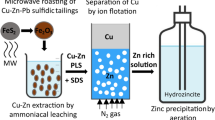
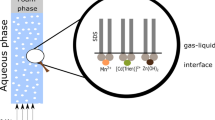
Abstract
Ion flotation is an efficient separation technique for the recovery of metal ions from dilute aqueous solutions. Provided that both the collected metal and the surfactant are recovered from the foam phase, it could be considered as an alternative to ion exchange, adsorption or solvent extraction in hydrometallurgy. However, most studies are focusing only on the metal recovery by ion flotation and neglect the development of a closed-loop ion flotation flow sheet with regeneration of the collector. As a consequence, the industrial implementation of this technique to hydrometallurgical operations is limited. The objective of this paper was to develop a simple solvometallurgical process for the decomposition of copper(II) tetraamine dodecyl sulfate sublates from foams generated after the ion flotation of dilute copper–zinc ammoniacal solutions with sodium dodecyl sulfate (SDS). It was possible to recover copper almost quantitatively as a copper-bearing precipitate, after mixing the foam phase with a 8 g L−1 NaOH in methanol solution. The collector was regenerated from the solution by removal of methanol by distillation and its reusability was tested in five subsequent ion flotation cycles.The efficiency of the regenerated SDS for the recovery of copper from dilute copper–zinc ammoniacal solutions was similar (ca. 80%) to that of fresh SDS solution without make-up. Based on the experimental results, a conceptual closed-loop flow sheet is proposed for the recovery of copper from dilute ammoniacal leachates by ion flotation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mineral processing and metallurgical industries generate vast amounts of waste such as waste rock, tailings, metallurgical slags and dusts, and these wastes have a negative environmental impact [1,2,3]. However, these industrial waste streams often contain low concentrations of valuable metals, which might be recovered in an economically feasible way if a suitable process would be available [4]. Therefore, the development of new metallurgical processes capable to recover efficiently valuable metals from mining waste has attracted a lot of attention from researchers over the last years [5]. The implementation of these technologies at industrial level will transform these materials from waste to low-grade secondary resources.
Ion flotation is a foam separation technique for removing or recovering metal ions from dilute aqueous solutions [6,7,8,9,10,11,12]. In this technique, surfactants (collectors) are added to a solution and compressed air or nitrogen gas is injected in the solution to create a mobile gas/liquid interface (bubbles). The presence of bubbles in the solution, in combination with the amphiphilic nature of the collectors, generates a charged interface. As a result, the targeted metal ions (colligends) are adsorbed to this interface due to interactions with the hydrophilic functional group of the collector. This interaction is usually electrostatic and forms a colloid (sublate), which is concentrated to a foam phase as the bubbles ascend to the surface of the solution [13].
Due to its simplicity and fast operation, ion flotation can be considered as an alternative hydrometallurgical process to recover metals from dilute aqueous solutions [14, 15]. The treatment of dilute solutions by solvent extraction, adsorption or ion exchange results in high extractant losses, high costs for selective adsorbent and large amounts of secondary wastes, respectively [16]. Since its first description by Sebba in 1959, numerous researchers have successfully implemented the technique of ion flotation to recover base metals (e.g. Cu, Zn, Al, etc.), precious metals (e.g. platinum group metals- PGMs) and to preconcentrate rare earths (e.g. Nd, La, Ho, etc.) from dilute simulated aqueous solutions [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Recent studies emphasized on the recovery of metals by using novel chelating surfactants because these can separate the colligends with better selectivity compared to commercial collectors [32, 33]. Finally, research has been also extended to the recovery or removal of metals from real dilute leachates or effluents [34,35,36].
Despite the promising results obtained at laboratory scale, ion flotation is not yet implemented in industry as an alternative hydrometallurgical process due to technical/practical limitations [8, 14, 37]. In particular, some of the surfactants used in ion flotation (e.g. quaternary ammonium compounds) may raise environmental concerns if released to water. Furthermore, the collectors must be present at least at stoichiometric equivalence with respect to the targeted metal ions concentration, which in combination with their high price makes the technique economically less attractive. Nevertheless, such constrains can be addressed by developing methods to strip and recover the metals from the loaded foam as well as to regenerate and re-use the surfactant.
