Abstract
Purpose of review
To describe the mechanisms involved in the heterogeneous drug hypersensitivity reactions and how a better understanding of them can help in the correct diagnosis, the improvement of the in vitro diagnostic methods, and the management of the reaction.
Recent findings
We know that drug hypersensitivity reactions are mediated by different mechanisms and until now some drugs have been reported to be able to activate the immune system by a single mechanism while other drugs can be involved in different mechanisms. Moreover, studies show that important clinical aspects such as risk factors, predictability, and cross-reactivity may depend on the drug action mechanism. In this way, recent genetic association studies have shown different human leukocyte antigen (HLA) associations with hypersensitivity reactions depending on the drug and/or the mechanism involved.
Summary
Mechanistically, drug hypersensitivity reactions (DHRs) are classified as allergic and non-allergic reactions. Allergic reactions have been further classified into reactions mediated by IgE, IgG, or IgM; immune complex/complement activation; and T cells. Non allergic reactions can be associated to nonspecific histamine release, bradykinin increase, complement activation, or changes in the metabolism of arachidonic acid. More recently, a classification based on the mode of action of drugs has been proposed, suggesting three mechanisms involved in DHRs: (i) drugs that bind covalently on macromolecules (e.g., proteins) (allergic/immune reaction); (ii) drugs that bind on immune receptors like HLA and T cell receptors (pharmacological interaction, p-i reactions); and (iii) drugs with the ability to stimulate or inhibit receptors or enzymes of inflammatory cells (pseudo-allergy). An extended knowledge on the mechanisms involved in the heterogeneous DHRs can help to understand differences in sensitization patterns, uncommon clinical manifestations, dependence on drug dose, predictability, and cross-reactivity. For that, a better understanding of them can help in the correct diagnosis and the management of the reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse drug reactions (ADR) are defined by the World Health Organization (WHO) as “any noxious, unintended and undesired effect of a drug that occurs at doses used for prevention, diagnosis or treatment” [1]. They are a relevant public health problem, involved in 3–6% of hospitalizations and in 10–15% of hospitalized patients [2, 3]. The pharmacological classification suggested by Rawlins and Thompson in 1974 [4] is the most widely applied and divided the ADRs in predictable (type A) and unpredictable (type B) reactions. Drug hypersensitivity reactions (DHRs) are type B reactions, defined by the WHO as “dose-independent, unpredictable, noxious, and unintended response to a drug taken at a dose normally used in humans” [1, 5].
DHR classification is complex because of the heterogeneity in terms of drug involved, clinical symptoms, and underlying mechanisms. Clinically, DHRs are usually classified as immediate or non-immediate/delayed based on the time interval between the drug exposure and the onset of the symptoms [6]. This classification has limitations and it is controversial because of the subjective cut-off point. Nowadays, a new cut-off point that classified these reactions into immediate (< 1–6 h after drug exposure) and non-immediate (> 1 h after drug exposure) has been proposed [7], overlapping immediate and non-immediate reactions from 1 to 6 h [6, 8•], originally defined by Levine [6] as “accelerated reactions.” Immediate reactions are mostly mediated by an IgE-mechanism and they commonly appear as isolated clinical manifestations like urticaria, angioedema, rhinitis, conjunctivitis, bronchospasm, or as systemic reactions like anaphylaxis or anaphylactic shock [9]. Non-immediate reactions are usually mediated by specific T cells; however, other mechanisms can be implicated [7] and the most usual clinical presentations are delayed urticaria and maculopapular exanthemas, as well as more severe reactions like acute generalized exanthematous pustulosis (AGEP), toxic epidermal necrolysis (TEN), Stevens–Johnson syndrome (SJS), and drug reactions with eosinophilia and systemic symptoms (DRESS). Though this classification is very useful in clinical routine, there are weaknesses due to other factors (e.g., administration route, drug metabolites, cofactors, coprescribed drugs) may increase or decrease the time interval between the drug administrations and the onset of the symptoms [10].
