Abstract
As environmental regulations are becoming stricter, new techniques must be developed for the removal of trace concentrations of heavy metals from mineral processing effluents. Foam separation techniques are an interesting alternative to more conventional processes such as ion exchange because of their efficiency to treat dilute aqueous streams. In this paper, the simultaneous removal of Cd2+, Mn2+, and Zn2+ from dilute aqueous solutions was investigated by using sodium dodecyl sulfate as collector and triethylenetetramine as auxiliary ligand via a series of batch-mode flotation experiments. Experimental results showed that Cd2+, Mn2+, and Zn2+ can be completely removed in one step under the following conditions: pH 9.50, flotation time = 120 min, auxiliary concentration 0.1 mmol L−1, collector-to-metals molar ratio 2:1, ethanol concentration 0.5% (v/v), and a nitrogen gas flowrate set at 25 mL min−1. An excess in auxiliary ligand concentration yielded to low removal efficiency. The modeled speciation of the examined system suggested that the metals are separated from the bulk solution to the foam phase via a combination of ion flotation and precipitate flotation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last decade, the legislative environmental framework has become stricter within the mineral processing industry, particularly in the case of management of mine or industrial waters, such as acid mine drainage, seepage mine waters, or process effluents. Conventional techniques for detoxifying aqueous effluents, such as chemical precipitation, ion exchange, adsorption, reverse osmosis, and membrane filtration, might not sufficiently be compliant with the threshold values of heavy metals for the safe disposal of industrial waters, as set by environmental legislations [1,2,3]. Therefore, extra post-treatment steps would be required for the removal of trace concentrations of heavy metals from dilute effluents.
Foam or adsorptive bubble separation techniques are promising for treating efficiently dilute wastewaters [4,5,6,7]. Ion and precipitate flotations are classified among these techniques and involve the metal extraction or removal from a dilute aqueous solution to a foam phase by adding surfactants (collectors), while nitrogen gas or air is bubbled through from the bottom [8, 9]. In ion flotation, the targeted metal ions (colligends) are adsorbed or adhered to the interface of the dispersed bubbles by interacting with the opposite charge collectors’ hydrophilic functional groups. They form insoluble precipitates (sublates) or soluble complexes, which are concentrated in a stable foam phase as the bubbles ascend to the surface of the solution [10,11,12]. In precipitate flotation, metal ions first are precipitated and then assisted by the surfactants to be separated from the bulk solution to the foam phase [13,14,15].
The advantages of foam separation techniques are their simplicity, relatively small space requirements, good recovery yields, and suitable for treating dilute aqueous solutions with low metal concentrations [16,17,18]. On the other hand, they are not suitable for concentrated solutions and the handling of chemicals and the generated foam might be difficult in larger scale. Additionally, some of the commercial available surfactants and the chemicals used might be expensive and/ or toxic [19, 20]. Nevertheless, laboratory studies showed that the regeneration of the chemicals used in foam separation techniques is possible, which can make them more attractive from an economical and environmental point of view [19, 21, 22]. To the best of our knowledge, only ion flotation has been employed so far on industrial scale for the purification of wastewater from textiles and for the recovery of tungsten and molybdenum from smelter wastewater [20]. Hence, studies should be extended at pilot plant scale, with realistic conditions, in order to assess the overall efficiency and estimate the costs [19].
For these reasons, there is a strong research interest in developing foam separation techniques for removing trace concentrations of heavy metals from dilute aqueous solutions. Several researchers have investigated the removal of cadmium (Cd2+) and zinc (Zn2+), from single- or multi-element solutions, via ion flotation or precipitate flotation by using either anionic or cationic surfactants as collectors and obtained high removal efficiencies [23,24,25,26,27]. Additionally, the removal of Cd2+, Zn2+, and manganese (Mn2+), from single- or multi-element solutions, via ion flotation by using collectors in combination with chelating ligands or chelating surfactants has been reported in several studies too [27, 28]. Thermodynamic calculations suggested that chelating ligands or chelating surfactants can enhance the efficiency and selectivity of ion flotation, since the overall Gibbs free energy for adsorption (ΔGads) is more negative in a system that is chelated compared to one that it is not [29,30,31,32].
