Abstract
Purpose of Review
Constructed wetlands (CWs) are engineered systems that have been proven as an alternative option to traditional wastewater treatment technologies because of their ability to provide cost-effective and energy-efficient solutions. This technology depends on natural microbial/biological, physical, and chemical processes to treat wastewater. Processes removing impurities in constructed wetlands are based on the combination of interactive systems such as selected plant species, the nature of substrate used for constructed wetlands, biofilm growth, microbial diversity, and several biogeochemically affected reaction cycles in wetland systems. Microorganisms play a vital role in these processes such as the degradation of pollutants and the transformation of nutrients. Microorganisms remove the pollutants from CWs by catalyzing chemical reactions, biodegrading, biosorbing, and supporting plant growth. An in-depth analysis of the function of microorganisms in CWs is important to understand. This review deals with the recent developments in constructed wetland systems from a microbiological perspective to treat impurities present in wastewater. It focuses on the studies of microbial diversity in CWs and the role of enzymes produced by microbes, the influence of the substrates of CWs on microbial diversity, the influence of the hydraulic design of CWs on the growth of microorganisms, the role of specific microbes in the removal of pollutants and the different software, analytical equipment, tools, and techniques used to measure/quantify the parameters of interest or to design and operate a wetland.
Recent Findings
The combination of different types of substrates in constructed wetlands can form different types of zones such as anaerobic and aerobic zones which can allow to form a diversity of microorganisms. In addition, plant diversity plays a vital role in microbial growth by providing O2 and increasing plant biomass production which influences the soil microbial community. Moreover, the influent carbon source influences the biomass as for example when the COD/N ratio is increased by 80%, the phospholipid fatty acids (PLFA) concentration of microbial biofilm in glucose constructed wetlands is increased by 50%. At the same time, the biomass of aerobic and anaerobic bacteria and fungi increased significantly. In addition, different microorganisms are responsible in removing different types of heavy metals and micropollutants.
Summary
This article provides useful information on the understanding of the diversity of microbes, influencing factors on the growth of microorganisms, and the efficiency of pollutant removal process in CWs. Overall, this review provides new ideas and directions for the improvement of constructed wetlands from a microbiological perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbiome constructed wetlands (CWs) are an alternative option to conventional systems for different types of wastewater treatment due to their cost-effectiveness, energy-efficient solutions, ease of maintenance, and operation. The performance of constructed wetlands depends on type of plant species present, hydraulic design, water depth, microorganisms, substrate type, and other environmental conditions (temperature, dissolved oxygen, etc.). The pollutants are removed by natural processes such as volatilization, sedimentation, sorption, photodegradation, plant uptake, transpiration flux, and microbial-mediated transformations. Microorganisms play a vital role in this system to remove nutrients/pollutants such as nitrogen, phosphorus, heavy metal, and micropollutants. Different researchers focus their research on the efficiency of the constructed wetlands on pollutant removal with different coupling methods. They also considered substrate types, plants, influent wastewater types, microorganisms, etc. Pelissari et al. [1] observed that nitrogen removal efficiency is higher in saturated vertical subsurface wetlands compared to unsaturated vertical subsurface wetlands because the denitrification potential is higher in the bottom layer of saturated wetlands compared to unsaturated wetlands. In addition, the saturated condition promoted the redox potential linked with carbon availability which helps to reduce more sulphate by enriching sulphate-reducing bacteria (SRB) (such as Desulfobacterales and Desulforomonadales). Fu et al. [2] showed in their research that different substrates can improve the dissolved oxygen supply to enhance nitrogen removal efficiency (because of their different porosities) in constructed wetlands. Fang et al. [3] investigated the influence of emergent plants on microbial communities in wetlands. But only a few research focuses on the influence of different variables (plants, substrates, wastewater types, solid accumulation) on the growth of microbial diversity and density. This review highlights the most recent findings about the influence of different variables (plants, substrates, wastewater types, biofilm) on the diversity of communities in microbial growth, the removal mechanisms associated with processes, techniques to measure the parameters to design and operate the wetland. In addition, the nutrients/pollutants removal processes by microorganisms in constructed wetlands are also highlighted in this paper.
Formation and Construction of Microbiome Wetlands
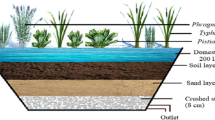
Constructed wetlands are vital engineered systems to treat different types of wastewaters such as stormwater, domestic sewage, agricultural runoff, mining water, and industrial drainage. They mainly consist of substrates, macrophytes (plants), and microorganisms. These constructed wetlands can be categorized into surface flow constructed wetlands, subsurface flow constructed wetlands, and hybrid systems. They can also be divided into vertical flow-constructed wetlands and horizontal flow-constructed wetlands based on the flow path. It is observed that the density and diversity of microorganisms vary with different types of wastewaters. As an example, Adrados et al. [4] observed Acinetobacter sp. (γ-Proteobacteria), Arthrobacter sp., Flavobacterium sp., Thauera terpenica, Xanthomonas sp., Dokdonella sp., Rhodanobacter sp., and Stenotrophomonas sp. microorganisms present in their domestic wastewater. Alternatively, Fu et al. [2] observed Vibrio, Candidatus Competibacter, Denitratisoma, Nitrospira, Thauera, Nitrosomonas, Planctomyces, Marinobacterium, Magnetospira, and Dechloromonas microorganisms present in sewage wastewater. This abundance of microorganisms depends on the influent COD/N ratio. In addition, these different types of microorganisms such as proteobacterial ammonia oxidizers (AOB) can carry out aerobic and anaerobic ammonia oxidation, nitrite-oxidizing bacteria (NOB) help to convert the nitrite to nitrate, Deltaproteobacteria sulfate-reducing bacteria (SRB) help to oxidize organic substrates anaerobically, and phylum Bacteroidetes helps to degrade complex organic matter [5]. A list of microorganisms along with the type of pollutants they remove is given in Table 1. In addition, this microbial diversity depends on environmental factors such as wetland plants, hydraulic design of the wetland, organic matter availability, and substrate types. The importance of vegetation on microbial diversity is highlighted in different research such as the diversity of microbial pollutants is more in planted constructed wetlands compared to unplanted constructed wetlands. This microbial density also varies with plant species, and for example, Phalaris arundinacea shows higher microbial density compared with Typha angustifolia and Phragmites australis [6]. In addition, the root provides an important role by providing oxygen to microbes and creating biofilm space. Substrate influences the diversity and density of microbial communities. The hydraulic configuration of constructed wetlands also has an impact on microbial communities. For example, subsurface flow wetlands provide an anoxic environment for the growth of denitrifying bacteria compared to the free water surface [7].
Microbiome wetlands can form naturally. This microbiome wetlands are enriched by different types of microorganisms and flora which play a crucial role in nutrient cycling, organic matter decomposition, and overall ecosystem functioning. The example of some natural wetlands is given in Table 2.