Mechanistically, DHRs are classified as allergic and non-allergic reactions [9]. ADRs with and immunological basis (ADRIBs) or allergic reactions have been furthermore classified in accordance with the classification system proposed by Gell and Coombs [11]: type I reactions, mediated by specific IgE with mast cells and/or basophils as main effector cells; type II reactions (cytolytic or cytotoxic), mediated by specific IgG or IgM; type III reactions (immune complex), mediated by immune complex and complement activation; and type IV reactions, mediated by specific T cells that can be subdivided (type IVa–IVd) according to the mechanism involved [12]. On the other hand, there are reactions with clinical symptoms compatible with an allergic reaction that are not true drug allergies. The proposed pathomechanisms for these reactions are (i) non-specific histamine release by masts cell and basophils, (ii) bradykinin accumulation, (iii) complement activation, (iv) alterations in arachidonic acid metabolism, and (v) bronchospasm induction by the pharmacological action of some drugs [7].
Finally, a classification based on the way drugs interact with the immunological system was proposed by Pichler et al. [13, 14••, 15••] to better comprehend different aspects of DHRs. According to this classification, three mechanisms can be involved in DHRs: (i) drugs that bind covalently on proteins and form carrier complexes (allergic/immune reaction); (ii) off-target action of drugs on immune receptors like human leukocyte antigen (HLA) and T cell receptors (TCR) (pharmacological interaction, p-i reactions); and (iii) drugs with the ability to stimulate or inhibit receptors or enzymes of inflammatory cells (pseudo-allergy). This classification is based on the drug action and is helpful in clinical practice, because it can clarify differences in sensitization patterns and can explain differences in sensitizations, uncommon clinical manifestations, dependence on drug dose, predictability, and cross-reactivity in DHRs. In this review, we focus on the description of mechanisms involved in the heterogeneous DHRs and how a better understanding of them can be helpful in the correct diagnosis with the improvement of the in vitro diagnostic methods and the management of the reaction.
Mechanisms involved and clinical manifestations
Allergic reactions: hapten hypothesis
Most of the drugs and their reactive metabolites are considered haptens, small molecules (< 1000 Da) too small to induce a specific immune response. The hapten hypothesis states that haptens are able to covalently bind on endogenous proteins, directly or previous drug metabolism [16], to form an antigenic hapten-carrier complex [17,18,19] (Fig. 1). The main protein targets for haptens could be serum proteins (e.g., albumin,hemoglobin), intracellular proteins, or specific tissue proteins. According to this hypothesis, both haptens and proteins are relevant for the immunological recognition by specific antibodies or specific T cells. Therefore, it is crucial to fully characterize the haptenic structures to better understand the molecular bases of DHRs. Hapten-protein adducts are processed to peptides and presented as peptide-HLA complexes by antigen-presenting cells (APCs), like dendritic cells and B cells and then finally recognized by TCR, eliciting drug-specific humoral or cellular immune responses [20, 21]. It is important to highlight that although the generation of drug-carrier proteins adducts are required to induce an immune response, more factors are needed, so different studies have shown circulating drug modified proteins are detected in tolerant subjects without hypersensitivity [22••, 23•]. Hapten-like drugs are capable to induce the four types of allergic reactions proposed by Gell and Coombs [11, 12], although the most clinically relevant immune-mediated DHRs are the types I and IV. Some of the drugs are able to bind stably and covalently to proteins as carrier molecule include betalactam antibiotics (benzylpenicillin [24], amoxicillin [25,26,27], piperacillin [28], flucloxacillin [29], and carbamazepine (CBZ) [30••], as well as other drug such as sulfanilamides, metamizole, quinolones, radiocontrast media, and muscle relaxants [14••, 15••].
IgE-mediated (type I) allergic reactions
Pathophysiology
IgE-mediated allergic reactions develop due to the production of specific IgE by specific B lymphocytes after a previous sensitization phase [7, 31, 32]. In this way, specific IgE molecules are released into the bloodstream and then, a proportion of these antibodies are reversible linked to specific high affinity receptors (FcεRI) expressed on the surface of basophils and mast cells [33, 34]. Then, after a new drug exposure, the antigen (hapten-protein adduct) interact with two or more adjacent specific IgE molecules bound on the surface of mast cells and basophils (cross-linking) triggering an intracellular signaling cascade leading to the cellular activation and degranulation, with the extracellular release of preformed inflammatory mediators (e.g., tryptase, histamine, TNF-α), and the synthesis and secretion of lipid mediators (e.g., PAF, prostaglandins, and leukotrienes) and cytokines (e.g., IL-4 and IL-13) [35,36,37,38,39]. All of them are responsible for the clinical manifestations (vasodilatation, smooth muscle contraction, and inflammation) [37]. Betalactam allergic reactions are the best defined IgE-mediated reactions [40].
