Abstract
Summary
In a cohort study of 182 consecutive patients with active endogenous Cushing’s syndrome, the only predictor of fracture occurrence after adjustment for age, gender bone mineral density (BMD) and trabecular bone score (TBS) was 24-h urinary free cortisol (24hUFC) levels with a threshold of 1472 nmol/24 h (odds ratio, 3.00 (95 % confidence interval (CI), 1.52–5.92); p = 0.002).
Introduction
The aim was to estimate the risk factors for fracture in subjects with endogenous Cushing’s syndrome (CS) and to evaluate the value of the TBS in these patients.
Methods
All enrolled patients with CS (n = 182) were interviewed in relation to low-traumatic fractures and underwent lateral X-ray imaging from T4 to L5. BMD measurements were performed using a DXA Prodigy device (GEHC Lunar, Madison, Wisconsin, USA). The TBS was derived retrospectively from existing BMD scans, blinded to clinical outcome, using TBS iNsight software v2.1 (Medimaps, Merignac, France). Urinary free cortisol (24hUFC) was measured by immunochemiluminescence assay (reference range, 60–413 nmol/24 h).
Results
Among enrolled patients with CS (149 females; 33 males; mean age, 37.8 years (95 % confidence interval, 34.2–39.1); 24hUFC, 2370 nmol/24 h (2087–2632), fractures were confirmed in 81 (44.5 %) patients, with 70 suffering from vertebral fractures, which were multiple in 53 cases; 24 patients reported non-vertebral fractures. The mean spine TBS was 1.207 (1.187–1.228), and TBS Z-score was −1.86 (−2.07 to −1.65); area under the curve (AUC) was used to predict fracture (mean spine TBS) = 0.548 (95 % CI, 0.454–0.641)). In the final regression model, the only predictor of fracture occurrence was 24hUFC levels (p = 0.001), with an increase of 1.041 (95 % CI, 1.019–1.063), calculated for every 100 nmol/24-h cortisol elevation (AUC (24hUFC) = 0.705 (95 % CI, 0.629–0.782)).
Conclusions
Young patients with CS have a low TBS. However, the only predictor of low traumatic fracture is the severity of the disease itself, indicated by high 24hUFC levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragility, or low traumatic fractures, which are fractures associated with either minimal or no discernible trauma, is a common complication of endogenous Cushing’s syndrome (CS) [1]. In fact, the first description of glucocorticoid-induced osteoporosis (GIO) was provided by Harvey Cushing in 1932, along with the clinical presentation of patients with endogenous hypercortisolism caused by an adrenocorticotropin (ACTH)-producing pituitary adenoma [2]. Currently, CS remains a relatively rare endocrine disorder characterized by specific clinical features associated with prolonged exposure to inappropriately high levels of cortisol produced by the adrenal glands due to a pituitary corticotroph tumor, ectopic ACTH production from tumor outside the pituitary, or autonomous adrenal overproduction [1]. Owing to the rarity of endogenous CS (2–10 cases per million per year), data related to the prevalence of low traumatic fractures, specific risk factors, intervention thresholds, and osteoporosis management in these patients vary considerably and are generally limited [3]. In contrast to exogenous hypercortisolism in which the rate of glucocorticoid (GC) prescription rises with age and is greatest in senior populations [4], patients with endogenous CS are usually young [1]. These patients can be treated, with up to 80 % achieving full recovery [5]. Fragility fractures significantly decrease quality of life and functional performance in patients with active CS, as compared with patients with CS without fractures, but with other health complications [6]. Furthermore, some bone deformities, functional decline, and decreased quality of life remain for at least 1 year after recovery in patients with CS who sustained fragility fractures during active disease [7].