Masuyama et al. used dichloromethane to separate the collector (amide oxime-based surfactant) from the collected metals (gallium, aluminium) after dissolving the generated foam into a mixture of methanol–water (1:5 ratio) solution and acidifying with nitric acid to pH = 2 [38]. Based on the reported experimental results, 68% of the surfactant was recovered after evaporation of the organic phase and 73% of gallium and 20% of aluminium remained in the aqueous phase. Nicol et al. reported a 20–60% recycling efficiency of the collector and > 90% of gold recovery after electrolysis at an ion flotation pilot plant in Australia [15]. Screenivasaro and Doyle investigated the decomposition of metal–dodecyl sulfate complexes by hydroxide precipitation, sulfide precipitation, chemical stripping and electrolysis, after letting the foam physically collapsed [39]. The most promising results were obtained by electrolysis, since copper and cadmium were deposited at current efficiencies of around 60–65% and the surfactant remained in the solution. Similarly, Eivazihollagh et al. researched the electrochemical treatment of copper from the surface active derivative of diethylenetriaminepentaacetic acid (C12DTPA) in alkaline solutions using a membrane cell [40]. Under optimized conditions, a copper recovery yield of 65% and a current efficiency of 53.6% was obtained. Considering all of these studies, electrolysis is indicated as the most suitable method to decompose the metal–surfactant sublate. However, the treatment of dilute aqueous solutions by electrolysis is usually a complicated process, which might result in energy inefficient, unwanted side reactions and products, gaseous emissions and costly apparatus [41].
Solvometallurgy might be an alternative route to recover the colligend and regenerate the collector from the foam phase. Solvometallurgy is a relatively novel metallurgical branch, through which water is partially or entirely replaced by organic solvents [42]. Solvometallurgical processes have been successfully developed for metal extraction (i.e. solvoleaching) and metal recovery operations (i.e. non-aqueous solvent extraction and non-aqueous electrodeposition) [43,44,45]. In a solvometallurgical approach, the generated foam carrying the sublate could be dissolved in an organic solvent, whereby the colligend and collector could be recovered from that solvent. Applying a non-aqueous (solvometallurgical) route for recovering the metal from the loaded foam and regenerating the surfactant from ion flotation foams might offer several advantages compared to aqueous systems. For instance limited water consumption, reduced acid consumption and better dissolution of the metal–surfactant colloid.
Overall, very few studies have been devoted to the recovery of the metal and the surfactant from the foam phase. The proposed methods involved intricate processing and limited metal recovery. In most studies, the regenerated collector was not tested in a moderate number of ion flotation cycles, which preclude a proper long-term assessment of ion flotation. Hence, a sustainable treatment should aim at quantitative recovering of the colligend and reusability of the collector as well. In a previous work of the authors, ion flotation was successfully implemented under realistic conditions for the recovery of copper from dilute Cu–Zn ammoniacal leachates of microwave-roasted Cu–Zn–Pb sulfidic tailings [34]. Although results were found to be very promising, the system could be further improved, for instance by stripping copper from the loaded foam, recovering and reusing the collector (sodium dodecyl sulfate). Therefore, the objective of this paper is to develop a straightforward solvometallurgical technique for the decomposition of copper(II) tetraamine dodecyl sulfate sublates from foams generated after the ion flotation of dilute Cu–Zn ammoniacal solutions. The novelty of the current work relies to the fact that an non-aqueous (solvometallurgical) treatment is introduced for the first time for stripping copper from the loaded foam and regenerating the collector (SDS).
Experimental
Chemicals
Nitric acid (65% a.r.), anhydrous copper(II) sulfate (a.r.), ammonia solution (25 wt%), aqueous standard solutions (1000 mg L−1 in 2–5% HNO3) of copper, zinc and scandium and oil standard solutions (1000 mg L−1 in hydrocarbon oil) of copper and lanthanum were purchased from Chem-Lab NV (Zedelgem, Belgium). Sodium dodecyl sulfate (SDS, ≥ 99%), 1-octanol (≥ 99%) and zinc(II) sulfate monohydrate (99%) were obtained from Acros Organics (Geel, Belgium). Ammonium carbonate (≥ 30% NH3 basis) was purchased from Sigma-Aldrich (Overijse, Belgium). Ethanol (EtOH, 99.5%), methanol (MeOH, 99.0% and HPLC grade) and sodium hydroxide (pearls, a.r.) were obtained from Fisher Scientific (Thermo Fisher Scientific, Loughborough, United Kingdom). Water was always of ultrapure quality, deionized to a resistivity of 18.2 MΩ cm with a Millipore ultrapure water system. All chemicals were used as received without any further purification.