Clinical manifestations
IgE-mediated reactions are related with two main clinical entities: urticaria, with or without angioedema, and anaphylaxis. Urticaria is characterized and previously described as “rapidly evolving transient pruriginous wheals occurring at different sites of the body, which may represent the first stage of an anaphylactic reaction” [40]. On the other hand, anaphylaxis is defined as “a serious allergic reaction with a rapid onset that may cause death” [41].
T cell-mediated (type IV) allergic reactions
Pathophysiology
The effector mechanism of these reactions include a previous expansion of T cells stimulated by hapten-peptide-HLA complexes, and then a recruitment of inflammatory effector cells to target organs, and finally, after a new antigen exposure, effector cells are activated to secrete cytokines that involved in the immunological reaction and cytotoxins that cause tissue damage [34].
Clinical manifestations
Contrary to IgE-mediated reactions, clinical manifestations in T cell-mediated reactions are heterogeneous and comprise a number of different clinical phenotypes [42•] that can either be diseases with multisystem involvement in which the main affected organ is the skin, such as DRESS [43], severe cutaneous diseases such as SJS and TEN [44], AGEP [45, 46], and generalized bullous fixed-drug eruption (FDE). Moreover, single-organ diseases are associated to this pathomechanism, affecting organs like the liver (drug-induced liver injury (DILI)) [47,48,49,50], the pancreas (drug-induced pancreatitis), the lungs (lung infiltrates with eosinophilia) [51, 52], and the kidney (interstitial nephritis) [53].
Pharmacological interaction with immune receptors: p-i concept
Pathophysiology
The pharmacological interaction (p-i) with immune receptors concept suggests that drugs/metabolites may directly, reversibly, and non-covalently bind to immune receptor proteins (TCR, HLA, or HLA peptides) [13, 54, 55]. This drug binding occurs through non-covalent bonds like hydrogen bonds, van der Waals forces, and electrostatic interactions. Drug interaction with TCR or HLA is frequently selective for a specific TCR or HLA molecule, due to only particular amino acid sequences and conformational structures enable relatively strong non-covalent drug interactions [56] that are spontaneous and could induce T cell activation within seconds after drug administration [57, 58]. Functional consequences of these interactions are influenced by the location and orientation of the drug binding site and the drug affinity [13, 59,60,61,62]. Moreover, non-covalent interaction of drug with immune receptors can result through different pathways (Fig. 1):
p-i TCR
Drugs can bind directly on TCR (p-i TCR), altering the TCR conformation and increasing the TCR binding affinity, giving them the potential to induce immune reactions [59]. A recent study has shown two types of sulfamethoxazole (SMX) reacting T cell clones that can be stimulated by the binding of SMX to CDR2 region of TCR-Vβ20-1 or CDR3 of the α-chain. Moreover, the study found that the stimulation was only dependent on SMX, with no requirement of peptide and HLA recognition [59].
p-i HLA
Drugs can bind directly to the binding groove of HLA (p-i HLA), generating new drug peptide-HLA complex (allo-HLA) or could alter the conformation of peptide-HLA complex that can be recognized as neoantigen by TCRs [13, 63]. For example, CBZ/aromatic antiepileptic drugs have been reported to interact directly with HLA-B*15:02 protein, with no intracellular antigen processing implicated in the HLA-B*15:02 presentation of CBZ [64]. As well as oxypurinol can directly activate specific T cells through HLA-B*58:01 with no intracellular processing [58].