Cd2+, Zn2+, and Mn2+ are heavy metals that may occur in dilute mining effluents, such as mine waste piles run off waters [33]. The contamination of surface or underground waters from these heavy metals can potentially pose a threat to human life even at low concentrations [34,35,36].
However, a simultaneous removal (co-flotation) of Cd2+, Zn2+, and Mn2+ has not been investigated yet either in chelated or in non-chelated flotation systems. Hence, the objective of this paper is to determine the optimum conditions for the co-flotation of Cd2+, Zn2+, and Mn2+ in one step, from dilute aqueous synthetic solutions at laboratory scale conditions. The anionic surfactant sodium dodecyl sulfate (SDS) was selected as collector and triethylenetetramine (Trien) as auxiliary ligand. Trien is a neutral chelating ligand, capable of chelating transition metal ions as metal–Trien complexes. It has been proven as an efficient auxiliary ligand for the ion flotation of copper and nickel [37].
Materials and Methods
Materials
Sodium dodecyl sulfate (≥ 95%) was purchased from TCI N.V. (Haven, Belgium). Triethylenetetramine hydrate (98%) and isopropanol (≥ 99.8%, p.a.) were obtained from Sigma-Aldrich (Overijse, Belgium). Cadmium chloride anhydrous (99%), manganese(II) chloride anhydrous (97%), and ethanol (EtOH, 99.8 + %, absolute) were purchased from Acros Organics (Geel, Belgium). Zinc chloride anhydrous (99.99%), nitric acid (65% a.r.), cadmium standard (1000 mg L−1 in 2–5% HNO3), zinc standard (1000 mg L−1 in 2–5% HNO3), manganese standard (1000 mg L−1 in 2–5% HNO3), and dysprosium standard (1000 mg L−1 in 2–5% HNO3) were supplied by ChemLab (Zedelgem, Belgium). Sodium hydroxide pearls (a.r.) were purchased from Fisher Scientific (Thermo Fisher Scientific, Loughborough, United Kingdom). All reagents were used without further purification. Ultrapure water (Milli- Q water resistivity 18.2 MΩ cm) was obtained from a Millipore device and used for the preparation of stock and flotation solutions.
Calculations with OLI Studio
The speciation of the metal ions in these solutions was modeled using the software OLI Studio: Stream Analyzer version 9.6.2 using the Mixed Solvent Electrolyte (MSE) database (OLI Systems Inc., USA).
Analytical Methods
Metal concentrations in the bulk solution before and after the flotation experiments were determined by inductively coupled plasma—optical emission spectroscopy (ICP-OES) using an Optima 8300 (Perkin-Elmer) spectrometer equipped with an axial/radial dual plasma view, a GemTip Cross-Flow II nebulizer, a Scott double pass with inert Ryton spray chamber, and a demountable one-piece Hybrid XLT ceramic torch with a sapphire injector (2.0 mm internal diameter). Dilutions were done with 2 wt% HNO3 solutions and all ICP-OES samples were measured in triplicate. Samples were 10 times diluted and dysprosium was used as internal standard. K100C tensiometer (Krüss) was used for the measurement of the equilibrium surface tensions of the solutions via a plate made of roughened platinum (Wilhelmy plate method) based on the following equation:
where γ is the surface tension (mN m−1), F is the force (mN) measured by the sensor when the plate attaches the surface of the solution, L is the wetted length of the plate (mm), and θ is the contact angle (°). Surface tensions measurements were carried out in 100-mL glass vessels (Krüss SV20, 70 mm internal diameter) at room temperature, containing approximately 80 mL of flotation solutions. Prior to the measurements, the surface tension of water was measured as a reference. Between the measurements, the platinum plate was cleaned with isopropanol followed by heating with a Bunsen burner and the vessels were cleaned with nitric acid and ultrapure water. The pH of the solutions was measured with a Mettler-Toledo pH meter SevenCompact pH/ ion S220 after calibration with standard buffer solutions of pH 1, 4, 7, and 12.