Instrumentation
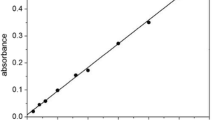
Ion flotation experiments were carried out in a glass column (45 cm high and 4.5 cm internal diameter) equipped with a sintered glass disc (D4 pore size, ~ 10–15 μm), described in earlier publications [7, 34]. Precipitation of copper from methanol solutions was set-up in a 250 mL round bottom flask at room temperature. After precipitation, the solid phase was separated from the liquid phase by vacuum filtration using a filtered paper of 1.6 μm pore size. The surfactant in the solution after the filtration was recovered by vacuum distillation using a standard set-up equipped with an Adixen pump (Pascal 2015SD) and a manometer (TPG 201 Pfeiffer Vacuum). Concentrations of elements in solutions were measured by an inductively coupled plasma-optical emission spectrometer (ICP-OES; Perkin Elmer Avio 500) equipped with an axial/radial dual plasma view and GemCone High Solids nebulizer. The standard solutions and the samples were prepared by dilution with 5 wt% HNO3 for the aqueous samples (i.e., ion flotation experiments) and with 1-octanol for the organic samples (i.e., precipitation experiments). Scandium (5 ppm) and lanthanum (5 ppm) were used as internal standards for the aqueous and organic ICP measurements, respectively. The concentration of the collector in methanol solutions was determined by liquid chromatography/mass spectroscopy (LC–MS; HPLC Agilent 1100 and MS Agilent 6110SQ). Elution was conducted with a mobile phase consisting of methanol (solvent D) and H2O + 0.1% formic acid (solvent A), which were pumped at a rate of 0.4 mL min−1. The gradient program was set as follows: 10 min from D:A = 80:20 to D:A = 100 and hold for 25 min. The column oven was set at 25 °C and the sample injection volume was 5 μL. Mass spectroscopy data were recorded in a negative electrospray ionization (ESI) mode from m/z 10 to 1500 and the analyzer was set in sensitivity mode. The samples were diluted 10 times with methanol and prior to their measurement a calibration curve was constructed by measuring samples of known SDS concentration (Figure S1). The concentration of SDS in the samples was calculated by integrating the surface area curve of the pseudo-molecular ion m/z 265.100 [M + H]− at retention time = 14.5 min. The mineralogical composition of the precipitates was characterized by X-ray diffraction analysis (XRD; Brucker D2 Phaser Diffractometer). Diffractograms were recorded in the measurement range of 5°–70° 2θ using CuKα radiation and applying an acceleration voltage of 45 kV, an electrical current of 30 mA, a step size of 0.020° and a counting of 1 s per step. The raw data were processed with EVA software with the ICDD database. The regenerated SDS was characterized by Fourier transform infrared-attenuated total reflectance (ATR-FTIR, Bruker Vertex 70), operating at room temperature. The equilibrium surface tension of the ion flotation solutions was measured by surface tensiometry (Krüss K100C tensiometer) with a plate made of roughened platinum (Wilhelmy plate method) based on the following equation:
where γ is the surface tension (mN m−1), F is the force (mN) measured by the sensor when the plate attaches the surface of the solution, L is the wetted length of the plate (mm) and θ is the contact angle (°). Surface tensions measurements were carried out in 100 mL glass vessels (Krüss SV20, 70 mm internal diameter) at room temperature, containing approximately 80 mL of solutions. Prior to the measurements the surface tension of water was measured as a reference. A Heraeus D-6450 oven was employed to dry the solid samples, operating at 40 °C. All experiments were done in duplicate and all data in figures represent averaged values of each duplicate.
Experimental Procedures
Ion flotation experiments were performed by mixing the appropriate amounts of metal ions, surfactant (fresh or regenerated product), ethanol and {ammonia + ammonium}-[NH3 + NH4+] stock solutions. The concentrations used for the experiments were simulating the ammoniacal leachates of the microwave roasted Cu–Zn–Pb sulfidic tailings under the optimized ion flotation conditions [34]. The solutions were stirred for 10 min with a magnetic stirring bar on a magnetic stirrer at low speed (200 rpm), in order to avoid the generation of foam and then poured slowly in the column. Nitrogen gas was bubbled through the solution from the bottom. Aliquots of approximately 3 mL were withdrawn for ICP-OES analysis from the bulk solution before and after the flotation experiments. The generated foam was collected in a glass beaker and then dissolved in 250 mL of methanol. The efficiency of ion flotation results was expressed as the recovery percent (Re%) according to Eq. (2):
where Ci and Cr are the initial and residual metal ion concentration of the bulk solution (mg L−1), respectively. In all the ion flotation experiments, 0.5% (v/v) of ethanol was added as a frother and the initial volume was 300 mL.
The precipitation of copper from methanol solutions was performed by adding sodium hydroxide (NaOH) either as a solid or dissolved in methanol at a concentration of 8 g L−1. At specified time intervals, a small aliquot of the sample was withdrawn for ICP-OES analysis. At the end of the experiments, the mixture was filtered by vacuum filtration. The precipitate was washed with methanol, dried at 40 °C for 24 h and analyzed by XRD. The precipitation efficiency EP (%) of copper was calculated based on Eq. (3):
where Ci(Cu) and Cf(Cu) are the initial and residual zinc concentration in the solution, respectively.
The filtrated solution after the precipitation was subjected to vacuum distillation (80 mbar at 40 °C for 2 h) using a rotary evaporator in order to separate methanol and the collector. For more accurate FTIR analysis, the regenerated product was dried overnight on the Schlenk line. The efficiency of the regenerated product was tested in five cycles. The distilled methanol was reused in order to dissolve the generated foams of the subsequent cycles.