Altered self-peptides repertoire hypothesis
Drug direct binding to HLA molecules can produce alteration of the regular repertoire of peptides presented by HLA [60,61,62]. The altered peptide repertoire model proposes that drugs interacting with the HLA peptide-binding groove can change the binding cleft and the specificity of peptide-HLA binding, which leads to T cell proliferation [65,66,67]. Hypersensitivity reactions to abacavir have been reported to be mediated by this model [60, 61]. Studies have shown that abacavir binds to HLA-B*57:01 F-pocket and modify the shape and chemistry of the antigen-binding cleft, altering the repertoire of endogenous peptides and inducing T cell activation and autoimmune-like systemic manifestations. It is important to remark that, until know, the altered peptide repertoire hypothesis has been only demonstrated for abacavir.
Clinical manifestations
Clinical manifestations in these reactions commonly appear > 5–7 days after the drug exposure and only after T cell have expended and migrated into tissues. In p-i reactions, drug doses are relevant for the induction of T cell reactions; however, in some cases, lower drug doses could be enough to elicit symptoms onset if a previous expansion of T cell has occurred [68]. p-i reactions are characterized by relevant clinical aspects like drug dose dependence and rate of symptoms onset depending on the number of stimulated T cells. [56]. Finally, in vitro studies suggest that p-i reactions are implicated in severe reactions like MPE [54], AGEP [69], DILI [70], SJS/TEN [64, 71], and DRESS [58, 72,73,74,75].
Pseudo-allergic/non-immunological reactions
Pathophysiology
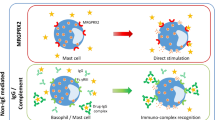
Pseudo-allergic reactions are heterogeneous reactions induced after drug binds directly to receptors or interacts with enzymes of effector cells without immune mechanisms involved, although the underlying pathomechanisms are not yet completely clarified [7, 9, 76]. No previous sensitization or cells expansion is required in these reactions because they are not mediated by the activation of the immune system [77]. The hypothesis proposed for these reactions is that certain drugs are capable to induce the direct stimulation of mast cells, basophils, eosinophils, and neutrophils with no demonstration of the presence of specific IgE, IgE, or T cells. This process can be mediated through a receptor on mast cells, the MAS-related G protein-coupled receptor-X2 (MRGPRX2), that has been reported to be relevant for the IgE-independent and direct mast cells activation [76, 78, 79]. The interaction of some drugs with MRGPRX2 receptor can elicit the release of histamine, TNF-α, β-hexosaminidase, and PGD2, responsible of the clinical manifestations. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most frequent elicitors of pseudo-allergic reactions [80••, 81], although other drugs like quinolones, vancomycin, radiocontrast media, opioids, dextrans, and neuromuscular blocking agents have been reported to directly stimulate mast cells [76, 79, 82] (Fig. 1).
Clinical manifestations
Most of pseudo-allergic reactions are mild reactions (e.g., acute urticaria), but other more severe even lethal reactions like anaphylaxis can be elicit by pseudo-allergic mechanisms. These reactions are frequently misdiagnosed as immediate hypersensitivity reaction, but the pathomechanism, drug cross-reactivity, and possible prevention strategies are significantly different to those involved in real allergic reactions. For example, pseudo-allergic reactions have been reported to be dose-dependent and higher drug concentrations are often needed to induce clinical symptoms compared with IgE-mediated reactions [83]. Interestingly, NSAIDs may boost the existing local inflammation in patients with eosinophilic inflammation, producing asthma exacerbations, urticaria, and rhinosinusitis [84]. Moreover, a higher risk to develop NSAID intolerance has been described in patients with severe asthma compared with subject with mild or no asthma [80••, 84,85,86,87,88]. According to this, inflammation is an important cofactor for the development of NSAID-induced pseudo-allergic reactions that can explain the appearance of symptoms in tissues suffering inflammation.
Management of drug hypersensitivity reactions
The mechanisms involved in DHRs entails differences related to different aspects such as drug cross-reactivity, drug dose, or reaction prediction, all of them relevant in the management of the hypersensitivity disease, as described below.
The first step and the most effective strategy for the management of DHRs must be the avoidance or discontinuation of the culprit drug, as well as the search for safe, effective, and non-cross-reactive alternative medication [89,90,91]. Cross-reactivity is common in the context of allergic, p-i as well as pseudo-allergic DHRs, although differences have been described.
