Abstract
Purpose
Hypercortisolism has detrimental effects on bone metabolism with the consequences of bone loss and bone fractures. We aimed to evaluate the frequency of vertebral fragility fractures and to determine the factors associated with Cushing’s syndrome (CS).
Methods
A total of 135 patients diagnosed with Cushing’s syndrome [108 patients with Cushing’s disease and 27 patients with adrenocortical adenoma] and 107 healthy controls were included in this cross-sectional study. The available clinical, laboratory, and radiologic data of patients with CS were recorded, retrospectively. Lateral vertebral radiograms were evaluated for vertebral fragility fractures according to Genant’s semi-quantitative method. Bone mineral density (BMD) was determined using a Dual-energy X-ray absorptiometry (DEXA).
Results
Vertebral fragility fractures (VFs) were observed in 75.3% (n = 61) of the patients. The median number of VFs was six (min–max: 2–12). All patients with vertebral fractures had thoracic VF, and 50.7% of the patients had lumbar fragility fractures. Thirty-three (40.7%) patients with vertebral fractures had normal bone densitometry values. Osteoporosis and osteopenia were observed in 16.2% and 40.7% of the patients, respectively. The duration of active disease, the presence of ACTH-secreting pituitary adenoma, and 24-h urinary cortisol did not influence the presence of vertebral fractures. Vertebral fractures were independently associated with age, FSH, LH levels, and lumbar BMD (R2 = 68.18%, p = 0.028). The femoral neck BMD (but not lumbar BMD) was independently associated with age, BMI, and PTH levels (R2 = 48.48%, p < 0.001).
Conclusion
Vertebral fracture frequency was higher in CS patients. Most of the patients with vertebral fractures had multiple fractures. Although low lumbar BMD was associated with VF, patients with CS with normal bone densitometry could experience VF. Vertebral radiograph evaluations as a part of routine evaluation for silent vertebral fractures may help to prevent further fractures in patients with CS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous and exogenous hypercortisolism is known to cause detrimental effects on bone metabolism. A high rate of bone loss and fractures has been reported in Cushing’s disease (CD). The prevalence of osteoporosis was reported as 40–70%, and osteopenia was reported as 80–85% at the time of the diagnosis in patients with endogenous hypercortisolism [1, 2].
Clinical studies have reported that there is a fivefold increase in vertebral and hip fragility in Cushing’s syndrome (CS). The rate of non-traumatic vertebral and peripheral fractures was reported as 30–70% [3], occurring more often at the vertebral level, especially in thoracic vertebras. Age, duration of the disease [4], bone mineral density (BMD) [5], and previous history of fracture [3] were found as predictors of fracture development. Fractures due to hypercortisolism usually appear in the first year, and improvements in bone densitometry are generally observed 2 years after the cure of the disease [3, 6, 7].
Symptomatic fracture frequency has been reported in a limited number of studies, ranging between 15 and 52% [5, 8]. In a study with subclinical CS, a clinically evident fracture was reported in 11.4% of patients [9]. In clinical studies, there is a wide range of distribution of vertebral fracture ratios, and there are conflicting results about predictors of fracture. The fracture rate, evaluated with vertebral radiographs, was reported to range between 15 and 76% [4, 5, 10,11,12].
We aimed to determine the frequency of non-traumatic vertebral fractures in patients with CS and the factors associated with vertebral fractures. The secondary objective was to assess bone mineral density in patients with CS.
Methods
Patients’ clinical and laboratory evaluation
We included 149 patients with CS who were followed up at Marmara University Medical School Endocrinology and Metabolism Department outpatient clinic between 1998 and 2020. Patients diagnosed with adrenocortical carcinoma, ectopic CS, subclinical CS, patients with chronic renal failure, primary hyperparathyroidism, and hyperthyroidism were excluded. A total of 135 patients [108 patients with CD and 27 patients with adrenocortical adenoma] were included in the final analysis.
The study protocol was approved by the Marmara University Medical School Ethics Committee (09.2020.628) and conducted following the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All subjects provided written informed consent.