Results and Discussion
Recovery of Copper from the Foam Phase by Precipitation
As mentioned in the introduction, copper can be selectively recovered from dilute copper–zinc ammoniacal leachates by ion flotation, with SDS as the collector [34]. Copper is adsorbed to the gas/liquid interface and concentrated to the foam phase as copper(II) tetraammine dodecyl sulfate sublate, with formula [Cu(NH3)4](DS)2. Pure solutions of lower alcohols, such as methanol (MeOH), ethanol (EtOH) and isopropanol (IPA) can rupture the bubbles of the foam phase and eventually dissolve the carried sublates [46]. Preliminary dissolution tests were conducted with MeOH, EtOH and IPA to select the most suitable solvent, having as sole criterion that the least amount of solvent should be used to dissolve the sublate. It was observed that MeOH performed the best among the examined solvents. In particular, 250 mL of MeOH was sufficient to decompose the whole amount of the generated sublate, whereas larger volumes of EtOH (350 mL) and IPA (450 mL) were required. Therefore, MeOH was selected as the solvent for the subsequent experiments.
The addition of a strong alkali, such as sodium hydroxide (NaOH) was expected to precipitate copper as oxide or hydroxide [47]. NaOH is a relatively inexpensive inorganic compound with a high solubility in MeOH (ca. 238 g L−1 at 25 °C) [48]. Finally, the addition of NaOH could provide the necessary sodium cations needed for regeneration of SDS sodium salt in the next stage. The precipitation efficiency of copper from MeOH solutions as a function of the NaOH concentration is illustrated in Fig. 1 and Table S1. An increase in sodium hydroxide concentration from 2 to 8 g L−1, gradually increased the precipitation efficiency of copper from 42% to 99%, respectively. Further increase in the NaOH concentration to more than 8 g L−1 had a negligible effect on the precipitation efficiency and therefore 8 g L−1 was selected as an optimum value.
Effect of NaOH concentration on the precipitation efficiency [Ep (%)] of copper from methanolic solutions. Conditions: [Cu2+]I = 150 mg L−1, steering speed = 550 rpm, V = 250 mL, T = 25 °C. Number of replicas: 2 (N = 2). Error bars were calculated as standard deviation. When the error bar is not visible, it is smaller than the marker
The effect of the precipitation time was investigated keeping the NaOH concentration constant at 8 g L−1 (Fig. 2). Within the first minute, 87% of copper precipitated and then reached a plateau (99%) after 15 min of precipitation. Adding NaOH pearls affected the precipitation kinetics by limiting the interphase solid–liquid transfer of reagents towards copper ions. As a result, some time was required for the NaOH pearls to dissolve and to enable them to react. It was assumed that the precipitation of copper could happen faster if NaOH was dissolved in MeOH prior to addition to the copper-containing foam. For this reason, a second series of precipitation experiments were carried out but this time the collected foam was dissolved with a methanolic solution containing 8 g L−1 of NaOH. The experimental results supported this hypothesis (Fig. 2 and Table S2), since copper was spontaneously precipitated within the first minute with an almost quantitative yield. Thus, prior dissolution of NaOH in MeOH resulted in shorter precipitation times, and this was selected as the optimum procedure.
Effect of time on the precipitation efficiency [Ep (%)] of copper by adding NaOH either a solid ( ) or dissolved in methanol (
) or dissolved in methanol ( ). Conditions: [Cu2+]I = 150 mg L−1, [NaOH] = 8 g L−1 steering speed = 550 rpm, V = 250 mL, T = 25 °C. Number of replicas: 2 (N = 2). Error bars were calculated as standard deviation. When the error bar is not visible, it is smaller than the marker
). Conditions: [Cu2+]I = 150 mg L−1, [NaOH] = 8 g L−1 steering speed = 550 rpm, V = 250 mL, T = 25 °C. Number of replicas: 2 (N = 2). Error bars were calculated as standard deviation. When the error bar is not visible, it is smaller than the marker
The X-ray diffraction analysis of the obtained precipitate (Figs. 3 and S1) showed that it is mainly consisted of copper oxide (tenorite and cuprite) and copper carbonate (malachite) phases. Small amounts of copper(II) sulfate phases (chalcanthite and bronchanthite) were also detected as well as sodium bicarbonate (nahcolite). The ion flotation solution contained carbonate and sulfate anions, derived from the salts used for the flotation experiments [copper(II) sulfate and ammonium carbonate]. To a certain extent these anions might be co-adsorbed to the foam phase during the ion flotation and then dissolved in the methanolic solution, resulting in the copper precipitate. Broad-low intensity peaks were also noticed and could be attributed to the formation of other copper-bearing amorphous phases in the precipitate [49]. A potential application of this precipitate is to be used as raw material for the production of metallic copper by solvent-extraction/electrowinning processes (SX-EW), after leaching with sulfuric acid [50].
Regeneration of the Collector
Based on the experimental results reported in the previous section, it can be concluded that copper could be precipitated almost quantitatively by addition of NaOH. As presented in Fig. 4 and Table S3, the addition of sodium hydroxide did not have any effect on the residual concentration of the collector in the MeOH solution. Even at relevant high NaOH concentrations (up to 10 g L−1), the surfactant concentration remained stable at around 6 mmol L−1, slightly below the critical micelle concentration in MeOH solutions (6.7 to 7 mmol L−1) [51]. Therefore, the solution after copper precipitation was still presenting suitable concentrations of collector to be further purified for potential reuse.