Cross-reactivity
Allergic reactions
Cross-reactivity is explained by the affinity of specific immune receptors (IgE, TCR) for the eliciting drug (hapten-protein or hapten-peptide complexes) but also related structures [14••]. Focusing on penicillins, the most frequent drugs involved in immunological DHRs [92], the treatment for patients with penicillin allergy is best limited to non-penicillin agents, even in patients with specific IgE directed against a specific side chain of the drug [93]. However, cross-reactivity between penicillins and carbapenems or monobactams are very rare [94,95,96,97,98], as well as third- or fourth-generation of cephalosporins, that may be considered as alternative treatment since the degree of cross-reactivity has been reported to be lower than with first-generation compounds [89, 99, 100]. Clinical cases with a history of DHRs to cephalosporin can be safely treated with negative skin test cephalosporins with a different side chain [101]. Regarding to NSAIDs, patients with a clinical history of ADRIBs should avoid the culprit drug and chemically related compounds, but NSAIDs not chemically related with the drug involved can be administered [102•].
Pharmacological interaction with immune receptors
In p-i reactions, cross-reactivity is common, but needs to be individually analyzed for each group of drugs [14••]. For example, in p-i HLA, abacavir has been reported to bind exclusively to B*57:01, but not to structurally similar HLA-proteins, and drug binding may be prevented by molecule modifications [14••, 103]. In contrast, HLA-B*15:02 protein can bind not only CBZ, but also some CBZ metabolites, and maybe other anticonvulsants (e.g., lamotrigine and phenytoin) [14••, 104].
Pseudo-allergic/non-immunological reactions
In pseudo-allergy reactions, MRGPRX2 receptor on basophils and mast cells is able to bind a diverse group with a common chemical structure motif with an extensive cross-reactivity [14••, 76]. On the other hand, non-immunologically mediated hypersensitivity reactions to NSAIDs are common and cross-reactivity has been reported between cyclooxygenase (COX)-1 enzyme inhibitory drugs and pyrazolones [80••], although structurally different, they can lead to cross-reactivity due to all have a common mode of action [80••, 85, 105]. The management of these reactions involves avoidance of COX-1 inhibitors to prevent further reactions. Interestingly, selective COX-2 inhibitors are tolerated by most of these patients and can be contemplated as treatment in emergency situations [14••].
Desensitization and dose dependence
Desensitization protocol is the only treatment for DHRs and it is critical for hypersensitivity patients to first-line therapy that needs to be administered [106•, 107•, 108•, 109•, 110]. This method enables the safe re-administration of the drug independently of the mechanism involved [111]. In general, desensitization has been reported to be helpful to prolong the first election treatment in numerous drugs, including chemotherapeutic (platinum agents) and biological agents [108•, 112,113,114,115], anticonvulsants [116], antituberculosis medication [117], antibiotics [118,119,120], NSAIDs [121•, 122, 123••], and others [124, 125]. Regarding to desensitization protocols, dose dependence in DHRs is a relevant factor [126] due to transient tolerance to a drug is induced by initially applying extremely low amounts, which are then steadily increased [14••]. Dose dependence is related with the mechanism involved in the reaction; thereby, immunologically mediated reactions can be elicited at lower doses than therapeutic doses, in contrast with pseudo-allergic reactions, where clinical manifestations usually appear at standard to high doses [14••]. Successful desensitization procedures are well documented in both allergic [107•, 111, 122, 125] and pseudo-allergic reactions [83, 127, 128].
Prediction of DHRs
At the moment, predictive tests to prevent DHRs are still limited to very few compounds such as abacavir and CBZ [129]. HLA associations to certain DHRs have been reported for an increasing number of compounds [130]; some of them with an almost exclusive association to a specific HLA allele and other drugs associated to different alleles. However, the main limitation for predictive testing is the low positive predictive value, because other risk factors should also be involved in the development of DHRs [14••].
