Abstract
Microbial community constitute a major component of constructed wetlands (CWs), playing a major role in these systems capacities for treating wastewater. Constructed wetland system has a hydraulic regime, although the volume of inflow in the wetland is never the same as the outflow. Wetland are either of Free Water Surface (FWS) or Subsurface Flow (SF). Nitrogen, the most important component in constructed wetlands undergoes transformation by various processes converting N into one to another form and by plant uptake. For instance, nitrification is more impactful for ammonia reduction and its removal relies on the configuration of the wetland and the dissolved oxygen (DO). The chapter discusses the types of wetlands and their physical, chemical and biological processes in the removal of various contaminants. It also gives an overview of different microbial processes and their mechanisms involved during the treatment of wastewater inside constructed wetland systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
“Constructed wetlands are engineered ecosystems that have been made to optimize the natural processes such as wetland flora, media, and associated microbial community for rejuvenating wastewater (Vymazal 2007)”. So wetlands created for treating effluents like domestic (rural or urban), industrial wastewater, stormwater runoff are termed as constructed wetlands (CW). These engineered systems are natural alternative that acts as biofilters and use natural functions of vegetation, microorganisms and media to treat different water streams. Wastewater purification can be governed by a number of factors including phytoremediation, adsorption or nitrification, filtration by gravel and gravitational sedimentation. Dominant factor among all is biological filtration through biofilm formation by aerobic or facultative bacteria.
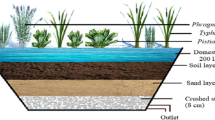
14.2 Constructed Wetlands
Constructed Wetlands are designed primarily for wastewater treatment with the aim to maximise pollutant storage and transformation. Components of wetland include water, media (gravel, sand), plants and microbes. Plants selected for use in CWs must have following characteristics:
-
Adaptation to local climatic conditions;
-
Adaptation to pollutants and waterlogged conditions;
-
Ecological acceptability;
-
Contaminant removal capacity, either through direct or indirect mechanisms.
-
Ability to establish, spread and growth
The constructed wetlands are of following types:
-
Horizontal surface flow systems;
-
Horizontal subsurface flow systems;
-
Vertical flow systems with upstream or downstream and undefined loading.
CWs functions are highly under the control of microorganisms (bacteria, fungi, algae, yeasts, protozoa) and their metabolism (Wetzel 1993). Wastewater treatment within CWs occurs as it passes through the wetland media (gravel/sand) and the plant rhizosphere. Major microbial process involved in wastewater treatment is transformation (which may be aerobic or anaerobic) of organic and inorganic substances into insoluble substances and alteration of redox potential of media. In general, microbes adapt to alternations with characteristics of the media and the water reached to them, but when environmental conditions are unsuitable, they remain dormant for years (Hilton 1993). However, toxic substances, such as pesticides and heavy metals may affect microbial community of a constructed wetland.
Metabolic activity of microorganisms in the root zone gets affected by the supply of oxygen. Rhizosphere, the most active reaction zone of constructed wetlands is responsible for physicochemical and biological processes induced plant-microbe interactions as well as with the soil and pollutants. In general, marsh plants give efficient results for wastewater treatment as they possess specific characteristics of growth physiology and able to survive even under extreme rhizosphere conditions such as acidic or alkaline pH, toxicity, salinity, etc. Many physical, chemical and biological mechanisms play important role in the treatment of wastewater.
14.2.1 Sedimentation
Sedimentation plays lead role in removal of contaminants and biological degradation pathways. Plant and leaf litter in the marsh slows down the water flow and allow sediments in the wastewater to be deposited into the bed of the marsh. While predominates are the anaerobic processes in subsurface flow systems, surface flow systems usually prevail with aerobic processes. Pollutant/contaminants accumulation is a prolonged process for phosphorus sink that only possible in a FWS system. Retention rates due to these accretion goes up to 75 g m−2 year.−1 (Vymazal 2007). Sedimentation plays lead role in the reduction of some microbes within the wetland (Gray 2004). Protozoan cysts and helminth eggs can automatically settle through gravity flow in a free-flowing surface water constructed wetland. Bacteria and viruses will not be removed unless they adhere to larger particles. Only some of viruses are known adhere to particles in stabilized ponds that get attached and observed to be too small to settle down on the surface of media (Characklis et al. 2005; Symonds et al. 2014). Thus, removal of pathogens can be directly correlated with particle removal in constructed wetlands (Chouinard et al. 2014; Wu et al. 2016).