Effects of Plants (Macrophytes) on Microbial Community Growth in Constructed Wetlands
Plants have a vital influence on microbial growth in constructed wetlands due to their root systems. Because roots act as suitable surfaces for the formation of biofilms which helps to extend the retention time of pollutants and provides them as feed for microbes. These roots also provide oxygen to rhizospheric bacteria which enhance the degradation of organic pollutants and removal of nutrients from wastewater. So, trapping particulate matter in the biofilm of the roots of macrophytes is an essential mechanism for particulate matter removal. In addition, plant roots help to create an aerobic region in the close vicinity of roots by penetrating atmospheric oxygen into the deeper layers of the substrate. So, these zones help the aerobic degradation of pollutants by Methanotrophs, Nitrosomonas, and Pseudomonas aeruginosa spp. In addition, anaerobic regions are created away from the aerobic regions and are dominated by methanogenic and sulfur reducing bacteria [16]. So, plants need to be selected very carefully based on their local availability, the nature of pollutants, and the climate zone. In addition, plants should be able to thrive in an extreme environment with an extensive root system, robust growth, and large aerenchyma in their roots and rhizomes. A literature survey also showed that plant diversity increases plant biomass production which also influences the soil microbial community patterns in constructed wetlands [17]. It is also noticed that plant growth depends on the influent nutrient load which, if high, can be harmful to plants and damage the root system at earlier plant stages [18]. Wetland plants come under the category of macrophytes which includes four types namely emergent macrophytes, floating-leaved macrophytes, submerged macrophytes, and freely floating macrophytes [16]. But the choice of types of plants is based on the constructed wetland types, wastewater type, local condition, and their availability. For example, plants such as Canna, Typha, Phragmites, Cyperus, Carex, Acorus, and Juncus genera types of plants are used in floating treatment wetlands (FTWs). In addition, some species of the Poaceae family (Lollium sp., Zizania sp., and Chrysopogon sp.) have been applied to develop FTWs in Italy, China, Singapore, and Thailand. A list of different types of plants with wetland types is given in Table 3. Different removal mechanisms such as microbial-mediated processes, chemical networks, volatilization, sedimentation, sorption, photo-degradation, plant uptake, and transpiration flux occur in constructed wetlands. Plants contribute significantly in combination of two or more of the above processes. Plant uptake, microbial-mediated photo degradation, etc. are a couple of examples [16]. The growth of rhizomes increases the porosity of constructed wetlands and transfer oxygen through the root systems. So, the microbial activity increases and provides enough time to oxidize NH4-N and NO3-N by nitrifying bacteria [19, 20]. In addition, the plant root serves as carbon source for heterotrophic denitrifying bacteria (e.g., Comamonadaceae and Gemmatimonadaceae) [21]. The plant also contributes to controlling hydraulic efficiency by modifying internal flow patterns in constructed wetlands as well as decreasing the wind effects. The plants also balance the water temperature by providing shade [22].
The overall influence of plant on microbial community can be summarized as (i) providing O2 to improve bacterial diversity of constructed wetlands [28], (ii) creating aerobic zone, (iii) decaying products of plant litter which play a part of the growth of microorganisms [29], (iv) improved sediment quality by providing deep-rooted macrophytes [30], and (v) controlling temperature.
Effects of Substrate on Microbial Community Diversity Indices in Constructed Wetlands
Different types of substrates have been used to provide support to the plants growing in constructed wetlands. These substrates can be coconut fiber, peat, soil, bamboo crush, sand, peat rice straw, compost, etc. But these substrate influences the pollutant removal process. Cheng et al. [19••] observed that the removal mechanism of phosphorus was different between two types of substrates such as ceramisite showed better performance compared to gravel and slag. Because ceramisite is composed of quartz (SiO2), large crystals containing Al, Fe, and Mg, it has enhanced cation absorption, and numerous microporous structures make phosphorus to be easily fixed to the substrate compared to gravel and slag [31]. They also completed a microbial community analysis, and found that ceramisite constructed wetlands (C-CWs) exhibited the highest relative abundance of Comamonadaceae, Planctomycetaceae, and Burkholderiaceae microorganisms among the four tested substrates (i.e., gravel (G-CWs), ceramsite (C-CWs), iron-carbon (I-CWs), and slag (S-CWs)). Another research conducted by Ge et al. [9] observed that Comamonadaceae can absorb the dissolved phosphorus to form polyphosphates for energy accumulation, which was considered as the main contributor to phosphorus removal. Thus, ceramisite may be more beneficial as a support for the growth of phosphorus accumulating bacteria such as Comamonadaceae. In another research, it was observed that the use of rice straw (composed of cellulose and lignin) compared to plastic filling (composed of polymethyl methacrylate) as a growth medium in ecological floating beds (EFBs) improved the removal process of total nitrogen. Because rice straw can break up into soluble matter by the bacteria present on its surface resulting in a thicker biofilm on the surface and providing carbon source. This thicker biofilm provided the anaerobic conditions necessary [32]. So, the important factors which need to be considered while selecting the substrate materials are high sorption capacity, efficiency, easy availability and economic factor/aspect, and absorption capacity. A list of different substrates used along with the microbial diversity (statistics or quantity of microorganisms per mL) is given in Table 4. Different techniques were used by different researchers to investigate the influence of substrate types on the formation of microbial biofilms such as fingerprint analysis, numerical analysis of DGGE profiles, and HTS technique (Illumina MiSeq) measurement of phosphate lipid fatty acid (PLFA). It was reported that the density and diversity of microbial communities depend on the filter media (expanded clay, sand, zeolite, gravel, etc.) [5, 33,34,35,36]. The combination of different substrates can form different types of zones which is suitable to grow different microorganisms. If the upper layer of CW is constructed with high porosity substrate and the lower layer with low porosity substrate, then the upper layer is suitable for growing aerobic organisms because of the availability of high DO. Alternatively, if the upper layer is constructed with low porosity substrate and the bottom layer is constructed with high porosity substrate then it can facilitate the formation of anaerobic environment which can help to improve the denitrification efficiency of wastewater treatment [2]. Several factors can influence the porosity of constructed wetlands and the oxygen transfer rates within these systems. The following are some key factors which play significant roles:
Media composition: The type and composition of the media or substrate used in the wetland can affect its porosity and oxygen transfer rates. Porous media, such as gravel, coarse sand, or specialized engineered materials, provide space for oxygen to diffuse into the wetland bed.
Media size and packing density: The size and arrangement of media particles can impact porosity and oxygen transfer rates. Larger media particles with less packing density generally have higher porosity and better oxygen diffusion compared to smaller, tightly packed particles.
Hydraulic loading rate: The hydraulic loading rate, which refers to the volume of water passing through the wetland per unit area and time, can affect porosity and oxygen transfer rates. High loading rates can cause compaction of the media and decrease porosity, limiting oxygen transfer.
Vegetation density: The presence and density of plants in the wetland can influence porosity and oxygen transfer. Plant roots create channels and macropores within the media, enhancing porosity and facilitating oxygen movement.
Water depth: The depth of water in the wetland affects the available surface area for oxygen exchange. Shallow water depths generally promote better oxygen transfer rates compared to deeper water.
Temperature: Temperature can influence both porosity and oxygen transfer rates. Warmer temperatures generally enhance microbial activity and oxygen consumption, potentially affecting oxygen availability within the wetland.
Water velocity: Water velocity or flow rate can impact the oxygen transfer rates. Higher velocities increase the turbulence and enhance oxygen exchange at the air–water interface.
Good examples of factors affecting porosity and oxygen transfer rates in constructed wetlands can be observed in real-world applications. For instance, in a vertical flow constructed wetland, the choice of media composition (e.g., gravel or specialized filter media), media size, and hydraulic loading rate will influence porosity and oxygen transfer efficiency [37]. In a free water surface constructed wetland, the presence of emergent plants like cattails (Typha spp.) or bulrushes (Scirpus spp.) contributes to enhanced porosity and oxygen transfer due to their dense root systems and the creation of oxygen pathways [38]. The design of a horizontal subsurface flow constructed wetland, including factors like media size, depth, and water flow velocity, will influence porosity and oxygen transfer rates.
Overall, these factors highlight the importance of careful design and optimization to ensure adequate porosity and efficient oxygen transfer within constructed wetlands, thereby supporting the desired treatment processes and ecological functions.