As described in numerous clinical case reports involving multiple severe fractures in CS patients, bone mineral density (BMD) decline is unexpectedly mild [8, 9]. The deleterious effects of GC on bone remodeling are mainly realized through a marked decrease in bone formation with a slight increased or unchanged bone resorption markers [10–12], which might explain the relatively subtle decreases observed in BMD. Consequently, non-invasive methods to assess bone microarchitecture and bone quality, rather than just BMD and bone mineral content, are clearly desirable in these patients. The trabecular bone score (TBS) is a relatively novel method that can be applied to dual-energy X-ray absorptiometry (DXA) images to perform gray-level textural analysis to estimate trabecular microarchitecture [13]. Using experimental variograms of two-dimensional (2D) projection images, TBS differentiates between three-dimensional (3D) bone structures that exhibit the same areal bone mineral density (aBMD) but with a different trabecular microarchitecture [14]. In clinical studies, TBS enhances the ability of DXA to predict fracture risk in postmenopausal women, is capable of predicting osteoporotic fractures independent of areal BMD, and, in some cases, helps to define a subset of non-osteoporotic women at high risk of fracture [15–19]. Recently, TBS was shown to be useful in the clinical assessment of trabecular microstructure in patients with diabetes [20], primary hyperparathyroidism [21, 22], and adrenal incidentalomas [23]. We hypothesized that TBS may be predictive for fractures as a marker of bone microarchitecture in patients with active endogenous CS.
The purposes of this study were to identify risk factors for fracture in patients with endogenous Cushing’s syndrome and to evaluate the value of bone microarchitecture as assessed by TBS in this cohort of patients.
Subjects and methods
The Institutional Review Board of the National Research Center for Endocrinology (NRCE) approved the study protocol.
In this retrospective cohort study, 182 consecutive patients with clinically evident and biochemically proven endogenous CS, who provided formal informed consent, were enrolled.
All patients were questioned and evaluated at the time of hospital admission, when the diagnosis of Cushing’s syndrome was biochemically established. The mean estimated time from the first symptoms to the final diagnosis of CS was 2–3 years.
Subjects has had CS confirmed by at least two of the following tests: 24-h urinary free cortisol (24hUFC; reference range, 60–413 nmol/24 h), late-night serum cortisol at 23:00 (reference range, 46–270 nmol/l), low-dose dexamethasone suppression test (DST; suppression threshold for serum cortisol level, 50 nmol/l) [1], and (in some patients only) late-night salivary cortisol (reference range, 0.5–9.4 nmol/l) [24]. Serum and saliva samples were assayed by electrochemiluminescence assay (ECLIA) Cobas e601 Roche. 24hUFC was measured by immunochemiluminescence assay (extracted with diethyl ether) on a Vitros ECi analyzer.
Exclusion criteria: any other cause of secondary osteoporosis at present or in a 2-year medical history [25] or any prolonged treatment with medicines documented to influence bone metabolism in humans [25, 26] during the previous 12 months, including treatment with antiresorptive or anabolic compounds for osteoporosis, or prolonged exogenous glucocorticoids, systematic alcohol abuse, pregnancy, and terminal conditions. In a subgroup of patients, who had provided serum samples for bone metabolism markers, any previous treatment to resolve hypercortisolism was excluded.
Height was measured by a stadiometer, and body mass index (BMI) was calculated as kilograms per square meter.
All patients had anteroposterior and lateral radiographs of vertebrae T4-L5 via a standardized protocol [27]. All available vertebrae were assessed by an experienced radiologist through the visual inspection of lateral spinal images, without direct vertebral measurement, as being either normal or deformed if visual inspection perceived at least a 20 % reduction in vertebral height (anterior, posterior, or middle) per the semi-quantitative method [27]. In all patients with a single vertebral deformity, or in event of hesitation, a consensus was reached between two radiologists. Axiom Icons R200 “Siemens” was used to perform the X-ray. Rib fractures were detected through routine chest X-rays, which are performed on all hospitalized patients at the NRCE.
Subjects were questioned about any other fractures they might have sustained associated with either no or minimal trauma, including falling from a standing height at the same level, which happened during an active stage of their CS. Only low traumatic fractures were registered. All non-vertebral fractures were cured at the time of admission and had happened 1–2 years before hospitalization. There was no precise data for the time of the vertebral fracture’s occurrence. Patients who had a fracture due to Cushing’s syndrome were compared with those patients who had not sustained a fracture while suffering from the same disorder.
DXA and any other medical tests provided were done during hospitalization.