Experimental Methods
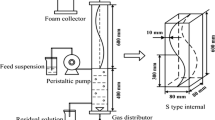
Flotation experiments were carried out in a home-built lab scale setup, which was a glass column of 45 cm in height and an internal diameter of 4.5 cm (Fig. 1). The column was equipped with an adjustable bubble generation mechanism of D4 porous size (~ 10–15 mm), a sampling port, and a port for collecting the foam. The whole setup was connected through a tube to a flowmeter rotameter for controlling the nitrogen gas introduced to the column. Flotation solutions of 300 mL volume were prepared in beaker by adding the appropriate amounts of collector, auxiliary ligand, and metal ions from the stock solutions. The solutions were stirred for 10 min on a magnetic stirrer (IKA RCT Basic) with a 20 × 10 mm magnetic stirring bar at low speed (200 rpm), in order to avoid the generation of foam and subsequently the pH was adjusted by using either 0.1 mol L−1 of HNO3 or 0.1 mol L−1 of NaOH. The solution was then poured slowly in the flotation column by using a funnel; nitrogen gas was bubbled through the solution from the bottom. Aliquots of approximately 3 mL were withdrawn for ICP-OES analyses from the bulk solution before and after the flotation experiments. The efficiency of flotation results was expressed as the removal percent (Re%) according to the following equation:
where Ci and Cr are the initial and residual metal ion concentration of the bulk solution (mmol L−1), respectively. The initial concentration of metal ions was the same for all the investigated conditions (0.1 mmol L−1). All experiments were carried out at room temperature (± 25 °C) and in duplicate. Data in Figs. 3, 4, 5, 6, 7, 8, and 9 represent averaged values. After every experiment, the flotation column was cleaned thoroughly with 1 mol L−1 HNO3 and rinsed five times with demineralized water.
Results and Discussion
Species Distribution Diagram Calculation
In order to elucidate the chelation mechanism of the auxiliary ligand to the targeted metal ions, the species distribution diagram of the Cd2+– Mn2+– Zn2+– Trien system, at stoichiometric conditions, was calculated as function of the pH by using OLI Systems software and is shown in Fig. 2. For its calculation, literature values of the equilibrium constants of triethylenetetramine for dissociation and formation of metal–Trien complexes were imported in the database of the software package [37, 38]. The hydrolysis constants of the examined metal ions were already available in the OLI Systems database.
Species distribution plot of Cd2+ − Mn2+ − Zn2+ − Trien system as a function of pH. Solution composition: [Cd2+] = [Mn2+] = [Zn2+] = 0.1 mmol L−1 and [Trien] = 0.4 mmol L−1. (1) [H4(Trien)]4+, (2) [H3(Trien)]3+, (3) Cd2+, (4) Zn2+, (5) Mn2+, (6) [Cd(Trien])2+, (7) [Zn(Trien)]2+, (8) [H2(Trien)]2+, (9) [H(Trien)]+, (10) [Mn(Trien)]2+, (11) [Cd(Trien)2]2+, (12) Mn(OH)2, (13) Cd(OH)2
As shown in Fig. 2, at pH < 5, the predominant species are the protonated forms of Trien, i.e., [H4(Trien)]4+ and [H3(Trien)]3+ (curves 1 and 2). Metal ions occur as free Cd2+, Mn2+, and Zn2+ (curves 3, 4, and 5, respectively), but in lower concentrations (0.1 mmol L−1) compared to the protonated forms of the auxiliary ligand (0.4 mmol L−1). At pH values between 5 and 8, Trien is deprotonating to [H2(Trien)]2+ (curve 8) and chelating with zinc and cadmium by forming the complexes [Zn(Trien)]2+ and [Cd(Trien)]2+ (curves 6 and 7, respectively), while manganese is still present as free Mn2+ (curve 5). At pH 8−9.50, the targeted metals are chelated by Trien as [Cd(Trien)]2+, [Zn(Trien)]2+, and [Mn(Trien)]2+ complexes, respectively (curves 6, 7, and 10). The monoprotonated [H(Trien)]+ species (curve 9) is also occurring at the same pH range. Above pH 10, the insoluble Mn(OH)2 and Cd(OH)2 species started to form (curves 12 and 13, respectively). Based on the above species distribution simulation, it can be concluded that at pH < 9.50, the targeted metal ions occur as positively charged species, which favors their floatability with an anionic collector like SDS.