Effects of Solid Accumulation and Water Flow on Biofilm Growth
Bacteria have a unique ability to form biofilms, and the formation can begin with the attachment of free-floating microbes to gas–liquid and solid–liquid interfaces. These biofilms consist of an extracellular matrix comprised of polysaccharide biopolymers, proteins, and DNA that hold the cell together. The nature of biofilms and associated matrices depends upon the types of substrates, medium, and growth conditions. Different methods are used to determine the development of biofilm. Ding et al. [40•] used total viable cell counts (TVC), extracellular polysaccharide (EPS), and extracellular protein (EP) to determine biofilm development. Total viable cell counts (TVC) represent the absolute number of bacterial cells that contribute to pollutant degradation. Ding et al. [40••] observed in their research that multilayer substrate in horizontal subsurface flow constructed wetlands (comprised of six equal layers with 0.1-m thickness, different sizes of sand, hydraulic conductivity K values of 26, 36, 43, 55, 75, and 176 m/d, respectively) showed higher concentrations of TVC, EPS, and EP than the monolayer (filled with quartz sand (0–6 mm) with hydraulic conductivity (K) of 65 m/d). Multilayer configuration promoted better biofilm growth by accumulating the solids homogenously between different layers. But most solids accumulated in the bottom layers of constructed wetlands. Another research conducted by Caselles-Osorio et al. [41] observed that most solids accumulated heterogeneously near the inlet of the constructed wetlands. But the inlet solids accumulation was rather homogeneous in Almatret north constructed wetlands which were treating the town’s wastewater flow. Thus, the flow pattern could impact this phenomenon and multi-layers can produce turbulence to disperse the solids uniformly. Sanchez-Huerta et al. [42] observed that the increase in biofilm thickness enhanced the system efficiency in removing the organic micropollutants through the development of a denser, more diverse and adapted microbial consortium in membrane-aerated biofilm reactor (MABRs). Biofilm formation is also influenced by the water flow of constructed wetlands. The moderate water velocities (5–45 cm/s) significantly enhanced the trapping and retention of fine particles by submerged macrophytes which increased the biomass. At the same time, water helps to provide oxygen profiles at leaf-biofilm. So, it can increase the chances to develop single colony-like biofilm bacterial biodiversity in submerged macrophytes [43]. But excessive water flow can also destroy this biofilm.
Effects of Hydraulic Design and Nutrients Load on Microbial Community Structure
The hydraulic configuration of constructed wetlands plays a crucial role in shaping the microbial community dynamics within these systems. The design and operation of constructed wetlands determine the flow patterns, residence times, and oxygen availability, which, in turn, influence the composition and activity of microbial populations. Water depth controls the oxygen transfer from the atmosphere to water where shallow horizontal subsurface constructed wetlands having higher redox value and produce more oxidized condition and are suitable to grow aerobic microorganisms [44]. Ding et al. [40•] mentioned in their research that the top layer of the horizontal surface constructed wetlands were mainly consisted of aerobic microorganisms, such as heterotrophic and nitrifying bacteria [45]. Another research conducted by Huang et al. [46•] showed that the DO concentration above 1.5 mg/L is essential for nitrification and the denitrification will be optimal at 0.5 mg/L [46, 47]. The following are some key aspects of hydraulic configuration and its relation to the microbial community in constructed wetlands:
Flow direction: The direction of flow, whether horizontal or vertical, affects the distribution of oxygen and other nutrients within the wetland. Horizontal flow wetlands (HFWs) typically have a shallower water depth, promoting more oxygen transfer to the root zone. Vertical flow wetlands (VFWs) have a deeper water column, leading to reduced oxygen availability in the root zone. These variations in oxygen levels shape the types of microbial communities that thrive in different sections of the wetland.
Retention time: The hydraulic retention time (HRT), which is the average time water spends within the wetland, influences the contact between the wastewater and the microbial community. Longer HRTs provide more opportunities for microbial processes, such as nutrient removal and organic matter degradation. Shorter HRTs may limit the efficiency of certain microbial transformations. The microbial community adapts to the specific HRT conditions, and their activities contribute to the wetland’s overall performance.
Substrate design: The presence of different substrates in constructed wetlands, such as gravel, sand, or organic media, creates variations in hydraulic flow paths and distribution of oxygen and nutrients. These substrates provide attachment surfaces for microbial biofilms, which play a significant role in pollutant removal processes. The composition of the biofilm communities can differ depending on the substrate type and the specific environmental conditions provided by the hydraulic configuration.
Plant influence: In wetlands with vegetation, the hydraulic configuration affects the spatial distribution of plant roots, which, in turn, influence the microbial community. Roots provide physical support, release oxygen, and supply organic matter as a substrate for microbial growth. The flow patterns within the wetland determine the availability of oxygen and nutrients to the rhizosphere, influencing the microbial diversity and activity associated with plant roots.
Influent carbon source (CS) or COD/N ratio has a great influence on microbial structure. This COD/N ratio is considered a critical representative energy that can impact nitrification and denitrification. Huang et al. [46••] observed a significant relationship between CS or COD/N ratio on microbial community structure in CWs. They used phospholipid fatty acids (PLFAs) to assess the changes in microbial biomass. They found that when the COD/N ratio is increased by 80%, then the PLFA concentration of microbial biofilm in glucose constructed wetlands is increased by 50%. At the same time, the biomass of bacteria, aerobic bacteria, anaerobic bacteria, and fungi increased significantly. Moreover, it was also observed that the average pH value decreased with increasing COD/N ratio. So, nitrification and anaerobic fermentation occurred at the initial stage because of the availability of sufficient DO and organic matter. But if a large number of acidic metabolites are produced, then the pH value decreases rapidly. So nitrifying bacteria activity will be disturbed, and denitrification will start to play a major role. Another research conducted by Lai et al. [48] observed that proper carbon sources combined with intermittent O2 supply enhanced bacterial diversity and density.
Overall, the hydraulic configuration of constructed wetlands and carbon source in the influent play critical roles in creating distinct microenvironments within the system. These microenvironments, characterized by variations in oxygen availability, nutrient gradients, and substrate characteristics, shape the microbial community composition and metabolic activities. Understanding the relationship between hydraulic design and microbial communities is important for optimizing the performance and efficiency of constructed wetlands for wastewater treatment and ecological restoration.
Mechanisms Involved in the Pollutant Removal Processes
Several removal processes may occur in constructed wetlands which are very complex. These removal processes can be divided broadly into two categories such as (a) non-destructive processes and (b) destructive processes. Volatilization and phytovolatilization, plant uptake and phytoaccumulation, sorption and sedimentation are classified as non-destructive processes. Phytodegradation and microbial degradation are classified as destructive process [49]. Each process is described below:
Volatilization: Volatilization refers to the process of pollutants being released into the atmosphere as vapors. This can occur for volatile compounds present in the water. For example, in a constructed wetland treating wastewater contaminated with volatile organic compounds (VOCs), such as benzene, toluene, and xylene, a fraction of these compounds may volatilize from the water surface into the air [49]. The rate of volatilization can be estimated using mathematical models that consider factors such as temperature, wind speed, and the physical and chemical properties of the compounds.
The volatilization of NH3 was measured once using the static chamber technique. To analyze the NH3 volatilization flux (F, µg N m−2 h−1) in the CWs, the gas was collected and the concentration of NH4+-N in the absorption liquid was detected by Nessler’s reagent colorimetry. The following equation was used to determine the NH3 volatilization flux: (Table 5)
where C is the concentration of NH4+-N in the absorption liquid (µg N mL−1); V is the volume of absorption liquid (mL); T is absorption time (16 h); S is the trapping area of the chamber (0.194 m2).