Bone mineral density measurements (BMD) were performed at L1–L4, the femoral neck, and total hip using a DXA Prodigy device (GEHC Lunar, Madison Wisconsin, USA). Quality control procedures were carried out, in accordance with the manufacturers’ recommendations. Since the vast majority of patients were of premenopausal age or younger than 50, in this paper, we presented the value of BMD loss as a Z-score using the criteria in the International Society for Clinical Densitometry’s official position statement: Z-score values of −2.0 standard deviations (SD) or lower are stated “below the expected range for age” and those above −2.0 SD “within the expected range for age” [28].
Blinded to clinical outcomes, TBS was derived retrospectively from existing BMD scans using TBS iNsight software v2.1 (Medimaps, Merignac, France). In all patients, TBS was assessed in the region of the lumbar spine BMD. The mean value of the vertebrae from L1 to L4 was calculated in an attempt to exclude any fractured and/or arthrosed vertebrae according to ISCD guidelines. In order to normalize TBS value for age, data was reported as a Z-score calculated based on the “Manufacturer European reference curve.” In addition to this, TBS values were classified as follows: TBS ≤1.2 were considered as degraded microarchitecture, between 1.20 and 1.35—partially degraded microarchitecture, and ≥1.35 normal [18, 19]. This classification was initially suggested for postmenopausal women. However, for research purposes, we considered it appropriate to apply this classification to our relatively young individuals as the prevalence of fractures in our cohort was even greater than expected in postmenopausal women.
Fasting serum samples were taken for osteocalcin (OC), carboxyterminal cross-linked telopeptide of type I collagen (CTx), and vitamin D (25OHD3) in a random subgroup of patients (n = 139). Both bone remodeling marker levels were assayed by electrochemiluminescence assay (ECLIA; Cobas e601 Roche). 25OHD3 levels were measured by immunochemiluminescence assay (Liaison).
Statistical analysis
Descriptive statistics are expressed as means, SD of the mean, and 95 % confidence intervals (CI) for the mean for all parameters with the exception of bone remodeling markers, whereby the values of which are presented as medians and interquartile ranges. Qualitative parameters are presented as percentages with exact binomial 95 % confidence intervals. The ANOVA test was utilized to compare continuous variables from two independent samples, while Fisher’s exact test was used for categorical variables. A Spearman’s rank correlation coefficient was calculated to assess the correlation between continuous variables. A binary logistic regression model was created with the presence/absence of fracture as the dependent variable, and patient age, gender, BMI, BMD, TBS, 24hUFC, and osteocalcin levels were entered as independent.
The threshold for 24hUFC level was calculated using receiver-operator curve (ROC) analysis. The choice of threshold was based on the highest test available, sum of sensitivity and specificity. Odds ratio (OR) for the identified threshold with 95 % confidence interval was calculated to quantitatively assess any factor influence according to the procedure described by Glas et al. [29].
All tests were two sided, with a p value of less than 0.05 considered statistically significant. SPSS 16.0 Inc software was used for all analyses.
Results
The general characteristics of the 182 enrolled patients with CS are summarized in Table 1. It was a typical cohort of patients in relation to comorbidities of CS, the most prevalent of which with the exception of osteoporosis was as follows: 77 % (arterial hypertension), 22 % (diabetes), 29 % had mental disorders mostly depression, and 11 % (infection). Practically, all females had some menstrual abnormalities (65.8 % amenorrhea, 31.5 % menstrual irregularity, and 2.7 % normal menses).
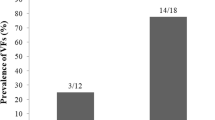
Fractures were identified in 81 (44.5 %) out of 182 patients, including vertebral fractures in 70 (in 17 patients, a single vertebra, and in 53, multiple vertebrae) and non-vertebral fractures in 24 subjects. All types of identified and reported fractures are listed in Fig. 1.
Eleven patients had non-vertebral fractures only. All others had vertebral fractures or both vertebral and non-vertebral. Rib fractures only were detected in four patients, a tibia was broken in one patient, while a fractured wrist was reported by three patients, fractured sternum and tibia by two patients, and both rib and metatarsal fractures by a single patient. Multiple vertebral and non-vertebral fractures together were registered in 13 cases: a single vertebral fracture coexisted with a metatarsal fracture in two patients and with a rib fracture in one case. Multiple vertebral fractures were present in eight patients with rib fractures. Similarly, multiple vertebral fractures coexisted with a hip fracture in one patient and a jaw fracture in another. In one patient, we verified two vertebral compressions as well as rib and tibia fractures.