Effect of the pH
The speciation of the targeted metal ions is influenced by the pH and affects in turn the interactions of the metals themselves with the functional group of the collector and the desired efficiency. Therefore, the pH of the solution should be carefully controlled. To study the effect of pH on the removal of Cd2+, Mn2+, and Zn2+ at the examined conditions ([SDS] = 0.6 mmol L−1, [Trien] = 0.4 mmol L−1, and [Cd2+]i = [Mn2+]i = [Zn2+]i = 0.1 mmol L−1, ethanol = 0.5% (v/v) and a gas flowrate set at 35 mL min−1), a series of tests was carried out at pH values varying from 2.50 to 9.50 (Fig. 3). The experimental results showed that the removal efficiency of all metals was increased with an increase in pH. The highest removal rates for Cd2+ (99.2%), Mn2+ (88.1%), and Zn2+ (80.2%) were observed at pH 9.50, which was selected for the subsequent experiments. This trend correlated well with the calculated species distribution plot (Fig. 2), since at pH < 6, the fractions [H4(Trien)]4+ and [H3(Trien)]3+ were the predominant species of the examined system and none of the metals was removed. At pH > 6, the ligand started to get deprotonated and the removal efficiency increased. At pH 9.50, where the maximum metal removal was achieved, the targeted metals were completely chelated by Trien as metal–Trien complexes.
Effect of the Flotation Time
The time that is required to remove heavy metals is another important parameter because it indicates how fast large volumes of dilute effluents can be treated. In foam separation, this time depends on the chemistry of the examined system and can vary from a few minutes to a couple of hours [11, 17,18,19]. By keeping the other conditions constant (flowrate and concentration of metal ions, surfactant, auxiliary ligand, and ethanol) and at pH 9.50, the effect of flotation time was investigated (Fig. 4). The maximum removal of Cd2+ (98.8%) was obtained during the first 60 min, while at the same time there was steep increase in the removal rates of Mn2+and Zn2+ (68.9% and 59.5%, respectively) and a plateau was reached after 120 min of flotation time (78.2% and 71.0%, respectively). Therefore, 120 min was necessary to achieve the simultaneous removal of the tested metal ions under the investigated conditions.
Effect of the Auxiliary Ligand Concentration
As mentioned in the introduction, it has been thermodynamically proven that the use of chelating agents as auxiliary ligands or chelating surfactants as collectors can enhance the efficiency or even the selectivity. Thus, it would be anticipated that an increase in the amount of Trien concentration as auxiliary ligand could potentially increase the removal efficiency. The effect of the auxiliary ligand (Trien) concentration on the removal efficiency of metal ions is presented in Fig. 5. Contrary to the expectations, the increase in Trien concentration yielded to low removal efficiencies for all the targeted metal ions. In particular, nearly quantitative (100%) removal efficiencies were achieved for Cd2+ (99.3%), Mn2+ (98.7%), and Zn2+ (98.9%) with low concentration of the Trien (0.1 mmol L−1). At the higher concentrations of Trien, the floatability of Mn2+ and Zn2+ gradually decreased, reaching a minimum value of 4.2% and 1.5%, accordingly. The removal rate of Cd2+ remained constant at its maximum value (above 99%) for Trien concentrations 0.1–0.8 mmol L−1. However, it significantly decreased to 1.2%, when the Trien concentration was 1.0 mmol L−1. It is apparent from Fig. 5, the importance of the auxiliary ligand to the examined system. The addition of 0.1 mmol L−1 Trien increased the removal efficiencies of Cd2+ Mn2+ and Zn2+ from 67.0%, 53.8% and 78.9% to almost 100%.