Clinical characteristics and demographic parameters (age and sex); laboratory data [fasting plasma glucose, HbA1c, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride, total cholesterol, uric acid, blood urea nitrogen (BUN), creatinine, calcium, phosphorus, albumin, 25-OH vitamin D, alkaline phosphatase (ALP), osteocalcin, c telopeptide (CTx), thyroid-stimulating hormone (TSH), free thyroxine (FT4), cortisol, 1 mg dexamethasone suppression test (DST), 24-h urine cortisol (UFC), 2 days 2 mg DST, adrenocorticotrophic hormone (ACTH), growth hormone (GH), insulin-like growth factor (IGF-1), prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH), total testosterone, estradiol (E2)]; imaging findings, and medical histories were obtained retrospectively from medical records.
Calcium levels were measured using a photometric color test, phosphorous levels were analyzed using a photometric ultraviolet (UV) test, and ALP and creatinine were analyzed using a kinetic color test (Jaffé method) with an AU5800 Clinical Chemistry Analyzer (Beckman Coulter, USA). Serum PTH was determined in all participants using an immunoassay method (Roche, modular system, E170, Germany). 25-OH vitamin D was analyzed using an immunoassay method on a Unicel DXI 800 automated analyzer (Beckman Coulter, USA). Cortisol levels were assessed using an enzyme-linked immunosorbent assay (ELISA) using an AU5800 Clinical Chemistry Analyzer (Beckman Coulter, USA). ACTH and osteocalcin concentrations were measured using a chemiluminescent immunometric assay (Immulite 2000, Siemens, LA, USA). The 24-h urine cortisol level was assessed using liquid chromatography-mass spectrometry. CTx was assessed using an electrochemiluminescence assay (Modular E, Roche Diagnostics, Mannheim, Germany).
Endocrinologic evaluation of hypercortisolism and pituitary function
One milligram DST, UFC, and midnight serum cortisol measurements were used as first-line screening tests. Low-dose DST was used for verification in specific cases. When two screening tests were positive, tests for differential diagnosis (plasma ACTH level, 8 mg DST, inferior petrosal sinus sampling) were performed according to the guidelines [13].
Hormonal remission was considered in cases that met the following criteria: 1 mg DST < 1.8 mcg/dL, < 100 mcg/day UFC, and lack or regression of clinical features [13]. Patients were evaluated for hormonal remission at least 3 months after surgery, every 3 months in the postoperative first year, and every 6 months thereafter.
FT4 and TSH levels, plasma total testosterone level (only for men), E2 level (only for women), and the presence of menstruation were used to evaluate the activity of the pituitary-thyroid and pituitary–gonadal axes, respectively. Adrenal insufficiency was defined as the presence of an ACTH stimulated peak cortisol value < 18 μg/dL after a low-dose ACTH stimulation test or insulin tolerance test (ITT) [14]. Growth hormone (GH) insufficiency was defined as the presence of two or more pituitary hormone deficiencies and an IGF level below the age-matched reference values or a peak GH level below 3 μg/L during the ITT [15].
Vertebral fracture evaluation
Vertebral fractures were assessed using lateral thoracolumbar X-ray radiograms. The semi-quantitative method defined by Genant et al. was used [16]. According to this clinical technique, vertebral fractures were graded on a visual inspection and without direct vertebral measurement as normal (grade 0), grade 1 (mild, approximately 20–25% reduction in anterior, middle, and/or posterior height and a reduction of area 10–20%), grade 2 (moderate, approximately 25–40% reduction in any height and a reduction in an area of 20–40%), and grade 3 (severe, approximately 40% reduction in any height and area).
Vertebral radiograms were independently performed by two blinded endocrinologists trained in osteoporosis management (TA and DGY).
Bone mineral density measurements
Bone mineral density (BMD) measurements of 107 age-matched controls were included in the analysis for the comparison of BMD and Z scores. None of the control subjects had chronic renal disease, primary hyperparathyroidism, thyroid or pituitary disease, and none of the controls had taken calcium, bisphosphonates, oral contraceptives, or hormonal replacement therapy.
Dual-energy X-ray absorptiometry (DEXA) was used to determine the BMD and measured in the lumbar spine (L1–L4) in the anteroposterior (AP) projection and the three sites of the right hip (femoral neck, Ward’s triangle, and trochanter) using a Lunar DPX-L. The coefficient of variation (CV) for three sets of measurements was 1.0%.