To identify significant risk factors for fragility fractures in patients with CS, we compared the clinical presentation of patients with and without fractures. These results are summarized in Table 2.
Male patients suffered from fractures significantly more frequently (22 of 33, 66.7 %) than females (58 of 149, 40.5 %; p = 0.004), although practically all females had some menstrual abnormalities. This gender difference might be explained by higher 24hUFC levels—3849 nmol/24 h (3046–4652) in males vs. female patients—2052 nmol/24 h (1788–2316; p < 0.001). Similarly, patients with ACTH-ectopic syndrome had statistically higher 24hUFC levels—3243 nmol/24 h (2421–4064) and fracture rate—77 % as compared with Cushing’s disease patients with 24hUFC—2225 nmol/24 h (1919–2530) and fracture rate—39.7 % (p = 0.023 and p < 0.001, respectively). The diagnostic performance of DXA parameter measurements was as follows: AUC: mean spine TBS, 0.548 (0.454–0.641); mean spine BMD, 0.637 (0.545–0.729); and neck BMD, 0.609 (0.517–0.700).
The combined effect of several variables presumably related to fracture risk in patients with CS (age, gender, mean spine TBS, L1–L4 BMD, femoral neck BMD, total hip BMD, and 24hUFC) was investigated using binary logistic regression. The analysis was performed separately for highly correlated variables like BMD in different parts of the skeleton, the Z-score, and BMI to avoid multicollinearity. In the final regression model, the only predictor of fracture occurrence was 24hUFC levels (p = 0.001), with an increase of 1.041 (95 % CI, 1.019–1.063) or 4.1 %, calculated for every 100 nmol free cortisol per 24-h increase. AUC for 24hUFC to identify patients with fractures was 0.705 (95 % CI, 0.629–0.782). A 24hUFC threshold of 1472 nmol/24 h was identified by ROC analysis with a sensitivity of 75.6 % (95 % CI, 65.1–83.8) and specificity of 54.0 % (95 % CI, 44.3–63.4). Adjusting for gender and BMD, the odds of fracture in patients with a 24hUFC more than 1472 nmol/24 h was 3.00 (95 % CI, 1.52–5.92; p = 0.002) greater vs. CS patients with a lower 24hUFC. The separate analysis of patients with non-vertebral fractures only (n = 11) did not give any additional information.
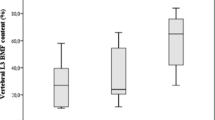
Despite the severe clinical presentation of GIO, as in so many cases with multiple vertebral and non-vertebral fractures, the mean of BMD Z-score was above -2.0 in all three measured regions of the skeleton, not “below the expected range for age.” Age-adjusted Z-score calculated for TBS was lower as compared with BMD Z-score, but similarly to BMD, it was not predictive of fractures. The BMD and TBS results for the whole cohort of patients are summarized in Table 3. Nevertheless, in these young patients, absolute values of TBS demonstrated a mainly degraded (49.5 %) or partially degraded (34 %) microarchitecture range using a classification previously defined for postmenopausal women [18, 19]. Meanwhile, the percentage of patients with BMD “below the expected range for age” was low, especially for the femoral neck and total hip (Fig. 2), it was even less if using a T-score (data is not presented).
In a subgroup of patients (n = 139; 65 with and 74 without fracture), markers of bone turnover were measured. Osteocalcin was lower in patients with factures (6.2 ng/ml, 3.3–8.0) vs. those without (8.3 ng/ml, 4.8–12.1; p = 0.005), whereas cross-linked telopeptide of type I collagen (CTx) levels did not differ (0.42 ng/ml, 0.23–0.53 vs. 0.36 ng/ml, 0.24–0.54; p = 0.45). A moderate inverse correlation was identified between 24hUFC and osteocalcin (ρ o = −0.5; p < 0.001), with a weak correlation noted between 24hUFC and CTx (ρ o = 0.33, p < 0.05). Note that osteocalcin dropped out of the final binary logistic model (p = 0.94), and therefore, it was entirely dependent on urinary free cortisol levels. In the same cohort of patients, vitamin D was measured, which was low (12.4 ng/ml (8–20.0)) but did not differ between subjects with or without fractures (p = 0.754).