Interestingly, the surface tension of the examined system showed a dependency on the concentration of the auxiliary ligand (Fig. 6). More specifically, for Trien concentrations between 0.1 and 0.4 mmol L−1, the surface tensions decreased progressively from 37.2 mN m−1 to 30.2 mN m−1 and remained stable (at around 29.5 mN m−1) for concentrations above 0.6 mmol L−1. This decrease in surface tension of the solution for various Trien concentrations indicated the presence of a more surface-active species, which was adsorbed favorably to the gas/ liquid interface and competed with the targeted metal ions. Based on the simulation of the species in the studied system (Fig. 2) at pH 9.50, the monoprotonated Trien species [H(Trien)]+ was still present in the solution. At higher concentrations of the auxiliary ligand, the [H(Trien)]+ was the predominant species, which most probably lowered the surface tension of the solution and it was concentrated to the foam phase due to interactions with the hydrophilic functional group of SDS. Hence, an excess of auxiliary ligand concentration should be avoided because it probably promotes the floatability of the [H(Trien)]+ species. A concentration of 0.1 mmol L−1 was found to be the optimum for the simultaneous flotation of the investigated metals.
Effect of the Collector Concentration
The effect of the concentration of the collector on the flotation efficiency of the targeted metals (Fig. 7) was investigated under optimized flotation time (Fig. 5) and concentration of the auxiliary ligand (Fig. 6). Cd2+ was most efficiently removed (86%), for SDS-to-metals molar ratio 1:2 (SDS:Mtotal), followed by Zn2+ (76%) and Mn2+ (29%). By increasing SDS:Mtotal ratio, the removal efficiencies also increased, reaching an optimum value close to 100% at a SDS:Mtotal ratio of 2:1. At higher SDS concentrations, no major differences were observed for the removal rates and therefore the selected optimum SDS concentration was 0.6 mmol L−1.
Effect of Polar Solvent Concentration and Nitrogen Gas Flowrate
The addition of a frother (polar solvent) in foam separation techniques reduces the bubbles size, which prevents their coalescence and leads consequently to more stable foam layers on the surface [35]. The effect of the ethanol concentration, as polar solvent, at pH 9.50, SDS:Mtotal ratio 2:1, Trien concentration of 0.1 mmol L−1, and 120 min of flotation time, is presented in Fig. 8. The change in the concentration of ethanol in the solution (0.0, 0.5, 3.0, and 5%, v/v) did not affect the removal rates of the targeted metal ions. However, visual observations during the experiments showed that the generated bubbles were dispersed better within the column and the generated foam was more stable in the presence of ethanol compared to a system without ethanol. Thus, for next studied parameter, 0.5% (v/v) of ethanol as frother was used.
Low aeration rates ensure small bubble size and large gas/ liquid interfaces, and they prevent the formation of turbulence flow. The combination with the addition of a polar solvent promotes the formation of a stable foam, which can be easily separated from the bulk solution [39]. The examined nitrogen gas flowrate values (25–100 mL min−1) did not significantly affect the removal efficiencies (Fig. 9). Nevertheless, the increase of the gas flowrate was proportional to the water loss. In particular, for low flowrates, i.e., 25 and 35 mL min−1 , the water loss could be kept low, at 16.7% and 20.1%, respectively. However, for flowrates of 50, 80, and 100 mL min−1, water loss was dramatically increased between 61.7% and above 80%. As a result, a flowrate of 25 mL min−1 was considered as optimum value.