Osteopenia was defined according to the classic World Health Organization (WHO) criteria, as a T score for the lumbar AP or femoral neck between − 1 SD and − 2.5 SD, accordingly.
Osteoporosis was defined as the BMD T score on DEXA at the lumbar spine or femoral neck less than or equal to − 2.5 SD for postmenopausal women and men aged over 50 years. For premenopausal women and men aged below 50 years, it was defined as a BMD Z score on DEXA at the lumbar spine or femoral neck less than or equal to − 2 SD.
Statistical analysis
Continuous variables are summarized using descriptive statistics presented as mean and standard deviation (SD). Categorical variables are summarized using counts and percentages. Categorical data were analyzed using the Chi-square (χ2) test or Fisher exact test as appropriate. Student’s t-test and analysis of variance (ANOVA) were used for parametric variables.
Multiple regression analysis was performed to define the predictors of vertebral fractures. Predictors with a possible influence on dependent variables were added as covariates (age, BMI, creatinine, PTH, 25-OH vitamin D, 1 mg DST, UFC, FSH, LH, total testosterone, estradiol, femoral neck, and lumbar BMD levels).
Multiple regression analysis was performed to define the relationship between BMD levels and the following variables: age, BMI, creatinine, PTH, 25-OH vitamin D, 1 mg DST, UFC, FSH, LH, total testosterone, and estradiol.
The results were evaluated at a 95% confidence interval, and p < 0.05 was considered statistically significant. All statistical analyses were performed using software (GraphPad InStat 3.0; GraphPad Software, Inc., San Diego, CA, USA).
Results
General characteristics of patients
Female preponderance [F/M: 111/24] was observed in our group of patients with CS. Type 2 diabetes mellitus, hypertension, and hyperlipidemia were observed in 49.6% (n = 67), 59.3% (n = 80), 41.5% (n = 56) of the patients, respectively. Clinical, anthropometric, laboratory parameters and DEXA results are presented in Table 1. The mean follow-up time was 46.6 ± 41.7 months. In the last visit, glucocorticoid deficiency was present in 17 (12.5%) patients, GH deficiency was in 6 (4.4%), and secondary hypogonadism was seen in 7 (5.1%) patients.
Although the number of cases with CS due to adrenal adenoma (n = 27) was less, there was no significant difference between the patients with ACTH-dependent pituitary tumors or independent CS regarding clinical and hormonal parameters [sex, BMI, cortisol, ACTH, 1 mg DST, 2 days 2 mg DST, PTH, 25-OH vitamin D, calcium, phosphorous, osteocalcin and CTx levels, DEXA, duration of symptomatic disease, time since remission, but not UFC (p = 0.013)].
Assessment of vertebral fracture and predictors of vertebral fractures
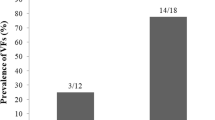
A history of any fracture was reported in ten cases. Radiologic vertebral fracture evaluations were available for 81 patients. Sixty-one (75.3%) patients had vertebral fragility fractures. Severe vertebral fractures (grade two and higher) were observed 69.1% (n = 56). Fourteen patients (22.2%) had grade 3 vertebral fractures. Symptomatic vertebral fractures were reported in 14 (17.2%) cases. All patients with vertebral fractures had thoracic fragility fractures, and 32 (50.7%) patients had lumbar fragility fractures. Fifty-four (85.7%) patients with vertebral fractures were women, 34 (62.9%) of whom were postmenopausal. Thirty-three (52.3%) patients with vertebral fractures had diabetes. There was no statistically significant difference between the vertebral fracture rate in patients with diabetes or without diabetes (p = 0.601).
There were no significant differences between patients with or without vertebral fractures and the clinical and laboratory parameters (sex, BMI, cortisol, ACTH, 1 mg DST, 2 days 2 mg DST, UFC, DHEAS, PTH, 25-OH vitamin D, calcium, the etiology of CS, and the duration of symptomatic disease) (p > 0.05, for all).