In addition, to estimate the volatilization of volatile organic compound (VOC) called trichloroethylene (TCE) from a constructed wetland surface, the air–water partitioning coefficient and Henry’s law constant can be used.
Sedimentation: Sedimentation is the process by which suspended particles in the water settle down and accumulate in the bed of a constructed wetland. This process is particularly important for the removal of suspended solids and associated pollutants. For instance, in a wetland treating stormwater, sedimentation can remove particulate matter and associated heavy metals. The settling velocity of particles and the hydraulic residence time of the wetland influence the efficiency of sedimentation.
Sorption: Sorption refers to the attachment or adsorption of pollutants onto the surfaces of soil particles, organic matter, or other solid materials present in the wetland. This process helps in the removal of contaminants from the water column. For example, in a wetland designed to remove nutrients like nitrogen and phosphorus, the pollutants can be sorbed onto the surface of the wetland substrate. The sorption capacity of the materials present in the wetland determines the extent of pollutant removal. This efficiency of sorption depends on the compound’s hydrophobic characteristics as well as on the organic carbon content, the chemical structure and composition of soil organic matter [49].
Langmuir sorption isotherm can be used to determine the phosphorus (P) sorption capacity of various substrates in constructed wetlands (CW). The linear form of the Langmuir equation to determine the P sorption capacity of substrates is
where, Ce is the concentration of P in solution at equilibrium, q is the mass of P adsorbed to the substrates, b is the apparent P sorption capacity, and a is a constant related to the binding strength of P. Luo et al. [50] used nine different substrates including four sands, two soils, bentonite, and two industrial by-products of furnace slag and fly ash to calculate the P sorption. Results showed that the furnace slag had the highest P sorption capacity (8.89 g P kg−1) followed by the fly ash (8.81 g P kg−1), and that of sand II was the lowest. Different kinds of sands also showed varying P sorption capacity (0.13–0.29 g P kg−1).
Plant uptake and transpiration flux: Constructed wetlands often include aquatic plants that can uptake pollutants from the water and release them into the atmosphere through transpiration. For example, plants in a wetland can uptake nutrients like nitrogen and phosphorus, as well as metals and organic contaminants, through their roots. These pollutants can then be transported to the above-ground parts of the plants and released into the air through transpiration. The extent of plant uptake and transpiration flux depends on factors such as plant species, pollutant concentrations, and hydrological conditions.
Photodegradation: Photodegradation involves the degradation or breakdown of pollutants through exposure to sunlight or UV radiation. This process is particularly relevant for the degradation of certain organic compounds, such as pesticides or pharmaceuticals that are susceptible to photolytic reactions. The rate of photodegradation depends on factors like sunlight intensity, water depth, and the specific chemical properties of the pollutants.
Phytodegradation: Phytodegradation is the metabolic degradation or breakdown of organic contaminants by plant enzymes or enzyme cofactors. Which plant degrade organic chemicals mainly depends on the specific compound of interest such as P. australis has been shown to possess enzymes degrading PCB with up to three chlorine atoms, whereas higher chlorinated PCBs were not transformed. This phytodegradation is very effective to contaminants removal such as it is a popular treatment to carbon tetrachloride contaminated water [49].
Microbial-mediated transformations: Microorganisms play a vital role in the transformation and removal of pollutants in constructed wetlands. They can degrade or transform various contaminants through processes such as biodegradation, nitrification, denitrification, and sulphate reduction. For example, microorganisms in a wetland can break down organic matter and convert ammonia to nitrate or nitrogen gas. The activity and diversity of microbial communities are influenced by factors such as oxygen availability, temperature, pH, and the nature of the pollutants.
It is important to note that the efficiency of these processes in constructed wetlands can vary depending on design factors, hydraulic loading rates, pollutant characteristics, and site-specific conditions. Numerical modeling and monitoring studies are often used to assess the performance of constructed wetlands and optimize their design for pollutant removal. Examples of different processes that are occurring in constructed wetlands are given in Table 6.
The Fate of Particulate Matter After Its Entrapment and the Role of Enzymes
In constructed wetlands, particulate matter that gets entrapped in the system undergoes a series of processes that contribute to its fate and transformation. Enzymes play a crucial role in these processes. Identifying and quantifying all possible enzymes in a constructed wetland is a complex task due to the diversity of microbial communities and the wide range of enzymatic activities involved in organic matter degradation. It is important to note that the specific enzyme composition and levels of presence can vary depending on the wetland’s design, operational conditions, and the types of organic substrates present. Some examples of enzymes are:
Cellulases: Produced by bacteria and fungi, cellulases break down cellulose, a major component of plant cell walls, into glucose units [57].
Ligninases: Produced by fungi and some bacteria, ligninases help in the decomposition of lignin, a complex polymer found in plant tissues [58].
Proteases: Produced by bacteria, proteases break down proteins into smaller peptides and amino acids [59].
Lipases: Produced by bacteria and fungi, lipases catalyze the hydrolysis of lipids (fats and oils) into fatty acids and glycerol [60].
Amylases: Produced by bacteria and fungi, amylases assist in the degradation of starch and other complex carbohydrates into simpler sugars [61].
Xylanases: Produced by bacteria and fungi, xylanases target xylan, a complex polysaccharide present in plant cell walls [62].
Phosphatases: Produced by bacteria and fungi, phosphatases help in the release of phosphate from organic compounds, making it available for plant uptake [63].
Nitrogenases: Produced by certain bacteria, nitrogenases convert atmospheric nitrogen (N2) into ammonia (NH3) through nitrogen fixation, contributing to nitrogen cycling in the wetland [64].
Sulfatases: Produced by bacteria and fungi, sulfatases catalyze the hydrolysis of sulfate esters, participating in sulfur cycling [65].
Enzymes involved in denitrification: Denitrifying bacteria produce various enzymes involved in the conversion of nitrate (NO3−) to gaseous nitrogen compounds (e.g., nitrous oxide, N2O, and nitrogen gas, N2) contributing to nitrogen removal in the wetland.
It is important to emphasize that the presence and levels of specific enzymes in a constructed wetland can vary depending on factors such as the composition of the microbial community, the availability of organic substrates, and the prevailing environmental conditions. Additionally, the identification and quantification of enzymes in a constructed wetland typically require specialized laboratory techniques, such as enzyme assays and metagenomic analyses, to accurately assess their presence and activities.
Different Software, Analytical Tools, and Techniques Used to Measure/Quantify in Biofilm Engineering
Different software, analytical equipment, tools, and techniques are required to measure/quantify parameters in wetlands. Geographic information systems (GIS) can be used to map wetland location and site characteristics, DNA sequencing and metagenomics can be used to studying microbial community composition and function, etc. A summarized table outlining different software, analytical equipment, tools, and techniques commonly used to measure/quantify parameters of interest, design, and operation of wetlands, including how they incorporate biofilm engineering and microbial community analysis are given in Table 7. The selection of specific software or equipment may vary depending on the objectives and scope of the wetland study or project.
