, sugars accumulate in the photosynthetic tissues, diminishing their drain to other tissues (Rinne et al. 2005). In contrast, sugar fluxes across the plasma membranes of the laticifers could not be altered. When the protoplasts of the laticifers were subjected to ethylene treatment simulating stress conditions, their sugar uptake was maintained (Bouteau et al. 1991). Therefore, these results suggest a critical influence of PMeV on papaya carbohydrate metabolism. Instead of being restricted to laticifers, the reduction of sugar concentration could reflect a systemic effect of PMeV in the papaya plant. Consequently, the arrival of sugars from photosynthetic cells in laticifers can be impaired as an indirect effect of the papaya defense response against the PMeV.
The sticky appearance of the diseased papaya latex is not caused by an increase in the sugar content. In fact, we observed a reduced level of total sugar in the diseased samples (Table 1). Indeed, the sticky aspect shall be related to the oxidative state of the latex. Our data showed an increased level of H2O2 in the infected laticifers cells (Fig. 3). At the same time, when the latex is exposed to the atmosphere, their components can react with the molecular oxygen. This process appears to be accelerated by high temperature as we usually observe much intense symptoms (including sticky appearance) in diseased papaya growing in the field during the hottest Brazilian year’s mouths (from January to March) and when the latex is heated, the diseased samples became dark brown while its healthy counterpart remains white (data not shown). The latex cysteine-proteases (in the sulphydril group) are good target molecules to be oxidized. Nowadays, the level of these and other papaya latex proteins, as well as their oxidation state, are in progress in our laboratory.
The reduction in the amount of latex particles (Fig. 4) could be related to the supposed PMeV-induced papaya carbohydrate translocation changes. We observed lipids, phenols, alkaloids, sugars and oxalate crystals occurring in the latex (Figs. 1, 2). Nevertheless, papaya latex is known to be mainly composed of proteins (Bravo et al. 1994; Moutim et al. 1999), and as observed for other species, polyisoprene molecules (Hunter 1994). The reduced amount of sugar in the papaya laticifers could down-regulate the biosynthesis of both proteins and polyisoprene molecules. Indeed, a smaller amount of protein was observed in the SDL than in the HL after the protein assay (Table 1) and the histochemical test (Fig. 1). The sugar content has an equivalent effect on isoprene biosynthesis (Chaykin et al. 1958), a highly active pathway in the laticifers of the rubber tree plant (Chow et al. 2007). For this plant, the process uses elevated amounts of sugar that are efficiently imported to the laticifers through a cell plasma membrane H+-sugar symport system (Bouteau et al. 1999).
In addition to the reduced biosynthesis of latex particles, the polymer shape was also altered, leading to the assumption that they had been disabled (Figs. 4, 5). In rubber trees, it has been demonstrated that, after tapping, latex coagulation was associated with adjacent polymer grains linking together (Wititsuwannakul et al. 2008a, b, c). The authors showed that HLL created multivalent bridges between rubber-particles through their binding to glycosilated (N-Acetyl-d-glucosamin, GluNAC) receptors (RP-HLLBP) located on the surfaces of the rubber-particles. The binding between HLL and GluNAC was Ca2+-dependent (Gidrol et al. 1994; Wititsuwannakul et al. 2008a). If this model also applies to papaya, the reduced amount of sugar and the morphological latex-particle changes could compromise the latex coagulation, perhaps compromising the perfect contact between individual grains. The PMeV also altered the levels of sugars and Ca2+, two additional important coagulation factors in papaya latex. The histochemical tests showed that not only the amount but also the distribution pattern of reducing sugars on the latex particles was changed (Fig. 1e, f). The water-insoluble calcium salt is the most commonly occurring oxalate in nature, and calcium oxalate crystals were previously reported in latex from different plant species (Hunter 1994). The virus increased the amount of oxalate crystals in the latex (Fig. 2), and oxalate calcium salt is important in regulating the calcium levels in higher plants (Borchert 1986). In addition, the SDL had only about half as much Ca2+ as healthy samples (Table 1). The combination of these effects could contribute to the increased latex fluidity typical of sticky diseased papaya.