The median number of lumbar and thoracic vertebral fractures was 6 (min–max: 2–12). The median numbers of grade 1, grade 2, and grade 3 fractures were 3 (min–max: 2–7), 4 (min–max: 1–9), and 2 (min–max:1–3), respectively. Thirty-three patients with vertebral fractures (40.7%) had normal lumbar and femoral neck BMD. There was no significant difference between vertebral fracture frequency in patients in remission or patients with active disease (p = 0.603).
Multiple regression analysis showed that the vertebral fractures were independently associated with age, FSH, LH levels, and lumbar BMD (R2 = 68.18%, p = 0.028).
Assessment of bone mineral density
There were 55 (40.7%) patients with osteopenia and 22 (16.2%) patients with osteoporosis. Patients with CS had statistically significantly lower BMD and Z scores, both in the lumbar spine and femoral neck compared with the control group (Table 2). One hundred twenty-seven patients (85.2%) were under 25 hydroxyvitamin D replacement. While the patients did not use a bone medication at the initial evaluation, respectively, 14 and 8 patients were treated with bisphosphonate and denosumab in the follow-up.
Osteopenia and osteoporosis were detected in 40.3% and 20.9% of patients with diabetes, respectively, while the frequencies were 41.2% and 11.8% in non-diabetic patients. There was no statistically significant difference in the frequency of osteoporosis (p = 0.169) or osteopenia (p = 0.999) according to the presence of diabetes. The presence of diabetes was not associated with both femoral neck (p = 0.074) and lumbar spine (p = 0.216) BMD levels.
There were no significant differences between patients with osteoporosis or patients with normal DEXA and the clinical and laboratory parameters (sex, BMI, cortisol, ACTH, 1 mg DST, 2 days 2 mg DST, UFC, DHEAS, PTH, 25-OH vitamin D, calcium, the etiology of CS, duration of symptomatic disease, time since remission, the presence of secondary hypogonadism or GH deficiency) (p > 0.05, for all).
Multiple regression analysis showed that the femoral neck BMD, not lumbar BMD, was independently associated with age, BMI, and PTH levels (R2 = 48.48%, p < 0.001).
Assessment of patients according to remission status
Seventy two patients (53.3%) were in remission and the median remission period was 36.5 months (min–max: 3–310). Mean follow-up time was higher in patients with remission than active disease group [55.6 ± 44.8 months; vs. 36.3 ± 35.6 months, (p = 0.006)]. Patients in remission had a shorter symptomatic disease period compared to active disease group [29.5 months (min–max: 3–205); vs. 50.3 months (min–max: 3–260), (p = 0.001)]. There was no significant difference between patients with remission status regarding clinical parameters (sex, age, BMI).
While patients in remission had higher femoral BMD compared with patients with active disease (p = 0.007), there were no significant differences between the lumbar spine BMD levels in these groups of patients. In the second year follow-up, patients in remission did not show a statistically significant increase in BMD compared to baseline (p = 0.807).
Discussion
In this cross-sectional study, vertebral fragility fracture frequency was 75.3%. All patients with vertebral fractures had thoracic fragility fractures, and 50.7% of the patients had lumbar fragility fractures. The median number of vertebral fractures was 6 (min–max: 2–12). Thirty-three (40.7%) patients with vertebral fractures had normal lumbar and femoral BMD levels on DEXA. Osteoporosis and osteopenia were observed in 16.2% and 40.7% of patients. Lumbar spine and femoral neck BMD were lower compared with the control group. The origin of CS, whether ACTH-dependent pituitary tumors or independent CS, did not influence the presence of vertebral fractures.
The prevalence of osteoporosis was reported to be higher in patients with adrenal CS than in those with pituitary CS in previously published reports [10, 17]. Normal levels of DHEAS have been held responsible for the protective effect in patients with pituitary-dependent CS [17]. Contrary to the literature, we did not find a difference between BMD levels in these two groups. DHEAS levels were not also differed in our study. While adrenal CS and pituitary CS were reported to be approximately equal in Asia, pituitary CS was reported to be 5 to 6 times more common than adrenal CS in Europe and the United States [10]. The small number of patients with adrenal Cushing's syndrome in our study renders it difficult to derive definitive conclusions. In addition, in Minetto et al.'s study patients with adrenal CS were older compared to our group. The diverse distribution of CS between populations may be responsible for different BMD levels and determining different prevalences of osteoporosis.