Allergic reactions
Some genetic associations have been found between IgE-mediated allergic reactions to BL antibiotics and single nucleotide polymorphisms (SNPs) of HLA alleles [131, 132]. Thereby, a recent study showed genetic variants in HLA-DRA and ZNF300 that predicted positivity to penicillin skin tests and SNPs rs7192 and rs8084 of HLA-DRA were found to be significantly associated with penicillin allergy in Italian and Spanish populations and with cephalosporin allergy in Spanish patients [131]. Another study focused on the association of LGALS3 polymorphisms with BL allergy in Spanish and Italian population showed a genetic association between rs11125 variant and IgE-mediated BL allergy [133]. On the other hand, a study evaluating allergic drug reactions to pyrazolones suggested a genetic predisposition linked to HLA-DQ locus, although a limited number of patients was included in the study [134]. In a more recent study, it was found that slow acetylation, related with slow alleles of arylamine N-acetyltransferases 2 (NAT2), was associated with an increased risk of developing selective hypersensitivity to metamizole, and particularly anaphylaxis [135]. Finally, two intronic variants (rs2241160 and rs2241161) for centrosomal protein of 68 kDa encodes gene (CEP68) have been recently described in single NSAID-induced urticaria and anaphylaxis [136•], pointing a potential use of this gene in the search and application of clinical biomarkers to detect patients at risk for these reactions. All these data suggest the important relationship between drug metabolism and specific individual genetic variability in the development of immunological reactions to drugs [137, 138], although studies including more participants as well as different geographical populations should be carried out.
Pharmacological interaction with immune receptors
A combination of genetic analysis of HLA and/or metabolizing enzymes could predict p-i-induced DHRs. As mentioned previously, the positive predictive value of HLA associations with DHRs reactions is low (< 3%), with the remarkable exception of abacavir, where about 50% of patients with HLA-B*57:01 allele developed a hypersensitivity reaction [139]. In this sense, HLA-B*57:01 screening previous to the administration of abacavir has been widely applied in clinical practice and is part of the US FDA and international human immunodeficiency virus treatment guidelines [140]. HLA association with DHRs has been demonstrated for other drugs with p-i mechanism; for example, it has been reported a strong association of HLA-B*15:02 allele with CBZ-induced SJS/TEN among Han Chinese [141,142,143,144]. However, in European and Japanese populations, CBZ-induced hypersensitivity reactions were found to be associated with HLA-A*31:01 [145, 146]. HLA-B*58:01 has been strongly associated to allopurinol-induced SJS/TEN [72, 147,148,149] and seems not to be ethnic dependent, as well as HLA-B*15:11 with CBZ [145, 146, 150, 151], HLA-B*15:02 with phenytoin [104, 144], and HLA-B*57:01 with abacavir and also associated to DILI development [152, 153].
Conclusions
Nowadays, diagnosis of DHRs is very complex and continues to be a challenge due to the clinical and mechanistic heterogeneity of them. Three clearly distinct mechanisms have been proposed to be involved in DHRs: allergic reactions, p-i with immune receptors, and pseudo-allergic reactions. Mechanisms involved entails differences related to drug cross-reactivity, drug dose, or prediction of the reaction, all of them important in the patient management. However, a better understanding of the underlying mechanisms involved in each clinical manifestation and each culprit drug is still needed.
Sources of funding
The present study has been supported by the Institute of Health ‘‘Carlos III’’ (ISCIII) of State Secretariat for Research, Development and Innovation of the Ministry of Economy and Competitiveness (MINECO) (grants cofounded by the European Regional Development Fund (ERDF): “Una manera de hacer Europa’’: grant nº. PI15/01206, PI18/00095, RETIC ARADYAL RD16/0006/0001); Andalusian Regional Ministry of Health (grant nº. PI-0241-2016 and PE-0172-2018). A.A. holds a ‘‘Sara Borrell’’ contract (grant no. CD17/0146), and G.B. holds a Juan Rodes research contract (JR18/00054), both by ISCIII of MINECO (grants cofunded by European Social Fund: ‘‘El FSE invierte en futuro’’). T.D.F. holds a “Ramon y Cajal” research contract from Ministry of Economy and Competitiveness (RYC-2013-13138). C.M. holds a ‘Nicolas Monardes’ research contract by Andalusian Regional Ministry Health (grant no. RC-0004-2016).