Proposed Foam Separation Mechanism
In order to unravel the foam separation mechanism, the species distribution plot was recalculated for the optimized conditions of the examined system ([Trien] = [Cd2+] = [Mn2+] = [Zn2+] = 0.1 mmol L−1). As presented in Fig. 10, at pH 9.50, the targeted metals were present as the [Cd(Trien)]2+ complex (curve 5), free Mn2+ (curve 4), and Zn(OH)2 precipitate (curve 6). It can thus be assumed that Cd2+ was concentrated to the foam phase as the [Cd(Trien)]2+ complex due to hydrophobic interactions with the hydrophilic head group of the collector (DS−), Mn2+ as Mn(DS)2 sublate, while Zn2+ was carried out as Zn(OH)2 precipitate [37]. As a consequence, the foam separation mechanism involved a combination of ion flotation and precipitate flotation.
Conclusions
The optimum conditions for the removal of Cd2+, Mn2+, and Zn2+ from dilute aqueous solutions were studied by foam separation, using the anionic surfactant SDS as collector in combination with Trien as auxiliary ligand. The most crucial parameters of foam separation techniques, i.e., pH, flotation time, auxiliary ligand concentration, molar ratio of the surfactant to metal ions, polar solvent concentration, and flowrate, were investigated via a series of batch-mode experiments, in a laboratory-scale flotation column setup. Experimental results showed that Cd2+, Mn2+, and Zn2+ can be completely removed in one step at pH 9.50, flotation time = 120 min, Trien concentration = 0.1 mmol L−1, SDS:Mtotal ratio 2:1, ethanol concentration = 0.5% (v/v), and a nitrogen gas flowrate set at 25 mL min−1. The increase in Trien concentration decreased the removal efficiency of the targeted colligends and the surface tension of the solution, probably due to an excess in the concentration of more surface-active Trien species. The calculated species distribution diagram of the examined system for the optimized conditions suggested that the targeted metals were concentrated to the foam phase by interacting with the hydrophilic group of the collector (DS−) either as metal–Trien complexes or as sublates or carried out as metal hydroxides. The residual metal ion concentrations obtained under the optimal operating conditions were 0.003 mg L−1 for Cd2+, 0.001 mg L−1 for Mn2+, and 0.004 mg L−1 for Zn2+, which are below the median threshold values for drinking water ([Cd2+] = 0.005 mg L−1, [Mn2+] = 0.1 mg L−1, [Zn2+] = 5 mg L−1) [40]. These results show great promise for treating dilute mine waters or industrial effluents containing traces of cadmium, zinc, and manganese. Nevertheless, in order to assess the efficiency and the environmental performance of the method, the regeneration of the surfactant and the auxiliary ligand should be investigated. Since these results were obtained under laboratory conditions, studies should be also extended at a pilot scale and on realistic conditions so as to get operating parameters and estimate the general costs.