14.2.2 Adsorption
Pollutants in the wastewater adhere to plant material and media colloids because of attractive forces, allowing them to settle to the base of the wetland. The movement of inorganic P from the soil to mineral surfaces and thereafter its accumulation at the media surface is known as adsorption. High presence of Al, Fe and Ca levels in media can be directly related with P adsorption.
14.2.3 Precipitation
Insoluble substances (heavy metals) can become insoluble settle onto media and plant material. Formation of phosphate ions with metallic cations, resulting in amorphous solids when both are at peak level in terms of their concentrations is regarded as precipitation reaction.
14.2.4 Nutrient Uptake
Plants growing in CWs use pollutants for growth due to abundance of nutrient availability in the wastewater. Ammonia and nitrate are utilized in the process of plant uptake or assimilation, which are regarded as spring process in temperate zones. In this process, wetland plants behave for long cycle of aboveground biomass like Typha spp. or Phragmites australis. Presence of nutrients and growth rate of plant tissues tapped the potential rate of uptake of nutrients by the plants. Likely, it impacts plant tissue in terms of nutrient storage and their concentrations and finally with accumulation of biomass which is maximum for standing vegetation.
14.2.5 Decomposition and Volatilisation
Various elements such as nitrogen and sulphur existing in gaseous form are released to the atmosphere which is important pathway for their removal in CWs. Water mass distinct the soluble organic matter, by the process of sorption to solid surfaces inside the constructed wetlands or through volatilization for volatile organic compounds (VOC) (US EPA 2000).
This chapter will dwell on the mechanisms of important processes carried out by microorganisms in the root zone of constructed wetlands which helps in removal of contaminants from wastewater.
14.3 Role of Microbes in Constructed Wetlands
A diverse array of microbial communities performs and impact the removal efficiency of contaminants in constructed wetlands. Also, microbial communities are mostly responsible for the improvement in water quality from effluents of constructed wetlands (Ibekwe et al. 2003; Dong and Reddy 2010; Long et al. 2016). Archaeal, bacterial, and fungal OTUs are always greater in the influent than in the effluent of the any constructed wetland (Ibekwe et al. 2017). Rhizosphere is the region where complex reactions take place due to close interaction between roots and the wetland media. Rhizosphere is also identified as zone of diverse substances as root exudates contains sugars, minerals, vitamins, polysaccharides, organic acids, phenol and other organic compounds (Miersch et al. 2001). Bacteria, fungi, algae and protozoa are the most common microorganisms responsible for the proper functioning of CWs. Rhizosphere with root exudates stimulates the microbial communities for the degradation of organic pollutants. Rhizodeposition products perform the mobilizing of nutrients. Organic acid excretion increased during nutrient limiting conditions which enhancing iron and phosphate solubility, thus the plant’s nutrient supply is boosted (Hoffland et al. 1992). Also, microorganisms use organic compounds as substrates (sugars and amino acids) and excreted vitamins stimulate microbial growth which is termed as rhizosphere effect. Microbial cells adheres to each other and to wetland surface to form biofilms. They are frequently embedded within a self-produced matrix of extracellular polymeric substance (EPS). However, wetland media is the main supporting material for the formation of microbial film and hence, responsible for removal of contaminants. In addition to contaminant/pathogen removal, there are other four major microbial reactions (fermentation, nitrification, denitrification and phosphorus removal) mainly responsible for the performance of constructed wetlands (Mitchell 1996). Nitrogen removal in constructed wetlands has been documented to be the result of the activities of microbial communities, which impacts Anammox (Oehl et al. 2004) and nitrification-denitrification (Kroger et al. 2012) pathways. In addition, microbial activities also effect P removal partially through mineralization (Truu et al. 2005) and immobilization.
14.3.1 Fermentation
With respect to climate change, constructed wetlands are also a major area of concern and need to focussed as they are the major significant producer of atmospheric methane. Designated by diverse microbial communities and water-logged surface constructed wetlands are emerged and acclimatized to continuous water presence. Oxygen poor environments favours methanogenesis and microbial communities living in moist and hot environments consume oxygen at much faster rate than it diffuses from air. Thus, wetlands are the most ideal candidates for fermentation processes with anoxic environments enabling easier decomposition of organic carbon and production of high energy compounds (e.g., alcohol, volatile fatty acids, methane). In a process of methanogenesis, microorganisms produce methane by fermenting acetate into methane and carbon dioxide.