Taking into account the low boiling point of methanol (~ 65 °C), distillation was considered to be a straightforward procedure to recover the collector from this solution [48]. Indeed distillation did generate a white crystalline solid precipitate (Figure S3), upon decreasing the volume of the solvent. The IR spectra of the regenerated product and fresh SDS were found to be almost identical (Fig. 5). More specifically, the CH3 (2956 cm−1), CH2 (2919 cm−1, 2851 cm−1), SO3 (1222 cm−1) and C–S–O (988 cm−1) infrared bands were present in both solids [21, 52]. Hence, all experimental evidence suggests that the regenerated product was SDS which could be recycled in a new ion flotation cycle.
Multiple Ion Flotation Cycles by Using the Regenerated Collector
To assess the efficiency of the regenerated collector on the ion flotation of copper from dilute ammoniacal leachates as well as the quality of the copper precipitate, the overall process was repeated five times. Firstly, copper was concentrated to the foam phase by ion flotation, then it was precipitated by mixing the foam with a methanolic solution containing 8 g L−1 NaOH and finally the surfactant was regenerated by removal of the methanol by distillation. The regenerated product was then reused as such in a new ion flotation experiment and the distilled methanol was also reused for the precipitation of copper. As presented in Figure S4 and Table S4, the performance of the regenerated surfactant on the recovery of copper was very similar to that of the fresh SDS. The recovery efficiency of copper remained stable at around 80% after regenerating the collector for 5 times. This implies that the collector can be successfully reused. Additionally, the surface tension of the ion flotation solutions over the cycles remained stable at about 25 mN m−1 (Fig. 6). A loss in the surfactant content during the process would be translated in an increase of the surface tension of the ion flotation solution. However, that was not the case for the examined system and at the scale on which the experiments were conducted (V = 300 mL).
Equilibrium surface tensions of ion flotation solutions as function of regeneration cycle. Conditions: [NH3 + NH4+] = 2 mol L−1, NH3:NH4+ = 2:1, [Zn2+]I = 6 mmol L−1, [Cu2+]I = 2 mmol L−1, [EtOH] = 0.5% (v/v), [SDS] = 5 mmol L−1, T = 23.4 °C, pH = 10.18. Number of replicas: 2 (N = 2). Error bars were calculated as standard deviation. When the error bar is not visible, it is smaller than the marker
Similarly no major differences were detected in the IR spectra of the regenerated SDS of every cycle (Figure S5). The quality of the copper-bearing precipitates were also studied by X-ray diffraction (Fig. 7). Interestingly, differences in the peak intensities of the copper(II) sulfate, oxide and carbonate phases were observed due to the amorphization of the precipitate [53]. In addition, these differences appeared to follow a specific trend. When the copper(II) sulfate and oxide phases peak intensities were higher (1st cycle precipitate) compared to the original precipitate, the peak intensities of the copper(II) carbonate phases were lower and vice versa (2nd, 3rd, 4th and 5th precipitate). However, this behavior did not affect the precipitation efficiency of copper, which remained stable at almost 100% in every cycle.
Conceptual Flowsheet
Based on the experimental results described in this paper, a conceptual closed-loop flow sheet can be proposed (Fig. 8). Copper is first selectively separated from a dilute copper–zinc ammoniacal leachates by ion flotation as copper(II) tetraamine dodecyl sulfate, with SDS as the collector. The zinc that remains in the solution after ion flotation can be recovered by sulfide precipitation or as basic zinc carbonate by aeration [34]. The foam phase is then mixed with a NaOH solution in methanol (8 g L−1) to precipitate copper. Copper can be almost quantitatively recovered as copper-containing precipitate, which mainly consists of copper oxide and carbonate phases. The copper-bearing precipitate can be used as raw material for the production of metallic copper in SX-EW processes, after leaching with sulfuric acid. The collector, which remains in the solution after precipitation, is regenerated by distillation. It was experimentally shown that the regenerated collector can be successfully reused in multiple ion flotation cycles without the need for SDS make-up. Nevertheless, a certain amount of SDS might be required to be added at larger scale to maintain the optimum surfactant concentration (5 mmol L−1) at the next ion flotation solution. The distilled methanol can also be recycled within this flowsheet after addition of NaOH for the precipitation of copper. This flowsheet could be considered as a solid basis for technical economic analysis. Furthermore, the suggested methods for the recovery of copper (chemical precipitation) and SDS (distillation) are well-established technologies in industry and can find minor limitations in scale-up activities. Such type of limitations might concern for instance the settling time and filterability of the cake (generated copper precipitate) during precipitation. Nonetheless, such constrains can be addressed and optimized at mini pilot scale stage. Finally, the suggested route is a sustainable process for the recovery of copper and SDS from the foam phase because all the compounds (with the exception of NaOH) are regenerated and reused within the flowsheet. In addition and from an environmental point of view, there is no generation of any solid or liquid waste and hence release of toxic compounds, such as methanol, to the environment.