Role of Microorganisms in the Removal of Nutrients and Contaminants from Wastewater in Wetlands
Nitrogen Removal
Nitrogen removal by microorganisms in constructed wetlands is a critical step. This removal process occurs through different steps such as amination, aerobic ammonia oxidation, nitrite oxidation, anoxic denitrification, heterotrophic nitrification, and aerobic denitrification in constructed wetlands. The required oxygen and nutrients to facilitate the growth of nitrifiers and required carbon and energy for denitrifiers are supplied from the roots to the enrichment of nitrogen-metabolizing microorganisms in the rhizosphere [66,67,68]. So, most of the nitrogen metabolism occurs at or near the roots in wetlands [69, 70]. In the first step, ammonia is first oxidized to nitrite and then to nitrate, and nitrate is next reduced to N2O or N2 step by step. Ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) are responsible for this ammonia oxidation but it depends on the temperature and nutrient content of the wastewater [71, 72, 26, 73,74,75]. The nitrification process occurred by two of the ammonia-oxidizing prokaryotes (AOP) coupled with nitrobacteria (NOB) (which is composed of four genera: Nitrocellulose, Nitrobacter, Nitrococuus, and Nitrocellubrium). In the next step, nitrogen reduction occurs in two different ways: denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Nitrate is finally reduced to N2 in the denitrification process, but nitrate is reduced to ammonium which can be used by other microorganisms in the DNRA process [72•]. Denitrifying microorganisms are facultative anaerobic heterotrophic and DNRA microorganisms are anaerobic. The two different pathways of denitrification and DNRA depend on electron donors, organic carbon, and ferrous compounds present [72, 76].
The nitrogen removal pathway is:
Nitrification: NH4+ → NH2OH → NO2− → NO3−
Denitrification: NH2OH → N2O → N2
Phosphorus Removal
Phosphorus removal is essential to reduce the eutrophication. Constructed and natural wetlands show good performances in removing phosphorus. Phosphorus can be present in different forms such as organic (phospholipids, nucleic acids, nucleoproteins, etc.) and inorganic (polyphosphates, etc.). There are different ways to remove phosphorus in wetlands such as (a) uptake by plant roots, (b) sorption onto or desorption from substrates, (c) the formation and accretion of new sediments and soils, (d) bacterial action, and incorporation into organic matter (OM). Phosphorus can interact strongly with soil and biota which can remove the phosphorus for short terms. On the other hand, phosphorus removal by biota (such as bacteria, algae, and macrophytes) can provide a long-term removal by a continuous cycling through growth, death, and decomposition [77]. In addition, vegetation in wetlands shows a better performance in phosphorus removal compared to unvegetated wetlands. Phosphorus can be removed from wastewater by polyphosphate-accumulating organisms (PAOs) present in constructed wetlands. PAOs contain three internal cell storage which is used for excess phosphorus removal: (1) polyphosphate, (2) polyhydroxy-alkanoates (mainly present as PHB), and (3) glycogen. The enzymes exopolyphosphatase (PPX) and polyphosphate kinase (PPK) are directly related to this activity. PPK can transfer a phosphate group from adenosine triphosphate (ATP) to the Poly-P chain [78]. PAOs utilize adenosine triphosphate (ATP) and volatile fatty acid (VFA) to produce polyhydroxyalkanoates (PHA) in the cell. Here, 3-nicotinamide adenine dinucleotide (NADH2) is used as an energy carrier released during the formation of PHB from glycogen in anaerobic condition [79]. This process is graphically displayed in Fig. 1. In addition, Cyanobacteria is one of the most important microorganisms to remove phosphorus in wetlands. Because it can enhance plant stress resistance by inducing signals, such as indole acetic acid, cytokinin, and other pro-growth regulators. Thus, it helps plants to promote phosphorus absorption [80]. In addition, Actinobacteria which is a phosphate solubilizing bacteria (PSB) can play an important role in immobilizing phosphate by producing phosphatase enzymes [81, 82]. In another study, it was found that Rhodocyclaceae which is a group of PAOs can produce extracellular polymeric substances that help to absorb an excessive amount of phosphate under aerobic conditions [83, 84]. Acidovorax and Paracoccus, as typical denitrifying phosphate-accumulating organisms (DPAOs), can remove P from water by utilizing nitrate as an electron acceptor to the absorbed phosphate [85, 86].
Removal of Heavy Metals
Heavy metal (HM) removal mechanisms in constructed wetlands by microbial processes are biosorption, bioaccumulation, microbe-mediated redox reactions, and plant–microbe interactions [87, 88]. Metal ions can destroy the microbiome cell membrane by destroying DNA and disturbing cell functions. But these challenges can be overcome by resistant microorganisms. Since resistant microorganisms have (i) better adaptability to the changing HM environment during the operation and (ii) they can accumulate more HM with the same biomass. The development of such HM resistant microorganisms depends on the evolution of microbial communities which can take time (e.g., 30 days). But the inoculation of heavy metals resistance microorganisms accelerates this process. For example, Yu et al. [89] observed in their research that Cd2+ and Zn2+ can be removed by resistant microorganisms (Serratia and Pseudomonas) through bioaugmentation within 15–20 days (at a shorter stabilization period) with 8–10% increased removal efficiency. This is because HM resistant microorganisms can produce specific proteins (e.g., HM–binding proteins and indole acetic acid (IAA)) and peptides (e.g., metallic-regulatory proteins and phytochelatins). These specific proteins and peptides can increase plant biomass and translocation of HM [8, 90, 91]. As an example, genetically modified Ralstonia eutropha can reduce the harmful Cd (II) by the production of metallothionein on the surface of the cell [8] (Table 8).
Removal of Micropollutants
Micropollutants pose potential risks to aquatic and public health worldwide as they are emerging contaminants and present at trace concentrations. Their origin can be households, hospitals, landfill leachates, and agricultural runoff, etc. So, it is essential to remove those micropollutants from water through appropriate treatments. Constructed wetlands show a good performance in removing micropollutants and their removal mechanisms include substrate sorption, plant uptake, and biodegradation. Biodegradation has been pointed as one of the major removal mechanisms in CWs but little is known about the effects of this mechanism on microbial community. Different researchers have investigated the effect of micropollutants on microorganism diversity and have identified that the response of microorganisms to micropollutants can be classified as subsidy responses and stress responses [96]. Subsidy responses include biodegradation, in which the microbes can utilize the compounds which belong to micropollutants as carbon sources. Alternatively, the stress responses include the inhibition of growth and survival of microorganisms. As an example, bisphenol A (BPA) and its substituted derivatives (e.g., bisphenol S, BPS) [97] are considered to produce subsidy responses, because of their relatively simple chemical structure and low toxicity. Microorganisms can use them as carbon sources. Alternatively, triclosan (TCS) and its substituted derivatives (e.g., triclocarban, TCC) [98] are considered to cause stress response because they inhibit the growth of several microorganisms. Another research by Zhang et al. [99] investigated the impact of micropollutants on organisms. They used community-level physiological profiling (CLPP) analysis which indicated that the presence of micropollutants can shape the microbial community metabolic function not only in the interstitial water but also in the biofilms. They observed that biofilm development decreases the sorption capacity of the packing materials with time. But the organic removal (COD, TOC, and BOD5) increased over time (between days 25 and 66), which suggests organic biodegradation likely occurred. Similar findings are also observed by Lv et al. [100], Zhang et al. [101], and Kurzbaum et al. [102] such as biofilm communities in CW had an important role in tebuconazole, ibuprofen, and phenol biodegradation. More studies are essential in order to understand the impacts of micropollutants on the microbial communities present in the wetlands and vice versa. Figure 2 summarizes the discussions made in the sections above for a quick view.
Conclusion and Future Research Prospects
Microorganisms play a vital role in wetlands to purify the water from the contaminants. Their diversity and density depend on surrounding factors such as substrate types, microphytes, influent wastewater quality, and hydraulic design. But a clear direction of inter-relationship among those factors is still missing such as the impact of substrate of CW and the influence of biofilm growth on diversity of micropollutants. In addition, the contribution of microorganisms to remove phosphorus among other mechanisms is not clear. Different microorganisms are responsible to remove nitrogen, phosphorus, heavy metals. But highly efficient microorganisms are not identified yet. The mechanism of removing micropollutants by microorganisms still needs to be investigated. So, the future research can be focused on:
-
a)
Investigating the internal mechanism of phosphorus removal by microorganisms along with influencing factors
-
b)
Identifying highly efficient microorganisms which are responsible for the removal of nitrogen, phosphorus, and heavy metals
-
c)
Identifying the causes of constraints (such as problems of clogging, and odors) by microorganisms to improve the sustainability of constructed wetlands.