Depending on the wetland type, in another process archaea oxidize hydrogen with carbon dioxide to produce methane and water.
14.4 Microbial Mediated Transformations in CWs
Microbial communities in wetland cells are the main cause for perturbation, concentration of dissolved organic matter, and stress related to chemical compounds (Wassel and Mills 1983; Hirayama et al. 2005; Bodtker et al. 2008; Nelson 2009). It has also been shown that, shifts in the bacterial communities structure can be related with alteration in many physico-chemical soil properties such as texture and availability of nitrogen (Frey et al. 2004; Lauber et al. 2008). Moreover, inside the constructed wetland, diverse microbial communities are the significant drivers greatly impacting the final quality of wetland effluents (Calheiros et al. 2009).
Biogeochemical cycling of nitrogen involves both biotic and abiotic transformation resulting in a wide variety of inorganic and organic nitrogen compounds. Most of the forms of the nitrogen are necessary for the existence of life as the nitrogen forms the structural constituent of cell. Nitrogen transformation in wetlands are necessary for functioning of wetland ecosystem and the transformation are enabled by a diverse group of micro-organisms. The important processes that leads to the transformation of nitrogen are ammonification, volatilization, nitrification, denitrification, nitrogen fixation, assimilation, Ammonia absorption and ANAMMOX (Vymazal 2007) occurring in the constructed wetlands.
14.4.1 Ammonification
Ammonification is a catabolic process involving biological conversion of organic nitrogen compounds like amino acids into ammonia. This is essentially a multi-step energy releasing biochemical process. The energy so released is utilised by the microbes involved in the transformations. The rate of ammonification is a function of pH, temperature, C/N ratio and soil physical conditions. However, the optimum temperature (40–60 °C) and pH (6.5–8.5) are reported for ammonification process. The important mechanism for the reduction of nitrogen content from constructed wetlands is by the ANAMMOX process. When anaerobic ammonium oxidized, ammonium and nitrite are converted to dinitrogen gas (Kuenen 2008). The ANAMMOX reaction is as follows:
Partial-nitrification in constructed wetlands with Anammox having huge deportation efficacy of total nitrogen has been investigated over conventional methods (Dong and Sun 2007), thus explaining microbial role in altering wastewater quality in engineered wetlands. It has been reported that Anammox utilize carbon dioxide as source of carbon to yield biomass and electron acceptor (nitrite) for oxidation of ammonium, and electron donor for the discharge of carbon dioxide (Kuenen 2008).
Ammonia formation from organic compounds during organic matter degradation is called ammonification, creating energy for growth, and then the ammonia is directly incorporated into cell biomass (Vymazal 2005). Various factors including temperature, pH, available nutrients, C/N ratio, such as texture and structure influencing ammonification rates (Han and Lee 2005; Gustavsson and Engwall 2012) and the values fluctuates between 0.004 and 0.53 g N/m2. d (Reddy et al. 2001; Scholz and Lee 2005). After formation, ammonium can also be absorbed by plants with their complex root systems (Forbes et al. 2010). The process occurs both under aerobic as well as anoxic conditions, but has been moderately slow with anaerobic conditions (Mitsch and Gosselink 2007). Ammonium (NH4 +) gets absorbed by plants with root and root hairs after the formation and immobilized in the sediments by ion exchange, volatilized as gas, anoxically changed back to organic matter by different microbial community (Bacillus, Clostridium, Proteus, Pseudomonas, and Streptomyces) called ammonifying bacteria. Then, process of deamination takes place which results in the removal of the amino groups and producing ammonia (NH3). In most soils, the ammonia dissolves in water and get converted into ammonium ions (NH4 +).