References
Chen T, Yan B, Lei C, Xiao X (2014) Pollution control and metal resource recovery for acid mine drainage. Hydrometallurgy 147–148:112–119. https://doi.org/10.1016/j.hydromet.2014.04.024
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev 4:37–59. https://doi.org/10.1002/cben.201600010
Azevedo A, Oliveira HA, Rubio J (2018) Treatment and water reuse of lead-zinc sulphide ore mill wastewaters by high rate dissolved air flotation. Miner Eng 127:114–121. https://doi.org/10.1016/j.mineng.2018.07.011
Somasundaran P (1975) Separation using foaming techniques. Sep Sci 10:93–109. https://doi.org/10.1080/00372367508057071
You-Cai Z, Zouboulis A, Matis K (1996) Flotation of molybdate oxyanions in dilute solutions. Part 1 Selective separation from arsenate. Hydrometallurgy 43:143–154. https://doi.org/10.1016/0304-386X(96)00018-7
Rubio J, Souza M, Smith R (2002) Overview of flotation as a wastewater treatment technique. Miner Eng 15:139–155. https://doi.org/10.1016/S0892-6875(01)00216-3
Zamboulis D, Peleka EN, Lazaridis NK, Matis KA (2011) Metal ion separation and recovery from environmental sources using various flotation and sorption techniques. J Chem Technol Biotechnol 86:335–344. https://doi.org/10.1002/jctb.2552
Pinfold T (1972) Ion flotation. In: Lemlich R (ed) Adsorptive bubble separation techniques. Academic Press, New York, pp 53–73
Grieves RB (1975) Foam separations: a review. Chem Eng J 9:93–106. https://doi.org/10.1016/0300-9467(75)80001-3
Sebba F (1959) Concentration by ion flotation. Nature 184:1062–1063. https://doi.org/10.1038/1841062a0
Matis KA, Mavros P (1991) Recovery of metals by ion flotation from dilute aqueous solutions. Sep Purif Rev 20:1–48. https://doi.org/10.1080/03602549108021407
Chang L, Cao Y, Fan G, Li C, Peng W (2019) A review of the applications of ion floatation: wastewater treatment, mineral beneficiation and hydrometallurgy. RSC Adv 9:20226–20239. https://doi.org/10.1039/C9RA02905B
Todd I, Distin P (1985) Precipitate flotation of nickel from acidic laterite leach solutions. Hydrometallurgy 14:309–316. https://doi.org/10.1016/0304-386X(85)90041-6
Caballero M, Cela R, Perez-Bustamante J (1990) Analytical applications of some flotation techniques- a review. Talanta 37:275–300. https://doi.org/10.1016/0039-9140(90)80056-L
Lazaridis N, Peleka E, Karapantsios T, Matis K (2004) Copper removal from effluents by various separation techniques. Hydrometallurgy 74:149–156. https://doi.org/10.1016/j.hydromet.2004.03.003
Charewicz W, Walkowiak W (1972) Selective floatation of inorganic ions. Sep Sci 7:631–646. https://doi.org/10.1080/00372367208057972
Deliyanni EA, Kyzas GZ, Matis KA (2017) Various flotation techniques for metal ions removal. J Mol Liq 225:260–264. https://doi.org/10.1016/j.molliq.2016.11.069
Peng W, Chang L, Li P, Han G, Huang Y, Cao Y (2019) An overview on the surfactants used in ion flotation. J Mol Liq 286:110955. https://doi.org/10.1016/j.molliq.2019.110955
Doyle FM (2003) Ion flotation- its potential for hydrometallurgical operations. Int J Miner Process 72:387–399. https://doi.org/10.1016/S0301-7516(03)00113-3
Lu S, Pugh RJ, Forssberg E (2005) Interfacial separation of particles. Elsevier, Amsterdam
Eivazihollagh A, Bäckström J, Norgren M, Edlund H (2018) Electrochemical recovery of copper complexed by DTPA and C12-DTPA from aqueous solution using a membrane cell. J Chem Technol Biotechnol 93:1421–1431. https://doi.org/10.1002/jctb.55103
Doyle FM, Duyvesteyn S, Sreenivasarao K (1995) The use of ion flotation for detoxification of metal-contaminated waters and process effluents. In: Herbst JA (ed) Proceedings of the XIX International Mineral Congress, vol 4. Society for Mining, Metallurgy, and Exploration, pp 176–179