In addition, robust models are required to predict the influence of substrate, plants, hydraulic design, wastewater type on microbial diversity and density to simulate the performance of CWs accurately and therefore they can be designed, constructed, and operated in a sustainable manner to remove the pollutants that are present in the water efficiently.
Data Availability
The authors declare that the data supporting the findings of this study are available in the references cited in the paper.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pelissari C, Guivernau M, Viñas M, García J, Velasco-Galilea M, Souza SS, Sezerino PH, Ávila C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018;141:185–95.
Fu G, Wu J, Han J, Zhao L, Chan G, Leong K. Effects of substrate type on denitrification efficiency and microbial community structure in constructed wetlands. Biores Technol. 2020;307:123222.
Fang J, Dong J, Li C, Chen H, Wang L, Lyu T, He H, Liu J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern China. Appl Soil Ecol. 2021;168:104141.
Adrados B, Sánchez O, Arias CA, Becares E, Garrido L, Mas J, Brix H, Morató J. Microbial communities from different types of natural wastewater treatment systems: vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014;55:304–12.
Sánchez O. Constructed wetlands revisited: microbial diversity in the–omics era. Microb Ecol. 2017;73(3):722–33.
Gagnon V, Chazarenc F, Comeau Y, Brisson J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci Technol. 2007;56(3):249–54.
Lin Y-F, Jing S-R, Lee D-Y, Chang Y-F, Shih K-C. Nitrate removal from groundwater using constructed wetlands under various hydraulic loading rates. Biores Technol. 2008;99(16):7504–13.
Shahid MJ, AL-surhanee AA, Kouadri F, Ali S, Nawaz N, Afzal M, Rizwan M, Ali B,Soliman MH. Role of microorganisms in the remediation of wastewater in floating treatment wetlands: a review. Sustainability. 2020;12(14):5559.
Ge H, Batstone DJ, Keller J. Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae. Water Res. 2015;69:173–82.
Lloyd J, Klessa D, Parry DL, Buck P, Brown N. Stimulation of microbial sulphate reduction in a constructed wetland: microbiological and geochemical analysis. Water Res. 2004;38(7):1822–30.
Desta AF, Assefa F, Leta S, Stomeo F, Wamalwa M, Njahira M, Appolinaire D. Microbial community structure and diversity in an integrated system of anaerobic-aerobic reactors and a constructed wetland for the treatment of tannery wastewater in Modjo, Ethiopia. PLoS ONE. 2014;9(12):e115576.
Yun J, Deng Y, Zhang H. Anthropogenic protection alters the microbiome in intertidal mangrove wetlands in Hainan Island. Appl Microbiol Biotechnol. 2017;101(15):6241–52.
Ajwang’Ondiek R, Kitaka N, Oduor SO. Assessment of provisioning and cultural ecosystem services in natural wetlands and rice fields in Kano floodplain, Kenya. Ecosyst Serv. 2016;21:166–173.
Friess DA, Yando ES, Alemu JB, Wong L-W, Soto SD, Bhatia N. Ecosystem services and disservices of mangrove forests and salt marshes. Oceanog Mar Biol. 2020.
Veettil BK, Pereira SFR, Quang NX. Rapidly diminishing mangrove forests in Myanmar (Burma): a review. Hydrobiologia. 2018;822:19–35.
Rajan RJ, Sudarsan J, Nithiyanantham S. Microbial population dynamics in constructed wetlands: review of recent advancements for wastewater treatment. Environ Eng Res. 2019;24(2):181–90.
Mina IA-P, Costa M, Matos A, Calheiros CSC,Castro P. Polishing domestic wastewater on a subsurface flow constructed wetland: organic matter removal and microbial monitoring. Int J Phytoremediation. 2011;13(10):947–958.
Hu S, Zuo X, Lv Z, He J, Wu Y, Liu H, Chen Z. Drained water quality in sludge treatment wetlands: effects of earthworm densities and plant species. J Clean Prod. 2020;247:119128.
•• Cheng R, Zhu H, Shutes B,Yan B. Treatment of microcystin (MC-LR) and nutrients in eutrophic water by constructed wetlands: performance and microbial community. Chemosphere. 2021;263:128139. This paper describes that microenvironment of ceramsite substrate based CWs is beneficial for the growth of functional microorganisms.
Du L, Trinh X, Chen Q, Wang C, Wang H, Xia X, Zhou Q, Xu D, Wu Z. Enhancement of microbial nitrogen removal pathway by vegetation in integrated vertical-flow constructed wetlands (IVCWs) for treating reclaimed water. Biores Technol. 2018;249:644–51.
Nie SA, Li H, Yang X, Zhang Z, Weng B, Huang F, Zhu GB, Zhu YG. Nitrogen loss by anaerobic oxidation of ammonium in rice rhizosphere. ISME J. 2015;9(9):2059–2067.
Kadlec R. The effects of wetland vegetation and morphology on nitrogen processing. Ecol Eng. 2008;33(2):126–41.
Wang W, Zhao Y, Jiang G, Wang Y. The nutrient removal ability and microbial communities in a pilot-scale horizontal subsurface flow constructed wetland fed by slightly polluted Lake water. Wetlands. 2020;40:2085–96.
Saleem H, Arslan M, Rehman K, Tahseen R, Afzal M. Phragmites australis—a helophytic grass—can establish successful partnership with phenol-degrading bacteria in a floating treatment wetland. Saudi J Biol Sci. 2019;26(6):1179–86.
Fu G, Zhao L, Huangshen L,Wu J. Isolation and identification of a salt-tolerant aerobic denitrifying bacterial strain and its application to saline wastewater treatment in constructed wetlands. Bioresour Technol. 2019;290:121725. https://doi.org/10.1016/j.biortech.2019.121725.
Li X, Zhang M, Liu F, Chen L, Li Y, Li Y, Xiao R, Wu J. Seasonality distribution of the abundance and activity of nitrification and denitrification microorganisms in sediments of surface flow constructed wetlands planted with Myriophyllum elatinoides during swine wastewater treatment. Biores Technol. 2018;248:89–97. https://doi.org/10.1016/j.biortech.2017.06.102.
Wang Q, Cao Z, Liu Q, Zhang J, Hu Y, Zhang J, Xu W, Kong Q, Yuan X, Chen Q. Enhancement of COD removal in constructed wetlands treating saline wastewater: intertidal wetland sediment as a novel inoculation. J Environ Manage. 2019;249:109398. https://doi.org/10.1016/j.jenvman.2019.109398.
Bahr M, Crump BC, Klepac-Ceraj V, Teske A, Sogin ML, Hobbie JE. Molecular characterization of sulfate-reducing bacteria in a New England salt marsh. Environ Microbiol. 2005;7(8):1175–85.
Ibekwe AM, Grieve CM, Lyon SR. Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl Environ Microbiol. 2003;69(9):5060–9.
Karajić M, Lapanje A, Razinger J, Zrimec A, Vrhovšek D. The effect of the application of halotolerant microorganisms on the efficiency of a pilot-scale constructed wetland for saline wastewater treatment. J Serb Chem Soc. 2010;75(1):129–42.
Shen Y, Zhuang L, Zhang J, Fan J, Yang T, Sun S. A study of ferric-carbon micro-electrolysis process to enhance nitrogen and phosphorus removal efficiency in subsurface flow constructed wetlands. Chem Eng J. 2019;359:706–12.