In van der Eerden et al.’s study, 82% of the patients had osteopenia, and 31% had osteoporosis [18]. The prevalence of osteoporosis is reported as ranging from 40 to 70% in patients with CS [6, 7, 19]. In our study, although osteoporosis and osteopenia rates were less compared with the literature, a higher prevalence of vertebral fractures (75.3%) was found, regardless of the origin of CS or the presence of osteoporosis and osteopenia. Tauchmanova et al. [5] also reported a high prevalence of vertebral fractures (76%). Valassi et al. reported the frequency of vertebral fractures as 65.1% (n = 56) in 86 patients with pituitary and adrenal CS. Randazzo et al. evaluated 14 patients with vertebral radiographs, grade 1 vertebral fractures were detected in all patients, and moderate fractures were present in 28% of the patients [20]. In a study with subclinical hypercortisolism conducted by Chiodini et al. [6], the vertebral fracture prevalence was reported as 43% in premenopausal and 78% in postmenopausal women. In a study by Tauchmanova et al. that only included women, vertebral fractures were detected in 69% and 57% of patients with overt and subclinical CS, respectively [9]. Faggiano et al. [21], Belaya et al. [11], Futo et al. [4], Ohmori et al. [10], and Trementino et al. [12] reported vertebral fractures as 55.5%, 38.4%, 37%, 21.4%, and 15.3% in patients with endogenous CS, respectively. In the European Registry study, 25% of patients with normal BMD had bone fractures [19], in our study, it was 40.7%.
The symptomatic fracture ratio was stated as 52% in Tauchmanova et al. [5] study. In Szappanos et al. study symptomatic vertebral fracture was detected in 25% of patients [8]. In a study with female CS, the clinically evident fractures were 28% and 11.4% in overt and subclinical CS, respectively [9]. Symptomatic vertebral fractures were present in 17.2% of cases in our study.
Similar to the literature, lower lumbar spine BMD was found as a predictor of vertebral fractures in our study [5, 9, 12]. In the European Registry study, bone fractures were detected more frequently in patients with osteoporosis [19]. Contrary Belaya et al. [11] found no association between lumbar BMD and vertebral fractures.
Trementino et al. stated that UFC levels were correlated with vertebral fractures and the duration of active disease was positively correlated with peripheral fractures, but not with vertebral fractures [12]. Futo et al. reported that the estimated duration of glucocorticoid excess was higher in patients with bone fractures [4]. While Futo et al. [4] and Valassi et al. [19] evaluated the presence of vertebral fractures with previous medical documentations, the rest of the studies [9, 11, 12] used the semi-quantitative method. In our study, there was no difference in the duration of active disease between patients with or without fractures (p = 0.129). On the other hand, the retrospective nature of our study renders it difficult to determine the exact timing of active disease and could have biased our results. The underlying causes of the discrepancy between the results might be due to the difference between the study groups and the difference between the number of cases of childhood-onset.
There are contradictory results in the literature about parameters that affect bone density and vertebral fractures. Tauchmanova et al. reported that morning cortisol and total testosterone levels were correlated with lumbar BMD but not with the prevalence of fractures [5]. Minetto et al. and Ohmori et al. stated that DHEAS levels were correlated with BMD both in the femoral neck and lumbar spine [17]. In a study by Tauchmanova et al. [9], lumbar spine BMD and the cortisol to DHEAS ratio were found as predictors of vertebral fracture. Futo et al. found no correlation between BMD or fractures and cortisol levels [4]. In the van der Eerden et al. study [18], UFC levels or estimated duration of symptomatic disease did not affect BMD and Z scores. We found no correlation between BMD and morning cortisol, UFC, and DHEAS levels.
There are limited numbers of studies that investigated the effect of remission on BMD in long-term follow-up, and it was found that only a small number of patients, including 10-year follow-up, recovered normal BMD [20, 22,23,24,25]. After the remission of CS, an increase in BMD, mostly lumbar spine, was reported as ranging from 14 to 79% [23, 26], and Kristo et al. stated that an increase in BMD was time-dependent [22]. In our study, femoral neck BMD was statistically higher in patients in remission than in patients with active disease, but it was not observed in the lumbar spine. We didn’t find a statistically significant increase in BMD in 2-year follow-up. In the previous reports, several factors such as female sex, the severity of the disease, time elapsed since cure, glucocorticoid exposure time have been reported to potentially affect bone damage and the time to bone recovery after hypercortisolism resolution [4, 23, 24, 27].