Conclusions
A solvometallurgical process to decompose the copper(II) tetraamine dodecyl sulfate sublate from foam phases generated after the ion flotation of dilute copper–zinc ammoniacal solutions was developed. After diluting the foam phase with a solution of 8 g L−1 NaOH in methanol it was possible to precipitate copper almost quantitively (yield close to 100%). The proposed route achieved higher metal recovery yield compared to previous studies dealing with the stripping of copper from the loaded foam [39, 40]. The copper-containing precipitate mainly consisted of copper(II) carbonate, oxide and sulfate phases. From the solution after the precipitation sodium dodecyl sulfate (SDS) collector was regenerated by removing methanol via distillation. The reusability of the regenerated collector was tested in five ion flotation cycles. Based on the experimental results, the efficiency of the regenerated SDS on recovering copper from dilute Cu–Zn ammoniacal solutions was comparable to that of fresh SDS. The distilled methanol was also reused for the precipitation of copper and a conceptual closed- looped flow sheet of a continuous process was proposed. This case study provided a reliable confirmation for the implementation of ion flotation as an alternative hydrometallurgical process for the recovery of copper from dilute Cu–Zn ammoniacal leachates, with regeneration of the collector. In addition, the suggested conceptual flowsheet gives very insightful laboratory scale results, which are very important for a strategic design of pilot plant scale, where the economic feasibility of the process could be thoroughly assessed. Nevertheless, future studies should emphasize on the development of similar closed-loop ion flotation methods for other colligends and collectors too.
Supporting Information
The supporting information is available on the Springer Nature website and includes: the residual metal concentration (mg L‒1) of the precipitation experiments (Tables S1–S2), the residual collector concentration (mmol L‒1) in methanol solutions (Table S3), the residual metal concentration (mg L‒1) of the ion flotation experiments (Figure S4 and Table S4), pictures of the copper precipitate (Figure S1) and the regenerated collector (Figure S2), the LC–MS calibration curve for SDS (Figure S3) and the comparative FTIR spectra of the regenerated collector (Figure S5).
References
Lottermoser B (2010) Mine wastes: characterization, treatment and environmental impacts. Springer, Berlin. https://doi.org/10.1007/978-3-642-12419-8
Kefeni KK, Titus AMM, Mamba BB (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Clean Prod 151:475–493. https://doi.org/10.1016/j.jclepro.2017.03.082
Kossoff D, Dubbin WE, Alfredsson M, Edwards SJ, Macklin MG, Hudson-Edwards KA (2014) Mine tailings dams: characteristics, failure, environmental impacts, and remediation. Appl Geochem 51:229–245. https://doi.org/10.1016/j.apgeochem.2014.09.010
Jamieson HE, Walker SR, Parsons MB (2015) Mineralogical characterization of mine waste. Appl Geochem 57:85–105. https://doi.org/10.3390/min9050303
Spooren J, Binnemans K, Björkmalm J, Breemersch K, Dams Y, Folens K, González-Moya M, Horckmans L, Komnitsas K, Kurylak W, Lopez M, Mäkinen J, Onisei S, Oorts K, Peys A, Pietek G, Pontikes Y, Snellings R, Tripiana M, Varia J, Willquist K, Yurramendi L, Kinnunen P (2020) Near-zero-waste processing of low-grade, complex primary ores and secondary raw materials in Europe: technology development trends. Resour Conserv Recycl 160(May):104919. https://doi.org/10.1016/j.resconrec.2020.104919
Sebba F (1959) Concentration by ion flotation. Nature 184:1062–1063. https://doi.org/10.1038/1841062a0
Xanthopoulos P, Binnemans K (2021) Removal of cadmium, zinc, and manganese from dilute aqueous solutions by foam separation. J Sustain Metall 7:78–86. https://doi.org/10.1007/s40831-020-00322-2
Chang L, Cao Y, Fan G, Li C, Peng WA (2019) Review of the applications of ion floatation: wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv 9(35):20226–20239. https://doi.org/10.1039/c9ra02905b
Somasundaran P (1975) Separation using foaming techniques. Sep Sci 10:93–109. https://doi.org/10.1080/00372367508057071
Pinfold T (1972) Ion flotation. In: Lemlich R (ed) Adsorptive bubble separation techniques. Academic Press, New York, pp 53–73. https://doi.org/10.1016/B978-0-12-443350-2.50009-5