Stalidis GA, Matis KA, Lazaridis NK (1989) Selective separation of Cu, Zn, and As from solution by flotation techniques. Sep Sci Technol 24:97–109. https://doi.org/10.1080/01496398908049754
Scorzelli I, Fragomeni A, Torem M (1999) Removal of cadmium from a liquid effluent by ion flotation. Miner Eng 12:905–917. https://doi.org/10.1016/S0892-6875(99)00077-1
Ulewicz M, Walkowiak W, Jang Y, Kim JS, Bartsch RA (2003) Ion flotation of Cadmium(II) and Zinc(II) in the presence of proton-ionizable lariat ethers. Anal Chem 75:2276–2279. https://doi.org/10.1021/ac026322y
Polat H, Erdogan D (2007) Heavy metal removal from waste waters by ion flotation. J Hazard Mater 148:267–273. https://doi.org/10.1016/j.jhazmat.2007.02.013
Yenial Ü, Bulut G (2017) Examination of flotation behavior of metal ions for process water remediation. J Mol Liq 241:130–135. https://doi.org/10.1016/j.molliq.2017.06.011
Yenidünya MD (2006) Recovery of Zn(II), Mn(II) and Cu(II) in aqueous solutions by foam fractionation with sodium dodecyl sulphate in combination with chelating agents. Sep Sci Technol 41:1741–1756. https://doi.org/10.1080/01496390600636959
Eivazihollagh A, Tejera J, Svanedal I, Edlund H, Blanco A, Norgren M (2017) Removal of Cd2+, Zn2+, and Sr2+ by ion flotation, using a surface- active derivative of DTPA (C12-DTPA). Ind Eng Chem Res 56:10605–10614. https://doi.org/10.1021/acs.iecr.7b03100
Liu Z, Doyle FM (2001) A thermodynamic approach to ion flotation. I. Kinetics of cupric ion flotation with alkylsulfates. Colloids Surf A 178:79–92. https://doi.org/10.1016/S0927-7757(00)00555-0
Liu Z, Doyle FM (2001) A thermodynamic approach to ion flotation. II. Metal ion selectivity in the SDS- Cu-Ca and SDS- Cu- Pb systems. Colloids Surf A 178:93–103. https://doi.org/10.1016/S0927-7757(00)00554-9
Liu Z, Doyle FM (2009) Ion flotation of Co2+, Ni2+, and Cu2+ using dodecyldiethylenetriamine (Ddien). Langmuir 25:8927–8934. https://doi.org/10.1021/la900098g
Schaider LA, Senn DB, Estes ER, Brabander DJ, Shine JP (2014) Sources and fates of heavy metals in a mining-impacted stream: temporal variability and the role of iron oxides. Sci Total Environ 490:456–466. https://doi.org/10.1016/j.scitotenv.2014.04.126
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:1–6. https://doi.org/10.1186/1745-6673-1-22
Plum LM, Rink L, Hajo H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365. https://doi.org/10.3390/ijerph7041342
O’Neal SL, Zheng W (2015) Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep 2:315–328. https://doi.org/10.1007/s40572-015-0056-x.Manganese
Doyle FM, Liu Z (2003) The effect of triethylenetetraamine (Trien) on the ion flotation of Cu2+ and Ni2+. J Colloid Interface Sci 258:396–403. https://doi.org/10.1016/S0021-9797(02)00092-9
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill, New York
Cho Y, Laskowski J (2002) Effect of flotation frothers on bubble size and foam stability. Int J Miner Process 64:69–80. https://doi.org/10.1016/S0301-7516(01)00064-3
World Health Organization (2018) A global overview of national regulations and standards for drinking- water quality. WHO CC BY-NC-SA 3.0 IGO, Geneva.
Acknowledgements
The research leading to these results has received funding from the European Community’s Horizon 2020 Programme under Grant Agreement No. 812580 (MSCA- ETN SULTAN). This publication reflects only the authors’ view, exempting the Community from any liability.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was D. Panias.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xanthopoulos, P., Binnemans, K. Removal of Cadmium, Zinc, and Manganese from Dilute Aqueous Solutions by Foam Separation. J. Sustain. Metall. 7, 78–86 (2021). https://doi.org/10.1007/s40831-020-00322-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-020-00322-2