The major limitation of this study is that the evaluation of vertebral fractures was made using a semi-quantitative method. Secondly, we evaluated retrospectively, and for comparing BMD scores, and making a precise interpretation between groups, larger prospective studies with longer follow-up are needed.
In conclusion, vertebral fracture frequency was higher in CS patients compared with age-matched controls. Most of the patients with vertebral fractures had multiple fractures, and thoracic vertebras were involved in all cases with vertebral fractures. Lumbar BMD was found as an independent factor associated with vertebral fractures. Patients with CS with normal BMD could experience vertebral fractures. Vertebral radiograph evaluations as a part of routine evaluation for silent vertebral fractures may help to prevent further fractures in patients with CS.
References
Kaltsas G, Manetti L, Grossman AB (2002) Osteoporosis in Cushing’s syndrome. Front Horm Res 30:60–72. https://doi.org/10.1159/000061073
Rahaman SH, Jyotsna VP, Kandasamy D, Shreenivas V, Gupta N, Tandon N (2018) Bone health in patients with Cushing’s syndrome. Indian J Endocrinol Metab 22(6):766–769. https://doi.org/10.4103/ijem.IJEM_160_18
Vestergaard P, Lindholm J, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, Brixen K, Kristensen LO, Feldt-Rasmussen U, Mosekilde L (2002) Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol 146(1):51–56. https://doi.org/10.1530/eje.0.1460051
Futo L, Toke J, Patocs A, Szappanos A, Varga I, Glaz E, Tulassay Z, Racz K, Toth M (2008) Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing’s syndrome. Osteoporos Int 19(7):941–949. https://doi.org/10.1007/s00198-007-0514-x
Tauchmanova L, Pivonello R, Di Somma C, Rossi R, De Martino MC, Camera L, Klain M, Salvatore M, Lombardi G, Colao A (2006) Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab 91(5):1779–1784. https://doi.org/10.1210/jc.2005-0582
Chiodini I, Torlontano M, Carnevale V, Trischitta V, Scillitani A (2008) Skeletal involvement in adult patients with endogenous hypercortisolism. J Endocrinol Investig 31(3):267–276. https://doi.org/10.1007/BF03345601
Arnaldi G, Mancini T, Tirabassi G, Trementino L, Boscaro M (2012) Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J Endocrinol Investig 35(4):434–448. https://doi.org/10.1007/BF03345431
Szappanos A, Patocs A, Toke J, Boyle B, Sereg M, Majnik J, Borgulya G, Varga I, Liko I, Racz K, Toth M (2009) BclI polymorphism of the glucocorticoid receptor gene is associated with decreased bone mineral density in patients with endogenous hypercortisolism. Clin Endocrinol 71(5):636–643. https://doi.org/10.1111/j.1365-2265.2009.03528.x
Tauchmanova L, Pivonello R, De Martino MC, Rusciano A, De Leo M, Ruosi C, Mainolfi C, Lombardi G, Salvatore M, Colao A (2007) Effects of sex steroids on bone in women with subclinical or overt endogenous hypercortisolism. Eur J Endocrinol 157(3):359–366. https://doi.org/10.1530/EJE-07-0137
Ohmori N, Nomura K, Ohmori K, Kato Y, Itoh T, Takano K (2003) Osteoporosis is more prevalent in adrenal than in pituitary Cushing’s syndrome. Endocr J 50(1):1–7. https://doi.org/10.1507/endocrj.50.1
Belaya ZE, Hans D, Rozhinskaya LY, Dragunova NV, Sasonova NI, Solodovnikov AG, Tsoriev TT, Dzeranova LK, Melnichenko GA, Dedov II (2015) The risk factors for fractures and trabecular bone-score value in patients with endogenous Cushing’s syndrome. Arch Osteoporos 10:44. https://doi.org/10.1007/s11657-015-0244-1
Trementino L, Appolloni G, Ceccoli L, Marcelli G, Concettoni C, Boscaro M, Arnaldi G (2014) Bone complications in patients with Cushing’s syndrome: looking for clinical, biochemical, and genetic determinants. Osteoporos Int 25(3):913–921. https://doi.org/10.1007/s00198-013-2520-5
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93(5):1526–1540. https://doi.org/10.1210/jc.2008-0125
Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH (2016) Hormonal replacement in hypopituitarism in adults: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101(11):3888–3921. https://doi.org/10.1210/jc.2016-2118
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S (2011) Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(6):1587–1609. https://doi.org/10.1210/jc.2011-0179
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Minetto M, Reimondo G, Osella G, Ventura M, Angeli A, Terzolo M (2004) Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int 15(11):855–861. https://doi.org/10.1007/s00198-004-1616-3
van der Eerden AW, den Heijer M, Oyen WJ, Hermus AR (2007) Cushing’s syndrome and bone mineral density: lowest Z scores in young patients. Neth J Med 65(4):137–141
Valassi E, Santos A, Yaneva M, Toth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM, Group ES (2011) The European Registry on Cushing’s syndrome: 2-year experience baseline demographic and clinical characteristics. Eur J Endocrinol 165(3):383–392. https://doi.org/10.1530/EJE-11-0272
Randazzo ME, Grossrubatscher E, Dalino Ciaramella P, Vanzulli A, Loli P (2012) Spontaneous recovery of bone mass after cure of endogenous hypercortisolism. Pituitary 15(2):193–201. https://doi.org/10.1007/s11102-011-0306-3
Faggiano A, Pivonello R, Filippella M, Di Somma C, Orio F Jr, Lombard G, Colao A (2001) Spine abnormalities and damage in patients cured from Cushing’s disease. Pituitary 4(3):153–161. https://doi.org/10.1023/a:1015362822901
Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J (2006) Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing’s syndrome: a prospective, long-term study. Eur J Endocrinol 154(1):109–118. https://doi.org/10.1530/eje.1.02067
Manning PJ, Evans MC, Reid IR (1992) Normal bone mineral density following cure of Cushing’s syndrome. Clin Endocrinol 36(3):229–234. https://doi.org/10.1111/j.1365-2265.1992.tb01437.x
Hermus AR, Smals AG, Swinkels LM, Huysmans DA, Pieters GF, Sweep CF, Corstens FH, Kloppenborg PW (1995) Bone mineral density and bone turnover before and after surgical cure of Cushing’s syndrome. J Clin Endocrinol Metab 80(10):2859–2865. https://doi.org/10.1210/jcem.80.10.7559865
Luisetto G, Zangari M, Camozzi V, Boscaro M, Sonino N, Fallo F (2001) Recovery of bone mineral density after surgical cure, but not by ketoconazole treatment, in Cushing’s syndrome. Osteoporos Int 12(11):956–960. https://doi.org/10.1007/s001980170025
Di Somma C, Pivonello R, Loche S, Faggiano A, Klain M, Salvatore M, Lombardi G, Colao A (2003) Effect of 2 years of cortisol normalization on the impaired bone mass and turnover in adolescent and adult patients with Cushing’s disease: a prospective study. Clin Endocrinol 58(3):302–308. https://doi.org/10.1046/j.1365-2265.2003.01713.x
Kawamata A, Iihara M, Okamoto T, Obara T (2008) Bone mineral density before and after surgical cure of Cushing’s syndrome due to adrenocortical adenoma: prospective study. World J Surg 32(5):890–896. https://doi.org/10.1007/s00268-007-9394-7
Acknowledgements
This study did not receive any specific grants from any funding agencies in the public, commercial, or nonprofit sector.
Funding
This study did not receive any specific grants from any funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data, participated in drafting the article or revising it critically for important intellectual content, and gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
Tugce Apaydin and Dilek Gogas Yavuz declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
All patients provided their consent for the use of their personal and medical information in the publication of this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Apaydın, T., Yavuz, D.G. Assessment of non-traumatic vertebral fractures in Cushing’s syndrome patients. J Endocrinol Invest 44, 1767–1773 (2021). https://doi.org/10.1007/s40618-020-01496-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01496-y