Effects of the PMeV on papaya laticifers defense responses and osmotic balance
Oxalate has been associated with different biological processes, although its precise mechanism of action is not completely understood. Recent studies suggest that oxalate impinges on plant signaling. Oxalate oxidase activity was first reported in barley extract, in which the conversion of oxalate into CO2 and H2O2 was demonstrated (Lane 2002). Therefore, oxalate-mediated signaling could involve the production of H2O2. After subjecting papaya to in situ H2O2 detection, laticifers were indicated as H2O2-producing sites (Fig. 3), and PMeV-infected papaya tissue showed an increase in H2O2 production (Fig. 3c, d). Thus, the accumulation of calcium oxalate crystals in sticky diseased papaya latex suggests that the oxalate is participating in the increased H2O2 production. This data is positively correlated to the contribution to plant stress response by the laticifers. Expressed sequence tags (ESTs) from rubber tree latex (Chow et al. 2007) and the proteomic analysis of Chelidonium majus latex (Nawrot et al. 2007) indicated the presence of highly expressed proteins with the ability to stress-response or defense against pathogens. Similarly, laticifers are known to participate during the synthesis and accumulation of secondary metabolic compounds, for example phenols and alkaloids (Hunter 1994; Samanani et al. 2006). This extensive group of molecules is related to abiotic and biotic stress responses. In this study, an accumulation of alkaloids was detected only in SDL samples (Fig. 1h), suggesting an antiviral papaya response. Consistent with this idea, An et al. (2001) proved the inhibitory activity of Cynanchum komarovii alkaloids against the Tobacco mosaic virus (TMV).
An accumulation of K+ was observed in the SDL (Table 1). In general, K+ serves several important functions in plant cells, such as neutralizing electrical anionic groups, controlling membrane polarization and participating in osmoregulation (Lebaudy et al. 2007). Inward-rectifying K+ channels were previously identified in guard cells (Schroeder et al. 1984), but can be found in several types of plant cells, including laticifers (Bouteau et al. 1996, 1999). Alternatively, K+ uptake could be related to the accumulation of calcium oxalate crystals. Sclerotinia sclerotiorum (Lib.) fungi are known to produce considerable amounts of oxalate in infected tissues as a virulence factor (Ferrar and Walker 1993). In this plant-fungi infection model, oxalate induces starch degradation in guard cells, followed by K+ accumulation (Guimarães and Stotz 2004). The osmotically active solutes lead to an increase of water uptake. The effect is likely to occur in papaya laticifers as well, since increased water content was observed in the SDL (Table 1). The authors further reported a swelling and, in some cases, a bursting of protoplasts in fungi-infected plants. Under normal conditions, the exudation of papaya latex requires tissue tapping, so the spontaneous latex exudation from sticky diseased papaya (Rodrigues et al. 1989; Ventura et al. 2001, 2004) indicates that the plant laticifers could be bursting as well.
The biological significance of the swelling and consequent cell rupture of the sticky diseased papaya laticifers could be related to the movement strategy of the virus. A recent review points to laticifers as an alternative plant tube system (Pickard 2008). It emphasizes the rupture and subsequent laticifer drainage as a special transport mechanism. As the PMeV is located only in the papaya laticifers (Kitajima et al. 1993), the results on latex fluidity and exudation presented in this work suggest that the virus uses host laticifers to move itself through the plant. Electron microscopy (Fig. 5) and molecular data (Fig. 6) show the virus in close association to latex particles, and thus support this idea. This association would allow the virus to flow more efficiently, since the PMeV could use this mechanism to move quickly from an inoculation site to non-infected cells.
This paper presents an important characterization of the effects of the PMeV on papaya latex. Overall, the presented results provide new insights on the interaction between the papaya and the PMeV, which could prove helpful when trying to understand and control the papaya sticky disease.