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972;498:25.
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356(9237):1255–9. https://doi.org/10.1016/S0140-6736(00)02799-9.
Thong BY, Leong KP, Tang CY, Chng HH. Drug allergy in a general hospital: results of a novel prospective inpatient reporting system. Ann Allergy Asthma Immunol. 2003;90(3):342–7. https://doi.org/10.1016/S1081-1206(10)61804-2.
Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. Oxford: Oxford University Press; 1977.
Davies DM, Ashton CH, Rao JG, Rawlins MD, Routledge PA, Savage RL, et al. Comprehensive clinical drug information service: first year’s experience. Br Med J. 1977;1(6053):89–90. https://doi.org/10.1136/bmj.1.6053.89.
Levine BB. Immunologic mechanisms of penicillin allergy. A haptenic model system for the study of allergic diseases of man. N Engl J Med. 1966;275(20):1115–25. https://doi.org/10.1056/NEJM196611172752009.
Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International consensus on drug allergy. Allergy. 2014;69(4):420–37. https://doi.org/10.1111/all.12350.
• Montanez MI, Mayorga C, Bogas G, Barrionuevo E, Fernandez-Santamaria R, Martin-Serrano A, et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol. 2017;8:614. https://doi.org/10.3389/fimmu.2017.00614 This review focuses on the mechanisms and diagnosis of drug-induced anaphylaxis.
Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–6. https://doi.org/10.1016/j.jaci.2003.12.591.
Bircher AJ, Scherer HK. Drug hypersensitivity reactions: inconsistency in the use of the classification of immediate and nonimmediate reactions. J Allergy Clin Immunol. 2012;129(1):263–4; author reply 5-6. https://doi.org/10.1016/j.jaci.2011.08.042.
Gell PGH, Coombs RRA. Clinical aspects in immunology. Oxford: Blackwell Scientific Publications; 1968.
Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139(8):683–93. https://doi.org/10.7326/0003-4819-139-8-200310210-00012.
Pichler WJ, Adam J, Watkins S, Wuillemin N, Yun J, Yerly D. Drug hypersensitivity: how drugs stimulate T cells via pharmacological interaction with immune receptors. Int Arch Allergy Immunol. 2015;168(1):13–24. https://doi.org/10.1159/000441280.
•• Pichler WJ, Hausmann O. Classification of Drug Hypersensitivity into Allergic, p-i, and Pseudo-Allergic Forms. Int Arch Allergy Immunol. 2016;171(3–4):166–79. https://doi.org/10.1159/000453265 This review discusses why drug hypersensitivity reactions have to be subclassfied into allergic-immune, pharmacological interactions, and pseudo-allergic reactions.
•• Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy. 2019;74(8):1457–71. https://doi.org/10.1111/all.13765This review focused on the classification of drug hypersensitivity reactions based on the action of drugs and the clinical utility.
Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, et al. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol. 2002;62(3):628–37. https://doi.org/10.1124/mol.62.3.628.
Martin S, Weltzien HU. T cell recognition of haptens, a molecular view. Int Arch Allergy Immunol. 1994;104(1):10–6. https://doi.org/10.1159/000236703.
Weltzien HU, Padovan E. Molecular features of penicillin allergy. J Invest Dermatol. 1998;110(3):203–6. https://doi.org/10.1046/j.1523-1747.1998.00122.x.
Faulkner L, Meng X, Park BK, Naisbitt DJ. The importance of hapten-protein complex formation in the development of drug allergy. Curr Opin Allergy Clin Immunol. 2014;14(4):293–300. https://doi.org/10.1097/ACI.0000000000000078.
Padovan E, Bauer T, Tongio MM, Kalbacher H, Weltzien HU. Penicilloyl peptides are recognized as T cell antigenic determinants in penicillin allergy. Eur J Immunol. 1997;27(6):1303–7. https://doi.org/10.1002/eji.1830270602.
White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136(2):219–34; quiz 35. https://doi.org/10.1016/j.jaci.2015.05.050.