Discussion
This study identifies the only predictor of fragility fractures in a sizeable number of young patients (n = 182; mean age, 37 years) with active endogenous Cushing’s syndrome and assesses the value of the TBS in this cohort of patients. A TBS Z-score (mean value, −1.86 (1.43)) presented in patients with endogenous cortisol excess seems lower as compared with spine BMD Z-score (−1.6 (1.26)) and hip BMD Z-score (−0.58 (1.12)). The absolute value of mean spine TBS at 1.207 (1.187–1.228) was also lower than expected in a healthy control (>1.350) reported elsewhere [18, 19]. Recently, Eller-Vanicher et.al. reported the TBS value in 34 patients with subclinical CS among 102 patients with adrenal incidentalomas in an older population (mean age, 67 years). The value of TBS was lower than even in subclinical CS as compared with patients without biochemical disturbances in cortisol levels [23]. Forty of these patients, including eight with subclinical hypercortisolism, were followed longitudinally for 24 months, and TBS was predictive for vertebral fracture incidence even after the exclusion of patients with subclinical CS, reflecting the predictive value of TBS in an elderly population with adrenal incidentalomas. Severe deterioration in TBS value at the diagnosis of a 34-year-old man with Cushing’s disease improved to normal value faster than BMD in a recently published single case report [30]. These findings support the theory that the predominant deleterious effect of GC on bone is upon its microstructure rather than quantity of bone (bone mass, bone mineral density).
However, neither BMD nor TBS was predictive of fractures in our young cohort of patients with active endogenous hypercorticism. The level of free cortisol overproduction measured in 24-h urine sample was the only predictor of low traumatic fracture occurrence among tested clinical risk factors in patients with active Cushing’s syndrome. This result differs from a few previous retrospective studies of patients with endogenous CS, which suggested a history of previous fractures [31], patient age, and estimated disease duration [32], as well as BMD at the lumbar spine [33] and forearm [34] predictive of fractures in case-control settings. Multivariable analysis was used only in the most populous of these studies by Tauchmanova et al. [33], estimating the best predictors of vertebral fracture among 80 patients with CS. According to this study, the best predictor of vertebral fractures was the lumbar spine BMD, irrespective of the etiology of hypercorticism or the gonadal status of the women [33]. However, this study focused on vertebral fractures only. In our multivariate evaluation in which patient’s gender, age, BMI, BMD, and TBS were entered as independent variables, the main predictor of fracture was the 24hUFC level (p < 0.001). Being male was clearly a risk factor; however, 24hUFC influenced fracture occurrence independently of gender. Moreover, in this cohort of patients, males had significantly higher 24hUFC levels than females, it being likely the adrenal glands’ ability to produce cortisol in response to high ACTH levels is greater in males than females. This explains the more severe clinical presentation of endogenous CS in men, including the development of GIO and fragility fractures. BMD was slightly lower in patients with fractures than in those without them, similarly to the previous study [33, 34], but it could not explain the extremely high fracture rate and was not predictive of fracture occurrence after applying multivariate analysis. It is interesting to note that in patients with exogenous hypercortisolism, the dose of GC may be not only the best but also the only significant predictor of fragility fracture [35, 36]. In a very large cohort of 42,500 men and women followed for 176,000 patient-years, previous corticosteroid use was associated with a significantly increased risk of any fracture, of osteoporotic fracture, and of hip fracture when adjusted for BMD. No significant difference in risk was evident between men and women, and the risk was independent of age and prior fracture. The risk was marginally and non-significantly upwardly adjusted when BMD was excluded from the model. The effect of GC on fracture risk was dose dependent: at a >7.5-mg daily dose of prednisolone or its equivalent, the relative risk of vertebral fracture was 5.2, whereas for a dose between 5 and 7.5 mg daily, the relative risk was half that, at 2.6 [36]. Exogenous CS differs from endogenous CS due to the various causative disorders, independently influencing the bone remodeling, types of synthetic glucocorticoids, dose and patients’ adherence to treatment, etc. Nevertheless, similarly, in the present study of endogenous CS, the odds of fracture were three times greater in patients with 24hUFC higher than 1500 nmol/24 h vs. the odds in patients with lower levels of 24hUFC.