14.4.2 Nitrification
Nitrification refers to the biological oxidation of ammonium to nitrate. Nitrification takes place in the occurrence of dissolved oxygen (DO), when microbes change ammonium to nitrate nitrogen, with an intermediate (nitrite) in the reaction sequence (Oopkaup et al. 2016). Soil organisms like Nistrospira, Nitrosomonas, Nitrosococcus, Nitrovibrio, Nitrosolobus are found to oxidize ammonia to nitrite and in the process generate energy for its growth (Oehl et al. 2004; Ipsilantis and Sylvia 2007). These organisms are chemolithotrophic aerobes. Subsequently, the facultative chemolitrotrophic bacteria (Nitrobacter) oxidize nitrite to nitrate. Two step nitrification process is as follows:
Nitrification process is influenced by both physico-chemical and environmental factors which includes temperature, pH, alkalinity, inorganic carbon source, moisture, microbial population, and ammonium-N and dissolved oxygen concentrations of (Vymazal 2007; Sundberg et al. 2007). Nitrate is either converted to biomass after subsequently absorbed by plants or microbes or be decreased through denitrification (Truu et al. 2005). Both the rhizosphere and biofilms (aerobic process) are the major spots of biofilms. Nitrogen removal by nitrification mediated by microorganisms in the presence of dissolved oxygen is a two-step process. Ammonium is converted to nitrite and nitrate nitrogen by microbial community either in the water columns or within the biofilms. The chemoautotrophic bacteria that accomplish the process are called nitrifying bacteria. The process occurs in the presence of oxygen in water column by suspended microbes and air within any aerobic biofilms. The water column or sediments pore water still remains with nitrate as it is not immobilized by wetland media which can further be utilized by plants or microbial cell factories in assimilatory nitrate reduction using them to produce additional plant biomass.
14.4.3 Denitrification
Denitrification occurs after nitrification as nitrate is one of the prerequisites for it. In denitrification nitrate get reduced in anoxic conditions to a gaseous dinitrogen. Denitrification is overruled by bacteria in the absence of oxygen with a terminal electron acceptor (nitrate) and electron donor in the form of organic carbon (Button et al. 2015). Nitrate is converted into N2 through intermediates nitrite, nitric oxide and nitrous oxide (Weber et al. 2011; Nolvak et al. 2013; Button et al. 2016). Biochemical conversion from nitrate to gaseous dinitrogen is as follows:
Also, the absence of O2, redox potential, temperature, pH, presence of denitrifiers, nitrate concentration impacts the denitrification rates (Vymazal 2007; Wang et al. 2012; Herbst et al. 2016). Denitrification reaction occurs mostly in the sediments of constructed wetlands and in the periphyton and phytoplankton films of water column where available carbon is very high and dissolved oxygen is very low. Denitrification or dissimilatory nitrate reduction again carried out by denitrifying bacteria under these anoxic conditions with the products (N2 and N2O gases) that will readily exit in the CWs.
Plant root exudates are act as potential sources of biodegradable organic carbon for the constructed wetland plants after they get decomposed. Further, microorganisms (bacteria and fungi) coagulate colloidal material, remove soluble and stabilize OM, and convert it into various gases and new cell tissue (Mitsch and Gosselink 1986). Dissimilatory nitrate reduction then supplies N2 for fixation by bacteria and for uptake by plants in the vicinity of roots of sediments (where system is nitrogen-lacking) with the remain as N2 in the water column.
14.4.4 Nitrogen Fixation
Atmospheric nitrogen is assimilated into organic compounds, especially by certain microorganisms (diazotrophs) that contain the enzyme nitrogenase. Diazotrophs are a diverse group of prokaryotes that includes cyanobacteria, green sulfur bacteria, and diazotrophs Azotobacteraceae, rhizobia and Frankia . Either aerobic or anaerobic bacteria and blue-green algae carried out nitrogen fixation in the overlying water in free water surface zones, sediment, oxidized rhizosphere, and on the surfaces of plants i.e. on the leaf and stems (Reddy and Graetz 1988).
14.5 Phosphates
Phosphorus is an important nutrient of CWs ecosystem which can have secondary effects by influencing eutrophication process that leads to algal blooms and other problems related to quality of water in constructed wetlands. Phosphorus in engineered wetlands occurs as phosphates in organic and inorganic compounds. Phosphorus elimination from water in CWs occurs takes place through plant use (assimilation/uptake) and microbes; aluminium, iron oxides and hydroxides adsorption; aluminum, iron, and calcium phosphates complexation; and removal of phosphorus adsorbed with sand/media sediments or organic matter (Richardson 1985; Johnston 1991; Walbridge and Struthers 1993). Also, until the transformation into a soluble inorganic form, dissolved organic phosphate remains unavailable to plants. Again, suspended microbes and biofilms in sediments are responsible for these transformations in the water column (Kaushal et al. 2016). Microbial removal (uptake by biofilm) of phosphorus from CWs is very fast and highly effective; however, following cell death, the phosphorus is discharged again. Polyphosphate-accumulating organisms are Acinetobacter spp., Lampropedia spp., Microlunatus phosphovorus and Tetrasphaera spp. Phosphate accumulating organisms (PAO) take up volatile fatty acids (VFAs), convert them to Polyhydroxybutyrate (PHB) and store as soluble organics. PAO break energy-rich Poly-P bonds to produce energy needed to produce PHB and thus Ortho-P is released into sediments. Accretion processes are also responsible for the loss of phosphate within the sediments.