Lemlich R (1972) Ion flotation. Adsorptive bubble separation techniques. Academic Press, New York, pp 53–68. https://doi.org/10.1016/b978-0-12-443350-2.x5001-1
Grieves RB (1975) Foam separations: a review. Chem Eng J 9:93–106. https://doi.org/10.1016/0300-9467(75)80001-3
Lu S, Pugh RJ, Forssberg E (2005) Gas/liquid interfacial separation. Studies in interface science. Elsevier, Amsterdam, pp 559–645. https://doi.org/10.1016/s1383-7303(05)80012-2
Doyle FM (2003) Ion flotation—its potential for hydrometallurgical operations. Int J Miner Process 72(1–4):387–399. https://doi.org/10.1016/S0301-7516(03)00113-3
Nicol S, Galvin K, Engel M (1992) Ion flotation- potential applications to mineral processing. Min Eng 5(10–12):1259–1275. https://doi.org/10.1016/0892-6875(92)90163-4
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Polat H, Erdogan D (2007) Heavy metal removal from waste waters by ion flotation. J Hazard Mater 148(1–2):267–273. https://doi.org/10.1016/j.jhazmat.2007.02.013
Mcdonald C, Suleiman A (1979) Ion flotation of copper using ethylhexadecyldimethyl-ammonium bromide. Sep Sci Technol 14(3):219–225. https://doi.org/10.1080/01496397908066960
Stoica L, Lacatusu I (2012) Cu(II) separation from diluted aqueous solutions by flotation with atypical collectors anti and syn 2-hydroxy-3,5-di-tert-butyl-benzaldoxime. Int J Environ Waste Manage 9(3–4):293–312. https://doi.org/10.1504/IJEWM.2012.046394
Strel’tsov KA, Abryutin DV (2010) Investigation of regularities of ion flotation of copper with the use of sodium diethyldithiocarbamate. Russ. J. Non-Ferrous Met. 51(2):85–88. https://doi.org/10.3103/S106782121002001X
Hoseinian FS, Rezai B, Kowsari E, Safari M (2018) Kinetic study of Ni(II) removal using ion flotation: effect of chemical interactions. Miner Eng 119(February):212–221. https://doi.org/10.1016/j.mineng.2018.01.028
McDonald CW, Ogunkeye OA (1981) Ion flotation of zinc using ethylhexadecyldimethylammonium bromide. Microchem J 26(1):80–85. https://doi.org/10.1016/0026-265X(81)90013-8
Ulewicz M, Walkowiak W, Jang Y, Kim JS, Bartsch RA (2003) Ion flotation of cadmium (II) and zinc (II) in the presence of proton-ionizable lariat ethers. Anal Chem 75(10):2276–2279. https://doi.org/10.1021/ac026322y
Lusher JA, Sebba F (1965) The separation of aluminium from beryllium by ion flotation of an oxalato-aluminate complex. J Appl Chem 15(12):577–580. https://doi.org/10.1002/jctb.5010151205
Thanh LH, Liu JC (2021) Ion flotation of palladium by using cationic surfactants–effects of chloride ions. Colloids Surf A 616:126326. https://doi.org/10.1016/j.colsurfa.2021.126326
Matis KA, Mavros P (1991) Recovery of metals by ion flotation from dilute aqueous solutions. Sep Purif Rev 20:1–48. https://doi.org/10.1080/03602549108021407
Yenial Ü, Bulut G (2017) Examination of flotation behavior of metal ions for process water remediation. J Mol Liq 241:130–135. https://doi.org/10.1016/j.molliq.2017.06.011
He XC (1991) Ion flotation of rhodium (III) and palladium (II) with anionic surfactants. Talanta 38(3):319–323. https://doi.org/10.1016/0039-9140(91)80054-4
Berg EW, Downey DM (1980) Ion flotation studies of the chlorocomplexes of some platinum group metals. Anal Chim Acta 120:237–248. https://doi.org/10.1016/S0003-2670(01)84367-1
Chirkst DE, Lobacheva OL, Dzhevaga NV (2012) Ion flotation of lanthanum (III) and holmium (III) from nitrate and nitrate-chloride media. Russ J Appl Chem 85(1):25–28. https://doi.org/10.1134/S1070427212010053
Micheau C, Diat O, Bauduin P (2018) Ion foam flotation of neodymium: from speciation to extraction. J Mol Liq 253:217–227. https://doi.org/10.1016/j.molliq.2018.01.022
Eivazihollagh A, Svanedal I, Edlund H, Norgren M (2019) On chelating surfactants: molecular perspectives and application prospects. J Mol Liq 278:688–705. https://doi.org/10.1016/j.molliq.2019.01.076
Liu Z, Doyle FM (2009) Ion flotation of Co2+, Ni2+, and Cu2+ using dodecyldiethylenetriamine (Ddien). Langmuir 25(16):8927–8934. https://doi.org/10.1021/la900098g
Xanthopoulos P, Kalebić D, Kamariah N, Bussé J, Dehaen W, Spooren J, Binnemans K (2021) Recovery of copper from ammoniacal leachates by ion flotation. J Sustain Metall. https://doi.org/10.1007/s40831-021-00363-1
Lazaridis NK, Peleka EN, Karapantsios TD, Matis KA (2004) Copper removal from effluents by various separation techniques. Hydrometallurgy 74(1–2):149–156. https://doi.org/10.1016/j.hydromet.2004.03.003