References
An T, Huang H, Yang Z, Zhang D, Li G, Yao Y, Gao J (2001) Alkaloids from Cynanchum komarovii with inhibitory activity against the Tobacco mosaic virus. Phytochemistry 58:1267–1269
Araújo MMM, Tavares ET, Silva FR, Marinho VLA, Souza Júnior MT (2007) Molecular detection of Papaya meleira virus in the latex of Carica papaya by RT-PCR. J Virol Methods 146:305–310
Azarkan M, Dibiani R, Baulard C, Baeyens-Volant D (2006) Effects of mechanical wounding on Carica papaya cysteine endopeptidases accumulation and activity. Int J Biol Macromol 38:216–224
Borchert R (1986) Calcium acetate induces calcium uptake and formation of calcium-oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 168:571–578
Bouteau F, Lacrotte R, Cornel D, Monestiez M, Bousquet U, Pennarun AM, Rona JP (1991) Electrogenic active proton pump in Hevea brasiliensis laticiferous cells its role in activating sucrose/H+ and glucose/H+ symports at the plasma membrane. Bioelectrochem Bioenerg 26:223–236
Bouteau F, Bousquet U, Pennarun AM, Convert M, Dellis O, Cornel D, Ronal JP (1996) Time dependent K+ currents through plasmalemma of laticifer protoplasts from Hevea brasiliensis. Physiol Plant 98:97–104
Bouteau F, Dellis O, Bousquet U, Rona JP (1999) Evidence of multiple sugar uptake across the plasma membrane of laticifer protoplasts from Hevea. Bioelectrochem Bioenerg 48:135–139
Bravo LM, Hermosilla J, Salas CE (1994) A biochemical comparison between latex from Carica candamarcensis and C. Papaya. Braz J Med Biol Res 27:2831–2842
Calvin M (1987) Fuel oils from euphorbs and other plants. Bot J Linn Soc 94:97–110
Chaykin S, Law J, Philipis AH, Tchen TT, Bloch K (1958) Phosphorylated intermediates in the synthesis of squalene. Proc Natl Acad Sci USA 44:998–1004
Chow KS, Wan KL, Isa MNM, Baharil A, Tan SH, Harikrishna K, Yeang HY (2007) Insights into rubber biosynthesis from transcriptome analysis of Hevea brasiliensis latex. J Exp Bot 58:2429–2440
Da Cunha M, Gomes VM, Xavier-Filho J, Attias M, Souza W, Miguens FC (2000) The laticifer system of Chamaesyce thymifolia: a closed host environment for plant Trypanosomatids. Biocell 24:123–132
Davis MJ, Kramer JB, Ferwerda FH, Brunner BR (1996) Association of a bacterium and not a phytoplasm with papaya bunchy top disease. Phytopathology 86:102–109
El Moussaoui A, Nijs M, Paul C, Wintjens R, Vincentelli J, Azarkan M, Looze Y (2001) Revisiting the enzymes stored in the laticifers of Carica papaya in the context of their possible participation in the plant defense mechanism. Cell Mol Life Sci 58:556–570
Esaú K (1976) Anatomía Vegetal, 3rd edn. Ediciones Omega, Barcelona
Ferrar PH, Walker JRL (1993) o-Diphenol oxidase inhibition—an additional role for oxalic acid in the phytopathogenic arsenal of Sclerotinia sclerotiorum and Sclerotium rolfsii. Physiol Mol Plant Pathol 43:415–422
Gidrol X, Chrestin H, Tan H, Kush A (1994) Hevein, a lectin-like protein from Hevea brasiliensis (rubber tree) is involved in the coagulation of latex. J Biol Chem 269:9278–9283
Guimarães RL, Stotz HU (2004) Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol 136:3703–3711
Hunter JR (1994) Reconsidering the functions of latex. Trees 9:1–5
Jensen WA (1962) Botanical histochemistry, principles and practice. W.H. Freeman, San Francisco
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kitajima EW, Rodrigues C, Silveira J, Alves FL, Ventura JA, Aragão FJL, Oliveira LHR (1993) Association of isometric vírus-like particles, restricted to laticifers, with “meleira” (“sticky disease”) of papaya (Carica papaya). Fitopatol Bras 18:118–122
Kraus JE, Arduin M (1997) Manual básico de métodos em morfologia vegetal. EDUR, Seropédica
Kronberg K, Vogel F, Rutten T, Hajirezaei MR, Sonnewald U, Hofius D (2007) The silver lining of a viral agent: increasing seed yield and harvest index in Arabidopsis by ectopic expression of the potato leaf roll virus movement protein. Plant Physiol 145:905–918
Lane BG (1994) Oxalate, germin, and extracellular matrix of higher plants. FASEB J 5:12239–12242
Lane BG (2002) Oxalate, germins, and higher-plant pathogens. IUBMB Life 53:67–75
Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC (1993) Germin, a marker protein of early plant growth, is an oxalate oxidase. J Biol Chem 268:12239–12242
Lebaudy A, Véry AA, Sentenac H (2007) K+ channel activity in plants: genes, regulations and functions. FEBS Lett 581:2357–2366