14.6 Heavy Metals
CWs receiving industrial wastewater are high in heavy metal content which can be removed by the plant material uptake, adsorption and precipitation through microbial behaviour. Fe (II) is oxidized to Fe (III) in surface flow CW by abiotic process and microbial oxidation; precipitation of other elements are also occur such as arsenic (Kaushal et al. 2017). Under anoxic conditions, by microbial dissimilatory sulfate reduction some of the heavy metals are immobilized to form H2S.
14.7 Conclusion
Constructed wetlands are low-rate biological treatment systems to for the treatment of variety of wastewaters including rural, urban, industrial and agricultural. CWs are less sophisticated in operation and maintenance. Although such treatment technologies tend to be land intensive, they are often more effective in removing pathogens and continuous to work with proper maintenance. Also in any CW, microbial processes are particularly needed in the conversion of nitrogen into different biologically appropriate forms, which are made available for plant metabolism. Microbes are also responsible for phosphorus uptake by plants which converts insoluble into soluble forms that again become available for the plants. In addition, microbes decompose the organic compounds, and release carbon dioxide in the oxygenic zones of CW and a variety of gases (CO2, H2S, and CH4) in anoxic zones. Microbial activity may be concentrated at solid surfaces provided by plants, biomass, and sediments. Microbial activities vary through seasonal influences, with the least during winters.
References
Bodtker, G., Thorstenson, T., Lillebo, B. L. P., Thorbjornsen, B. E., Ulvoen, R. H., et al. (2008). The effect of long-term nitrate treatment on SRB activity, corrosion rate and bacterial community composition in offshore water injection systems. Journal of Industrial Microbiology & Biotechnology, 35, 1625–1636.
Button, M., Weber, K. P., Nivala, J., Aubron, T., & Muller, R. A. (2015). Community-level physiological profiling of constructed wetland microbial communities: Effects of sample preparation. Applied Biochemistry and Biotechnology, 178, 960–973.
Button, M., Auvinen, H., Van Koetsem, F., Hosseinkhani, B., Rousseau, D., Weber, K. P., & Du Laing, G. (2016). Susceptibility of constructed wetland microbial communities to silver nanoparticles: A microcosm study. Ecological Engineering, 97, 476–485.
Calheiros, C. S. C., Duque, A. F., Moura, A., Henriques, I. S., Correia, A., et al. (2009). Substrate effect on bacterial communities from constructed wetlands planted with Typha latifolia treating industrial wastewater. Ecological Engineering, 35, 744–753.
Characklis, G. W., Dilts, M. J., Simmons, O. D., Likirdopulos, C. A., Krometis, L.-A. H., & Sobsey, M. D. (2005). Microbial partitioning to settleable particles in stormwater. Water Research, 39, 1773–1782.
Chouinard, A., Balch, G. C., Jorgensen, S. E., Yates, C. N., & Wootton, B. C. (2014). Tundra wetlands: The treatment of municipal wastewaters. RBC blue water project: performance & operational tools. CWAT, Fleming College pp. 380.
Dong, X., & Reddy, G. B. (2010). Soil bacterial communities in constructed wetlands treated with swine wastewater using PCR-DGGE technique. Bioresource Technology, 101, 1175–1182.
Dong, Z., & Sun, T. (2007). A potential new process for improving nitrogen removal in constructed wetlands-promoting coexistence of partial-nitrification and ANAMMOX. Ecological Engineering, 31, 69–78.
Forbes, D. A., Reddy, G. B., Hunt, P. G., Poach, M. E., Ro, K. S., et al. (2010). Comparison of aerated marsh-pond-marsh and continuous marsh constructed wetlands for treating swine wastewater. Journal of Environmental Science and Health, 45, 803–809.