Cao W, Zhang Y. Removal of nitrogen (N) from hypereutrophic waters by ecological floating beds (EFBs) with various substrates. Ecol Eng. 2014;62:148–52.
Calheiros CS, Duque AF, Moura A, Henriques IS, Correia A, Rangel AO, Castro PM. Substrate effect on bacterial communities from constructed wetlands planted with Typha latifolia treating industrial wastewater. Ecol Eng. 2009;35(5):744–53.
Guan W, Yin M, He T, Xie S. Influence of substrate type on microbial community structure in vertical-flow constructed wetland treating polluted river water. Environ Sci Pollut Res. 2015;22:16202–9.
Li M, Zhou Q, Tao M, Wang Y, Jiang L, Wu Z. Comparative study of microbial community structure in different filter media of constructed wetland. J Environ Sci. 2010;22(1):127–33.
Vacca G, Wand H, Nikolausz M, Kuschk P, Kästner M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005;39(7):1361–73.
Stefanakis AI, Tsihrintzis VA. Effects of loading, resting period, temperature, porous media, vegetation and aeration on performance of pilot-scale vertical flow constructed wetlands. Chem Eng J. 2012;181:416–30.
Vymazal J. Plants used in constructed wetlands with horizontal subsurface flow: a review. Hydrobiologia. 2011;674(1):133–56.
Yang X, He Q, Guo F, Sun X, Zhang J, Chen Y. Impacts of carbon-based nanomaterials on nutrient removal in constructed wetlands: microbial community structure, enzyme activities, and metabolism process. J Hazard Mater. 2021;401:123270.
•• Ding Y, Lyu T, Bai S, Li Z, Ding H, You S, Xie Q. Effect of multilayer substrate configuration in horizontal subsurface flow constructed wetlands: assessment of treatment performance, biofilm development, and solids accumulation. Environ Sci Pollut Res. 2018;25:1883–1891. This paper highlights that multilayer substrate configuration significantly influenced biofilm growth and solids accumulation in constructed wetlands.
Caselles-Osorio A, Puigagut J, Segú E, Vaello N, Granés F, García D, García J. Solids accumulation in six full-scale subsurface flow constructed wetlands. Water Res. 2007;41(6):1388–98.
Sanchez-Huerta C, Fortunato L, Leiknes T, Hong P-Y. Influence of biofilm thickness on the removal of thirteen different organic micropollutants via a membrane aerated biofilm reactor (MABR). J Hazard Mater. 2022;432:128698.
Han B, Zhang S, Wang P, Wang C. Effects of water flow on submerged macrophyte-biofilm systems in constructed wetlands. Sci Rep. 2018;8(1):1–12.
Morató J, Codony F, Sánchez O, Pérez LM, García J, Mas J. Key design factors affecting microbial community composition and pathogenic organism removal in horizontal subsurface flow constructed wetlands. Sci Total Environ. 2014;481:81–9.
Samsó R, García J. Bacteria distribution and dynamics in constructed wetlands based on modelling results. Sci Total Environ. 2013;461:430–40.
•• Huang L, Wang N, Deng C, Liang Y, Wang Q, Liu M, Chen Y. Interactive effect of carbon source with influent COD/N on nitrogen removal and microbial community structure in subsurface flow constructed wetlands. J Environ Manage. 2019;250:109491. This paper spotlights that microbial community structure is affected significantly by carbon source, influent COD/N ratio and their interaction.
Ye F, Li Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol Eng. 2009;35(7):1043–50.
Lai X, Zhao Y, Pan F, Yang B, Wang H, Wang S, He F. Enhanced optimal removal of nitrogen and organics from intermittently aerated vertical flow constructed wetlands: relative COD/N ratios and microbial responses. Chemosphere. 2020;244:125556. https://doi.org/10.1016/j.chemosphere.2019.125556.
Imfeld G, Braeckevelt M, Kuschk P, Richnow HH. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere. 2009;74(3):349–62.
Luo B, Ge Y, Han W, Fan X, Ren Y, Du Y, Shi M, Chang J. Decreases in ammonia volatilization in response to greater plant diversity in microcosms of constructed wetlands. Atmos Environ. 2016;142:414–9.
Di Luca GA, Maine MA, Mufarrege M, Hadad HR, Pedro MC, Sánchez GC, Caffaratti SE. Phosphorus distribution pattern in sediments of natural and constructed wetlands. Ecol Eng. 2017;108:227–233.
Xu D, Xu J, Wu J, Muhammad A. Studies on the phosphorus sorption capacity of substrates used in constructed wetland systems. Chemosphere. 2006;63(2):344–52.
Mathon B, Coquery M, Miège C, Vandycke A, Choubert J-M. Influence of water depth and season on the photodegradation of micropollutants in a free-water surface constructed wetland receiving treated wastewater. Chemosphere. 2019;235:260–70.
Schierano MC, Panigatti MC, Maine MA, Griffa CA, Boglione R. Horizontal subsurface flow constructed wetland for tertiary treatment of dairy wastewater: removal efficiencies and plant uptake. J Environ Manage. 2020;272:111094.
Dittrich E, Salamon-Albert É, Somfai D, Dolgos-Kovács A, Kiss T. Transpiration effect of Tufted sedge for a horizontal subsurface flow constructed wetland. Water Sci Technol. 2019;79(10):1905–11.
Zhang W, Guan A, Peng Q, Qi W,Qu J. Microbe-mediated simultaneous nitrogen reduction and sulfamethoxazole/N-acetylsulfamethoxazole removal in lab-scale constructed wetlands. Water Res. 2023:120233.
Wilson DB. Three microbial strategies for plant cell wall degradation. Ann N Y Acad Sci. 2008;1125(1):289–97.
Vicuña R. Bacterial degradation of lignin. Enzyme Microb Technol. 1988;10(11):646–55.
Sharma KM, Kumar R, Panwar S, Kumar A. Microbial alkaline proteases: optimization of production parameters and their properties. J Genet Eng Biotechnol. 2017;15(1):115–26.
Negi S. Lipases: A promising tool for food industry. Green Bio-processes: Enzyme Ind Food Process. 2019:181–198.
Gurung N, Ray S, Bose S, Rai V. A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. BioMed Res Int. 2013;2013.
Talamantes D, Biabini N, Dang H, Abdoun K, Berlemont R. Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol Biofuels. 2016;9:1–11.
Khan AA, Jilani G, Akhtar MS, Naqvi SS, Rasheed M. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci. 2009;1(1):48–58.
Zhu Y-G, Peng J, Chen C, Xiong C, Li S, Ge A, Wang E,Liesack W. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023.
Kertesz MA, Fellows E, Schmalenberger A. Rhizobacteria and plant sulfur supply. Adv Appl Microbiol. 2007;62:235–68.
Jun-Xing Y, Yong L, Zhi-Hong Y. Root-induced changes of pH, Eh, Fe (II) and fractions of Pb and Zn in rhizosphere soils of four wetland plants with different radial oxygen losses. Pedosphere. 2012;22(4):518–27.
Lamers LP, Van Diggelen JM, Op den Camp HJ, Visser EJ, Lucassen EC, Vile MA, Jetten MS, Smolders AJ, Roelofs JG. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: a review. Front Microbiol. 2012;3:156.
Wu H, Wang X, He X, Zhang S, Liang R, Shen J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci Total Environ. 2017;598:697–703.