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Ma Y, Zhou T, Hong Y, Fan Z, Li H (2008) Decreased level of ferredoxin I in Tobacco mosaic virus-infected tobacco is associated with development of the mosaic symptom. doi:10.1016/j.pmpp.2008.05.004
Miller RO (1997) Nitric-perchloric acid wet digestion in an open vessel. In: Kalra YP (ed) Soil and plant analysis. CRC Press, Edmonton
Moutim V, Silva LG, Lopes MTP, Wilson Fernandes G, Salas CE (1999) Spontaneous processing of peptides during coagulation of latex from Carica papaya. Plant Sci 142:115–121
Nawrot R, Kalinowski A, Gozdzicka-Jozefiak A (2007) Proteomic analysis of Chelidonium majus milky sap using two-dimensional gel electrophoresis and tandem mass spectrometry. Phytochemistry 68:1612–1622
Orozco-Cárdenas ML, Ryan C (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and system in via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Pickard WF (2008) Laticifers and secretory ducts: two other tube systems in plants. New Phytol 177:877–888
Purvis MJ, Collier DC, Walls D (1964) Laboratory techniques in botany. Butterwoths, London
Radwan DE, Fayez KA, Mahmoud SY, Hamad A, Lu G (2007) Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol Biochem 45:480–489
Rinne PL, van den Boogaard R, Mensink MG, Kopperud C, Kormelink R, Goldbach R, van der Schoot C (2005) Tobacco plants respond to the constitutive expression of the tospovirus movement protein NS(M) with a heat-reversible sealing of plasmodesmata that impairs development. Plant J 43:688–707
Rodrigues CH, Ventura JA, Marin SLD (1989) Ocorrência e sintomas da meleira do mamoeiro (Carica papaya) no Estado do Espírito Santo. Fitopatol Bras 14:118
Rodrigues SP, Galvão OP, Andrade JS, Ventura JA, Fernandes PMB (2005) Simple molecular method to papaya sticky disease detection from latex and tissues samples. Summa Phytopatol 31:273–275
Sajnani C, Zurita JL, Roncel M, Ortega JM, Baron M, Ducruet JM (2007) Changes in photosynthetic metabolism induced by tobamovirus infection in Nicotiana benthamiana studied in vivo by thermoluminescence. New phytol 175:120–130
Samanani N, Alcantara J, Bourgault R, Zulak KG, Facchini PJ (2006) The role of phloem sieve elements and laticifers in biosynthesis and accumulation of alkaloids in opium. Plant J 47:547–563
Schroeder JI, Hedrich R, Fernandez JM (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312:361–362
Scott TA Jr, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 11:1656–1661
Stuart NW (1936) Adaptation of the micro-Kjedahl method for the determination of nitrogen in plant tissues. Plant Physiol 11:173–179
Ventura JA, Costa H, Tatagiba JS (2001) Sintomatologia da meleira do mamoeiro e sua importância para o “roguing”. Fitopatol Bras 26:536
Ventura JA, Costa H, Tatagiba J, da S (2004) Papaya diseases and integrated control. In: Naqvi SAH (ed) Diseases of fruits and vegetables: diagnosis and management. Kluwer, London
Wititsuwannakul R, Pasitkul P, Kanokwiroon K, Wititsuwannakul D (2008a) A role for a Hevea latex lectin-like protein in mediating rubber particle aggregation and latex coagulation. Phytochemistry 69:339–347
Wititsuwannakul R, Rukseree K, Kanokwiroon K, Wititsuwannakul D (2008b) A rubber particle protein specific for Hevea latex lectin binding involved in latex coagulation. Phytochemistry 69:1111–1118
Wititsuwannakul R, Pasitkul P, Jewtragoon P, Wititsuwannakul D (2008c) Hevea latex lectin binding protein in C-serum as an anti-latex coagulating factor and its role in a proposed new model for latex coagulation. Phytochemistry 69:656–662
Yasue T (1969) Histochemical identification of calcium oxalate. Acta Histochem Cytochem 2:83–95
Zambolim EM, Alonso SK, Matsuoka K, Carvalho MG, Zerbini FM (2003) Purification and some properties of Papaya meleira virus, a novel virus infecting papayas in Brazil. Plant Pathol 52:389–394
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Financiadora de Estudos e Projetos (FINEP) and Fundação de Apoio à Ciência e Tecnologia do Estado do Espírito Santo (FAPES). We thank Prof. Antônio Alberto R. Fernandes for helpful comments on this manuscript. We also thank João G.Z. Piccin for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Judelson.
Rights and permissions
About this article
Cite this article
Rodrigues, S.P., Da Cunha, M., Ventura, J.A. et al. Effects of the Papaya meleira virus on papaya latex structure and composition. Plant Cell Rep 28, 861–871 (2009). https://doi.org/10.1007/s00299-009-0673-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0673-7