Frey, S. D., Knorr, M., Parrent, J. L., & Simpson, R. T. (2004). Chronic nitrogen enrichment affects thee structure and function of the soil microbial community in temperate hardwood and pine forests. Forest Ecology and Management, 196, 159–171.
Gray, N. F. (2004). Biology of wastewater treatment, Series in Environmental Science and Management. London: Imperial College Press.
Gustavsson, L., & Engwall, M. (2012). Treatment of sludge containing nitro-aromatic compounds in reed-bed mesocosms- water, BOD, carbon and nutrient removal. Waste Management, 32, 104–109.
Han, H. S., & Lee, K. D. (2005). Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Research Journal of Agriculture and Biological Sciences, 1, 176–180.
Herbst, F. A., Lunsmann, V., Kjeldal, H., Jehmlich, N., Tholey, A., von Bergen, M., Nielsen, J. L., Hettich, R. L., Seifert, J., & Nielsen, P. H. (2016). Enhancing metaproteomics-the value of models and defined environmental microbial systems. Proteomics, 16, 783–798.
Hilton, B. L. (1993). Performance evaluation of a closed ecological life support system (CELSS) employing constructed wetlands. In G. A. Moshiri (Ed.), Constructed wetlands for water quality improvement (pp. 117–125). Boca Raton: CRC Press.
Hirayama, H., Takai, K., Inagaki, F., Yamato, Y., Suzuki, M., et al. (2005). Bacterial community shift along a subsurface geothermal water stream in a Japanese gold mine. Extremophiles, 9, 169–184.
Hoffland, E., Van Den Boogaard, R., Nelemans, J., & Findenegg, G. (1992). Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. The New Phytologist, 122, 675–680.
Ibekwe, A. M., Grieve, C. M., & Lyon, S. R. (2003). Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Applied and Environmental Microbiology, 69, 5060–5069.
Ibekwe, A. M., Ma, J., Murinda, S., & Reddy, G. B. (2017). Microbial diversity in continuous flow constructed a wetland for the treatment of swine waste. Hydrology Current Research, 8, 277.
Ipsilantis, I., & Sylvia, D. M. (2007). Abundance of fungi and bacteria in a nutrient impacted Florida wetland. Applied Soil Ecology, 35, 272–280.
Johnston, C. A. (1991). Sediment and nutrient retention by freshwater wetlands: Effects on surface water quality. Critical Reviews in Environmental Control, 21(5), 491–565.
Kaushal, M., Wani, S. P., Patil, M. D., & Datta, A. (2016). Monitoring efficacy of constructed wetland for treating domestic effluent-microbiological approach. Current Science, 110, 1710–1715.
Kaushal, M., Patil, M. D., & Wani, S. P. (2017). Potency of constructed wetlands for deportation of pathogens index from rural, urban and industrial wastewater. International Journal of Environmental Science and Technology, 15, 637–648. https://doi.org/10.1007/s13762-017-1423-y.
Kroger, R., Pierce, S. C., Littlejohn, K. A., Moore, M. T., & Farris, J. L. (2012). Decreasing nitrate-N loads to coastal ecosystems with innovative drainage management strategies in agricultural landscapes: An experimental approach. Agricultural Water Management, 103, 162–166.
Kuenen, J. G. (2008). Anammox bacteria: From discovery to application. Nature Reviews. Microbiology, 6(4), 320–326.
Lauber, C. L., Strickland, M. S., Bradford, M. A., & Fierer, N. (2008). The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biology and Biochemistry, 40, 2407–2415.
Long, Y., Yi, H., Chen, S., Zhang, Z., Cui, K., et al. (2016). Influences of plant type on bacterial and archaeal communities in constructed wetland treating polluted river water. Environmental Science and Pollution Research, 23, 19570–19579.
Miersch, J., Tschimedbalshir, M., Barlocher, F., Grams, Y., Pierau, B., Schierhorn, A., & Kraus, G. J. (2001). Heavy metals and thiol compounds in Mucor racemosus and Articulospora tetracladia. Mycological Research, 105, 883–889.
Mitchell, C. (1996). Pollutant removal mechanisms in artificial wetlands: Course notes for the IWES 96. Gold Coast: International Winter Environmental School.
Mitsch, W. J., & Gosselink, J. G. (1986). Wetlands. New York: Van Nostrand Reinhold.
Mitsch, W. J., & Gosselink, J. G. (2007). Wetlands (4th ed.). Hoboken: Wiley p 582.