Trias Mansilla R, Ruiz Rueda O, García Lledó A, Vilar Sanz A, López i Flores R, Quintana Pou X, Hallin S, Bañeras Vives L. Emergent macrophytes act selectively on ammonia-oxidizing bacteria and Archaea. © Appl Environ Microbiol. 2012;78(núm. 17):6352–6356.
Shen J-P, Zhang L-M, Di HJ, He J-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front Microbiol. 2012;3:296.
Ji M, Hu Z, Hou C, Liu H, Ngo HH, Guo W, Lu S, Zhang J. New insights for enhancing the performance of constructed wetlands at low temperatures. Biores Technol. 2020;301:122722.
• Tang S, Liao Y, Xu Y, Dang Z, Zhu X, Ji G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour Technol. 2020;314:123759. This paper explains the microbial coupling mechanisms of nitrogen removal conserves oxygen and energy and the activity and diversity of nitrifiers and denitrifiers are affected significantly by plant species.
He S, Wang Y, Li C, Li Y, Zhou J. The nitrogen removal performance and microbial communities in a two-stage deep sequencing constructed wetland for advanced treatment of secondary effluent. Biores Technol. 2018;248:82–8.
Song S, Wang P, Liu Y, Zhao D, Leng X, An S. Effects of Oenanthe javanica on nitrogen removal in free-water surface constructed wetlands under low-temperature conditions. Int J Environ Res Public Health. 2019;16(8):1420.
Srithep P, Pornkulwat P, Limpiyakorn T. Contribution of ammonia-oxidizing archaea and ammonia-oxidizing bacteria to ammonia oxidation in two nitrifying reactors. Environ Sci Pollut Res. 2018;25:8676–87.
Rahman MM, Roberts KL, Grace MR, Kessler AJ, Cook PL. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci Total Environ. 2019;666:608–17.
Kadlec RH. Phosphorus removal in emergent free surface wetlands. J Environ Sci Health. 2005;40(6–7):1293–306.
Du L, Chen Q, Liu P, Zhang X, Wang H, Zhou Q, Xu D, Wu Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by integrated vertical-flow constructed wetlands (IVCWs). Biores Technol. 2017;243:204–11. https://doi.org/10.1016/j.biortech.2017.06.092.
Hei S, Xu H, Liu Y, Liu B, Zhang S, Zhu X, Lin W, Chen L, Jiang H, Cheng X, Yong X, Wu X, Huang X. Redox environment inducing strategy for enhancing biological phosphorus removal in a full-scale municipal wastewater treatment plant. J Clean Prod. 2022;376:134237. https://doi.org/10.1016/j.jclepro.2022.134237.
Rady MM, Taha SS, Kusvuran S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Sci Hortic. 2018;233:61–9.
Hamdali H, Hafidi M, Virolle MJ, Ouhdouch Y. Rock phosphate-solubilizing Actinomycetes: screening for plant growth-promoting activities. World J Microbiol Biotechnol. 2008;24(11):2565–75. https://doi.org/10.1007/s11274-008-9817-0.
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimarães CT, Schaffert RE, Sá NMH. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem. 2009;41(9):1782–7. https://doi.org/10.1016/j.soilbio.2008.01.012.
Xu F, Cao FQ, Kong Q, Zhou LL, Yuan Q, Zhu YJ, Wang Q, Du YD, Wang ZD. Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem Eng J. 2018;339:479–486. https://doi.org/10.1016/j.cej.2018.02.003.
Świątczak P, Cydzik-Kwiatkowska A. Performance and microbial characteristics of biomass in a full-scale aerobic granular sludge wastewater treatment plant. Environ Sci Pollut Res. 2018;25(2):1655–69. https://doi.org/10.1007/s11356-017-0615-9.
Wang Q, Ding J, Xie H, Hao D, Du Y, Zhao C, Xu F, Kong Q, Wang B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J Clean Prod. 2021;288:125199.
Sun L, Zhao X, Zhang H, Zhang Y. Biological characteristics of a denitrifying phosphorus-accumulating bacterium. Ecol Eng. 2015;81:82–8. https://doi.org/10.1016/j.ecoleng.2015.04.030.
Rahman Z. An overview on heavy metal resistant microorganisms for simultaneous treatment of multiple chemical pollutants at co-contaminated sites, and their multipurpose application. J Hazard Mater. 2020;396:122682.
Yu G, Peng H, Fu Y, Yan X, Du C, Chen H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Biores Technol. 2019;280:337–44.
Yu G, Wang G, Li J, Chi T, Wang S, Peng H, Chen H, Du C, Jiang C, Liu Y. Enhanced Cd2+ and Zn2+ removal from heavy metal wastewater in constructed wetlands with resistant microorganisms. Biores Technol. 2020;316:123898.
Huguenot D, Bois P, Cornu J, Jezequel K, Lollier M, Lebeau T. Remediation of sediment and water contaminated by copper in small-scaled constructed wetlands: effect of bioaugmentation and phytoextraction. Environ Sci Pollut Res. 2015;22:721–32.
Yu G, Wang G, Chi T, Du C, Wang J, Li P, Zhang Y, Wang S, Yang K, Long Y. Enhanced removal of heavy metals and metalloids by constructed wetlands: a review of approaches and mechanisms. Sci Total Environ. 2022:153516.
Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Biores Technol. 2016;218:388–96.
Prum C, Dolphen R, Thiravetyan P. Enhancing arsenic removal from arsenic-contaminated water by Echinodorus cordifolius− endophytic Arthrobacter creatinolyticus interactions. J Environ Manage. 2018;213:11–9.
Ashraf S, Afzal M, Rehman K, Naveed M, Zahir ZA. Plant-endophyte synergism in constructed wetlands enhances the remediation of tannery effluent. Water Sci Technol. 2018;77(5):1262–70.
Hussain Z, Arslan M, Shabir G, Malik MH, Mohsin M, Iqbal S, Afzal M. Remediation of textile bleaching effluent by bacterial augmented horizontal flow and vertical flow constructed wetlands: a comparison at pilot scale. Sci Total Environ. 2019;685:370–9.
Izabel-Shen D, Li S, Luo T, Wang J, Li Y, Sun Q, Yu C-P, Hu A. Repeated introduction of micropollutants enhances microbial succession despite stable degradation patterns. ISME Commun. 2022;2(1):48.
Staples CA, Dome PB, Klecka GM, Oblock ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–73.
McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394(6693):531–2.
Zhang L, Lyu T, Vargas CAR, Arias CA, Carvalho PN, Brix H. New insights into the effects of support matrix on the removal of organic micro-pollutants and the microbial community in constructed wetlands. Environ Pollut. 2018;240:699–708.
Lv T, Carvalho PN, Zhang L, Zhang Y, Button M, Arias CA, Weber KP, Brix H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: influence of system design and sampling strategy. Water Res. 2017;110:241–51.
Zhang L, Lv T, Zhang Y, Stein OR, Arias CA, Brix H, Carvalho PN. Effects of constructed wetland design on ibuprofen removal – A mesocosm scale study. Sci Total Environ. 2017;609:38–45. https://doi.org/10.1016/j.scitotenv.2017.07.130.
Kurzbaum E, Kirzhner F, Armon R. Performance comparison of plant root biofilm, gravel attached biofilm and planktonic microbial populations, in phenol removal within a constructed wetland wastewater treatment system. Water Sa. 2016;42(1):166–70.
Author information
Authors and Affiliations
Contributions
SM drafted the manuscript and MB, SM, JF and VJ provided supervised SM and feedback. VJ revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moazzem, S., Bhuiyan, M., Muthukumaran, S. et al. Microbiome Wetlands in Nutrient and Contaminant Removal. Curr Pollution Rep 9, 694–709 (2023). https://doi.org/10.1007/s40726-023-00280-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-023-00280-9