The prevalence of low traumatic fractures, 44.5 % reported in our study, differs from that previously established, which varies from 21 % [37] to 76 % [33] among published studies. A certain difference in the prevalence of fragility fractures in patients with CS reported in the literature might be related to discrepancies in the primary outcomes and design of the various studies. In an Italian clinical study in which routine thoraco-lumbar spine radiographs were systematically examined focusing specifically on vertebral deformities in patients with endogenous CS, vertebral fractures were detected in 61 of 80 (76 %) consecutively evaluated patients with CS; in 52 patients, there were multiple fractures, though they were only clinically evident in 32 [33]. Tauchmanova et al. [33] used a different method to assess vertebral body deformities than we did, including a spine deformity index [38], whereas we applied the semi-quantitative assessment scheme suggested by Genant et al. [27]. Investigators behind a Hungarian study reported 23 symptomatic vertebral and 25 non-vertebral fractures, which occurred in 24/68 (35 %) of their patients with CS over a 5-year period of time before the diagnosis of endogenous CS, but no fractures once CS was successfully treated [32]. Similar to both these results and the presently reported findings, we recently published a chapter documenting fragility fractures in 45 % of 217 Russian patients with CS [39]. In the ERCUSYN database, fractures were evident in 21 % of the CS patients [37]; however, this last low figure might have underestimated the real prevalence due to the asymptomatic nature of some fractures and the fact that bone complications were not the primary goal of the ERCUSYN study. Undoubtedly, endogenous CS increases the risk of fracture relative to that observed in those who are healthy. This was confirmed in a survey in which self-administered questionnaires were sent to 125 patients suffering from CS in Denmark. With an overall response rate of 83 %, the authors identified a markedly increased risk within the 2 years prior to diagnosis (incidence rate ratio (IRR), 6.0; 95 % CI, 2.1–17.2). The subjects also had more low-energy fractures than the control (relative risk (RR), 5.4; 1.4–20.1). The risk of fracture did not depend upon the underlying etiology of hypercorticism [31].
Similar to other studies [10–12], we detected decreased levels of bone formation markers, which were more profound in patients with low-traumatic fractures. Nevertheless, the osteocalcin levels were entirely dependent on the 24hUFC levels and its suppression, thereby secondary to the severity of hypercorticism.
The current study has certain strengths and limitations. A reasonably sized cohort of patients, the largest to date, specifically designed to estimate fractures in patients with CS, enabled us to perform multilevel analysis of fracture predictors despite the rarity of this disorder. Focusing on bone complications in patients with CS helps to estimate the prevalence of fractures, which might be underestimated even in studies involving larger cohorts in which lateral X-rays were not performed for every patient. On the other hand, we must also admit to certain obvious limitations, the foremost being that the study was retrospective as we registered the fractures that had occurred before admission to our hospital and retrospectively evaluated TBS values, which were likely falsely elevated by the vertebral body deformities that were so common in these patients. Moreover, we enrolled newly diagnosed patients, but the duration of disease, though always more than 6 months, might have varied considerably thereby influencing fracture rate. At the same time, some of these limitations are inevitable. Given the severity and life-threatening clinical presentation of endogenous CS, the treatment is provided along with the diagnosis in the vast majority of patients. This makes the prospective evaluation of patients with active endogenous CS without treatment unethical and clearly impossible. Any estimate of the exact duration of hypercorticism is usually speculative.
In conclusion, patients with endogenous CS have a very high prevalence of fragility fractures (44.5 %), which predominantly involves vertebral body compression. In addition, once one fracture occurs, the likelihood of more is great as the vast majority of our patients with fractures had multiple ones. At the time of clinical presentation, CS patients generally appear to have a low TBS value, decreased levels of bone formation markers, and yet just a minimal decrease in BMD. Neither TBS nor BMD was predictive for fractures. The only predictor of prior low-trauma fracture appears to be the severity of hypercorticism measured via 24hUFC levels, out-influencing all other factors, including patient’s gender, age, BMI, BMD, TBS, and the degree of bone formation marker suppression. Consequently, patients with 24hUFC value higher than 1500 nmol/24 h should be considered candidates for antiosteporotic treatment in the event of hypercorticism not being able to be urgently resolved.