Jafari M, Abdollahzadeh AA, Aghababaei F (2017) Copper ion recovery from mine water by ion flotation. Mine Water Environ 36:323–327. https://doi.org/10.1007/s10230-016-0408-2
Peng W, Chang L, Li P, Han G, Huang Y, Cao Y (2019) An overview on the surfactants used in ion flotation. J Mol Liq. https://doi.org/10.1039/c9ra02905b
Masuyama A, Okano T, Okahara M, Section E (1990) Application of surface-active amide oximes to the collectors for gallium ion in an ion-flotation system. Ind Eng Chem Res 29:290–294. https://doi.org/10.1021/ie00098a02
Doyle FM, Duyvesteyn S, Sreenivasarao K (1995) The use of ion flotation for detoxification of metal-contaminated waters and process effluents. In: Proceedings of the XIX international mineral processing congress. San Francisco, CA (United States), pp 22–27
Eivazihollagh A, Bäckström J, Norgren M, Edlund H (2018) Electrochemical treatment of copper complexed by chelating agent and chelating surfactant in alkaline solution using a membrane cell. J Chem Technol Biotechnol 93(5):1421–1431. https://doi.org/10.1002/jctb.5510
Kammel R (1984) Metal recovery from dilute aqueous solutions by various electrochemical reactors. In: Bautista RG (eds) Hydrometallurgical process fundamentals. NATO conference series (VI materials science), vol 10, Springer, Boston. https://doi.org/10.1007/978-1-4899-2274-8_24
Binnemans K, Jones PT (2017) Solvometallurgy: an emerging branch of extractive metallurgy. J Sustain Metall 3:570–600. https://doi.org/10.1007/s40831-017-0128-2
Peeters N, Binnemans K, Riaño S (2020) Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem 22(13):4210–4221. https://doi.org/10.1039/D0GC00940G
Li Z, Li X, Raiguel S, Binnemans K (2018) Separation of transition metals from rare earths by non-aqueous solvent extraction from ethylene glycol solutions using Aliquat 336. Sep Purif Technol 201:318–326. https://doi.org/10.1016/j.seppur.2018.03.022
Li X, Monnens W, Li Z, Fransaer J, Binnemans K (2020) Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals. Green Chem 22(2):417–426. https://doi.org/10.1039/C9GC02983D
Bi PY, Dong HR, Dong J (2010) The recent progress of solvent sublation. J Chrom A 1217(16):2716–2725. https://doi.org/10.1016/j.chroma.2009.11.020
Tünay O, Kabdaşli NI (1994) Hydroxide precipitation of complexed metals. Water Res 28(10):2117–2124. https://doi.org/10.1016/0043-1354(94)90022-1
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill, New York
Bevandić S, Blannin R, Vander Auwera J, Delmelle N, Caterina D, Nguyen F, Muchez P (2021) Geochemical and mineralogical characterisation of historic Zn–Pb mine waste, plombières. East Belg Miner 11(1):28. https://doi.org/10.3390/min11010028
Prasad MS, Kenyen VP, Assar DN (1992) Development of SX-EW process for copper recovery—an overview. Miner Process Extr Metall Rev 8(1–4):95–118. https://doi.org/10.1080/08827509208952680
Niraula TP, Chatterjee SK, Bhattarai A (2018) Micellization of sodium dodecyl sulphate in presence and absence of alkali metal halides at different temperatures in water and methanol-water mixtures. J Mol Liq 250:287–294. https://doi.org/10.1016/j.molliq.2017.12.014
Viana RB, da Silva AB, Pimentel AS (2012) Infrared spectroscopy of anionic, cationic, and zwitterionic surfactants. Adv Phys Chem. https://doi.org/10.1155/2012/903272
Zhao P, Lu L, Liu X, De la Torre AG, Cheng X (2018) Error analysis and correction for quantitative phase analysis based on rietveld-internal standard method: whether the minor phases can be ignored? Curr Comput-Aided Drug Des 8(3):110. https://doi.org/10.3390/cryst8030110
Acknowledgements
The research leading to these results has received funding from the European Community’s Horizon 2020 Programme under Grant Agreement No. 812580 SULTAN) (MSCA- ETN SULTAN). This publication reflects only the authors’ view, exempting the Community from any liability. The authors thank Bart van Huffel (KU Leuven) for helping with the LC/MS analysis. Thomas Abo Atia (KU Leuven), Srećko Bevandić (KU Leuven) and Demian Kalebić (KU Leuven) are gratefully acknowledged for the fruitful scientific discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Yongxiang Yang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xanthopoulos, P., Binnemans, K. Closing the Loop in Ion Flotation: Recovery of Copper, Regeneration and Reuse of Collector from the Foam Phase by a Solvometallurgical Process. J. Sustain. Metall. 7, 1565–1574 (2021). https://doi.org/10.1007/s40831-021-00463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-021-00463-y