Nelson, C. E. (2009). Phenology of high-elevation pelagic bacteria: The roles of meteorologic variability, catchment inputs and thermal stratification in structuring communities. The ISME Journal, 3, 13–30.
Nolvak, H., Truu, M., Tiirik, K., Oopkaup, K., Sildvee, T., Kaasik, A., Mander, U., & Truu, J. (2013). Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Science of the Total Environment, 1, 636–644.
Oehl, F., Frossard, E., Fliessbach, A., Dubois, D., & Oberson, A. (2004). Basal organic phosphorus mineralization in soils under different farming systems. Soil Biology and Biochemistry, 36, 667–675.
Oopkaup, K., Truu, M., Nõlvak, H., Ligi, T., & Preem, J. K. (2016). Dynamics of bacterial community abundance and structure in horizontal subsurface flow wetland mesocosms treating municipal wastewater. Water, 8, 457.
Reddy, K. R., & Graetz, D. A. (1988). Carbon and nitrogen dynamics in wetland soils. In D. D. Hook (Ed.), Ecology and management of wetlands. Ecology of Wetlands Portland (pp. 307–318). Portland: Timber Press.
Reddy, G. B., Hunt, P. G., Phillips, R., Stone, K., & Grubbs, A. (2001). Treatment of swine wastewater in marsh-pond-marsh constructed wetlands. Water Science and Technology, 44, 545–550.
Richardson, C. J. (1985). Mechanisms controlling phosphorus retention capacity in freshwater wetlands. Science, 228, 1424–1427.
Scholz, M., & Lee, B. H. (2005). Constructed wetlands: A review. International Journal of Environmental Studies, 62, 1256–1261.
Sundberg, C., Tonderski, K., & Lindgren, P. E. (2007). Potential nitrification and denitrification and the corresponding composition of the bacterial communities in a compact constructed wetland treating landfill leachates. Water Science and Technology, 56, 159–166.
Symonds, E. M., Verbyla, M. E., Lukasik, J. O., Kafle, R. C., Breitbart, M., & Mihelcic, J. R. (2014). A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Research, 65, 257–270.
Truu, J., Nurk, K., Juhanson, J., & Mander, U. (2005). Variation of microbiological parameters within planted soil filter for domestic wastewater treatment. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 40, 1191–1200.
US EPA. (2000). Constructed wetlands treatment of municipal wastewaters (1st ed.). Cincinnati: United States Environmental Protection Agency.
Vymazal, J. (2005). Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecological Engineering, 25(1), 478–490.
Vymazal, J. (2007). Removal of nutrients in various types of constructed wetlands. Science of the Total Environment, 380(1–3), 48–65.
Walbridge, M. R., & Struthers, J. P. (1993). Phosphorus retention in non-tidal palustrine forested wetlands of the Mid-Atlantic region. Wetlands, 13(2), 84–94.
Wang, Y., Hayatsu, M., & Fujii, T. (2012). Extraction of bacterial RNA from soil: Challenges and solutions. Microbes and Environments, 27, 111–121.
Wassel, R. A., & Mills, A. L. (1983). Changes in water and sediment bacterial community structure in a lake receiving acid-mine drainage. Microbial Ecology, 9, 155–169.
Weber, K. P., Mitzel, M. R., Slawson, R. M., & Legge, R. L. (2011). Effect of ciprofloxacin on microbiological development in wetland mesocosms. Water Research, 45, 3185–3196.
Wetzel, R. G. (1993). Constructed wetlands: Scientific foundations are critical. In G. A. Moshiri (Ed.), Constructed wetlands for water quality improvement (pp. 3–7). Boca Raton: CRC Press.
Wu, S., Carvalho, P. N., Muller, J. A., Manoj, V. R., & Dong, R. (2016). Sanitation in constructed wetlands: A review on the removal of human pathogens and fecal indicators. Science of the Total Environment, 541, 8–22.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kaushal, M., Wani, S.P., Patil, M.D. (2019). Harnessing Microbial Potential for Wastewater Treatment in Constructed Wetlands. In: Shah, S., Venkatramanan, V., Prasad, R. (eds) Sustainable Green Technologies for Environmental Management. Springer, Singapore. https://doi.org/10.1007/978-981-13-2772-8_14
Download citation
DOI: https://doi.org/10.1007/978-981-13-2772-8_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2771-1
Online ISBN: 978-981-13-2772-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)