References
Nieman LK, Biller BMK, Finding JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540
Cushing H (1932) The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp 50:137–195
Kaltsas G, Makras P (2010) Skeletal diseases in Cushing’s syndrome: osteoporosis versus arthropathy. Neuroendocrinology 92(suppl 1):60–64
Diez-Perez A, Hooven FH, Adachi JD, Adami S, Anderson FA, Boonen S, Chapurlat R, Compston JE, Cooper C, Delmas P, Greenspan SL, Lacroix AZ, Lindsay R, Netelenbos JC, Pfeilschifter J, Roux C, Saag KG, Sambrook P, Silverman S, Siris ES, Watts NB, Nika G, Gehbach SH (2011) Regional differences in treatment for osteoporosis. The Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 49:493–498
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat M, Buchfelder J, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JAH, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. Clin Endocrinol Metab 93:2454–2462
Dragunova NV, Belaya ZE, Solodovnikov AG, Rozhinskaya LY, Melnichenko GA (2014) Fractures in patients with endogenous Cushing’s syndrome and their influence on quality of life and functional performance. Osteoporos Int 25(suppl 2):260–261
Dragunova NV, Belaya ZE, Rozhinskaya LY, Melnichenko GA (2014) Bone mineral density, markers of bone remodeling and quality of life in patients with Cushing’s syndrome after 12 months of remission. Endocr Abstr 35:69–70. doi:10.1530/endoabs.35.P24
Lee HJ, Je JH, Seo JH, Na YJ, Yoo HJ (2014) Multiple spontaneous rib fractures in patient with Cushing’s syndrome. J Bone Metab 21:277–282
Papadakis G, Uebelhart B, Goumaz M, Zawadynski S, Rizzoli R (2013) An unusual case of hypercortisolism with multiple weight-bearing bone fractures. Clin Cases Miner Bone Metab 10:213–217
Kristo C, Jemtland R, Ueland T, Godang K, Bollerslev J (2006) Restoration of the coupling process and normalization of bone mass following successful treatment of endogenous Cushing’s syndrome: a prospective long-term study. Eur J Endocrinol 154:109–111
Szappanos A, Toke J, Lippai D, Patocs A, Igaz P, Szucs N, Futo L, Glaz E, Racz K, Toth M (2010) Bone turnover in patients with endogenous Cushing’s syndrome before and after successful treatment. Osteoporos Int 21:637–645
Belaya ZE, Rozhinskaya LY, Melnichenko GA, Solodovnikov AG, Dragunova NV, Iljin AV, Dzeranova LK, Dedov II (2013) Serum extracellular secreted antagonists of the canonical Wnt/β-catenin signaling pathway in patients with Cushing’s syndrome. J Osteoporos Int 24:2191–2199
Pothuaud L, Carceller P, Hans D (2008) Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 42:775–787
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 42:775–787
Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D (2009) Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study. J Clin Densitom 12:170–176
Rabier B, Heraud A, Grand-Lenoir C, Winzenrieth R, Hans D (2010) A multicentre, retrospective case-control study assessing the role of trabecular bone score (TBS) in menopausal Caucasian women with low areal bone mineral density (BMDa): analysing the odds of vertebral fracture. Bone 46:176–181
Winzenrieth R, Dufour R, Pothuaud L, Hans D (2010) A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int 86:104–109
Hans D, Goertzen AL, Krieg MA, Leslie WD (2011) Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res 26:2762–2769
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Leslie WD, Aubry-Rozier B, Lamy O, Hans D (2013) Trabecular bone score and diabetes-related fracture risk. J Clin Endocrinol Metabol 98:602–609
Silva BC, Boutroy S, Zhang C, McMahon DJ, Zhou B, Wang J, Udesky J, Cremers S, Sarquis M, Guo XE, Hans D, Bilezikian JP (2013) Trabecular bone score (TBS)—a novel method to evaluate bone microarchitectural texture in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 98:1963–1970
Eller-Vainicher C, Filopanti M, Palmieri S, Ulivieri FM, Morelli V, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Verga U, Scillitani A, Beck-Peccoz P, Chiodini I (2013) Bone quality, as measured by trabecular bone score, in patients with primary hyperparathyroidism. Eur J Endocrinol 169:155–162
Eller-Vainicher C, Morelli V, Ulivieri FM, Palmieri S, Zhukouskaya VV, Cairoli E, Pino R, Naccarato A, Scillitani A, Beck-Peccoz P, Chiodini I (2012) Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J Bone Miner Res 27:2223–2230
Belaya ZE, Iljin AV, Melnichenko GA, Rozhinskaya LY, Dragunova NV, Dzeranova LK, Butrova SA, Troshina EA, Dedov II (2012) Diagnostic performance of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay in obese and overweight patients referred to exclude Cushing’s syndrome. Endocrine 41:494–500
Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM (2010) AACE Osteoporosis Task Force American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract 16(Suppl 3):1–37
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizolli R, Reginster R, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semi-quantitative technique. J Bone Miner Res 8:1137–1148
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16:455–466
Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PMM (2003) The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 56:1129–1135
Kim S-Y, Davydov O, Hans D, Bockman R (2015) Insights on accelerated skeletal repair in Cushing’s disease. Bone Rep. doi:10.1016/j.bonr.2015.03.001
Vestergaard P, Lindholm J, Jorgensen JOI, Hagen C, Hoeck HC, Laurbe P, Rejnmark L, Brixen K, Kristensen L, Feldt-Rasmussen U, Mosekilde L (2002) Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur J Endocrinol 146:51–56
Futo L, Toke J, Patocs A, Szappanos A, Varga I, Glaz E, Tulassay Z, Racz K, Toth M (2008) Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing’s syndrome. J Osteoporos Int 19:941–949
Tauchmaonova L, Pivonello R, Di Somma C, Rossi R, De Martino MC, Camera L, Klain M, Salvatore M, Lombardi G, Colao A (2006) Bone demineralization and vertebral fractures in endogenous cortisol excess: role of disease etiology and gonadal status. J Clin Endocrinol Metab 91:1779–1784
Eerden AW, den Heijer M, Oyen WJ, Hermus AR (2007) Cushing’s syndrome and bone mineral density: lowest Z-scores in young patients. Neth J Med 65:137–141
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton J, Tenenhouse A, Reeve J, Silman AJ, Pols HAP, Eisman JA, McCloskey EV, Mellsrom D (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C (2000) Fracture and oral corticosteroids: relationship to daily and cumulative dose. Rheumatology (Oxford) 39:1383–1389
Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM, ERCUSYN Study Group (2011) The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. European J Endocrinology 165:383–392
Minne HW, Leidig G, Wuster C, Siromachhkostov K, Bakdauf G, Bickel R, Sauer P, Lojen M, Zeigler R (1988) A newly defined spine deformity index (SDI) to quantitative vertebral crush fractures in patients with osteoporosis. Bone Miner J 3:225–349
Belaya ZE, Rozhinskaya LY, Solodovnikov AG, Dragunova NV, Melnichenko GA: Glucocorticoid-induced osteoporosis: fractures and bone remodeling in patients with endogenous Cushing’s syndrome (2013) Series: Endocrinology Research and Clinical Developments. Online Book, Published by Nova Science Publishers, Inc New York, 55 pp
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
МД-3332.2015.7
Conflict of interest
Zhanna Belaya, Liudmila Rozhinskaya, Natalia Dragunova, Natalia Sasonova, Alexander Solodovnikov, Timur Tsoriev, Larisa Dzeranova, Galina Melnichenko, and Ivan Dedov declare that they have no conflict of interest. Didier Hans is co-owner of the TBS patent and has corresponding owner shares and position in Medimaps group.
Rights and permissions
About this article
Cite this article
Belaya, Z.E., Hans, D., Rozhinskaya, L.Y. et al. The risk factors for fractures and trabecular bone-score value in patients with endogenous Cushing’s syndrome. Arch Osteoporos 10, 44 (2015). https://doi.org/10.1007/s11657-015-0244-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-015-0244-1