•• Meng X, Al-Attar Z, Yaseen FS, Jenkins R, Earnshaw C, Whitaker P, et al. Definition of the nature and hapten threshold of the beta-lactam antigen required for T cell activation in vitro and in patients. J Immunol. 2017;198(11):4217–27. https://doi.org/10.4049/jimmunol.1700209 This paper shows that the levels of piperacillin-human serum albumin adducts are equivalent in hypersensitivity and tolerant patients and it suggests that other factors should be involved in the propensity to develop hypersensitivity.
• Meng X, Yerly D, Naisbitt DJ. Mechanisms leading to T-cell activation in drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2018;18(4):317–24. https://doi.org/10.1097/ACI.0000000000000458 This review highlights the requirement to reach consensus that the formation of drug protein adduct as well as the direct drug binding on immune receptors are relevant for T-cell activation.
Christie G, Kitteringham NR, Park BK. Drug-protein conjugates—XIII. The disposition of the benzylpenicilloyl hapten conjugated to albumin. Biochem Pharmacol. 1987;36(20):3379–85. https://doi.org/10.1016/0006-2952(87)90314-5.
Ariza A, Collado D, Vida Y, Montanez MI, Perez-Inestrosa E, Blanca M, et al. Study of protein haptenation by amoxicillin through the use of a biotinylated antibiotic. PLoS One. 2014;9(3):e90891. https://doi.org/10.1371/journal.pone.0090891.
Meng X, Earnshaw CJ, Tailor A, Jenkins RE, Waddington JC, Whitaker P, et al. Amoxicillin and Clavulanate form chemically and immunologically distinct multiple haptenic structures in patients. Chem Res Toxicol. 2016;29(10):1762–72. https://doi.org/10.1021/acs.chemrestox.6b00253.
Torres MJ, Montanez MI, Ariza A, Salas M, Fernandez TD, Barbero N, et al. The role of IgE recognition in allergic reactions to amoxicillin and clavulanic acid. Clin Exp Allergy. 2016;46(2):264–74. https://doi.org/10.1111/cea.12689.
Whitaker P, Meng X, Lavergne SN, El-Ghaiesh S, Monshi M, Earnshaw C, et al. Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. J Immunol. 2011;187(1):200–11. https://doi.org/10.4049/jimmunol.1100647.
El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012;341(3):597–610. https://doi.org/10.1124/jpet.111.190900.
••Yip VLM, Meng X, Maggs JL, Jenkins RE, Marlot PT, Marson AG, et al. Mass spectrometric characterization of circulating covalent protein adducts derived from epoxide metabolites of carbamazepine in patients. Chem Res Toxicol. 2017;30(7):1419–35. https://doi.org/10.1021/acs.chemrestox.7b00063 This paper provides the first chemical evidence for microsomal production of monoxygenated carbamazepine metabolites ([O]CBZ) able to react covalently with proteins.
Monticelli S, De Monte L, Vercelli D. Molecular regulation of IgE switching: let’s walk hand in hand. Allergy. 1998;53(45 Suppl):6–8. https://doi.org/10.1111/j.1398-9995.1998.tb04932.x.
Vercelli D. Immunology of IgE. In: Adkinson NF, Brochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, editors. Middleton’s allergy. 7th ed: Elsevier; 2009.
Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366(6454):421–8. https://doi.org/10.1038/366421a0.
Park BK, Naisbitt DJ, Demoly P. Drug hypersensitivity. Allergy. New York: Elsevier Ltd; 2012. p. 321–330.
Schnyder B, Pichler WJ. Mechanisms of drug-induced allergy. Mayo Clin Proc. 2009;84(3):268–72. https://doi.org/10.1016/S0025-6196(11)61145-2.
Simons FE. 9. Anaphylaxis. J Allergy Clin Immunol. 2008;121(2 Suppl):S402–7; quiz S20. https://doi.org/10.1016/j.jaci.2007.08.061.
Williams KW, Sharma HP. Anaphylaxis and urticaria. Immunol Allergy Clin N Am. 2015;35(1):199–219. https://doi.org/10.1016/j.iac.2014.09.010.
Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11(4):319–25. https://doi.org/10.1097/ACI.0b013e3283481ab6.
Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, et al. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clin Exp Allergy. 2009;39(1):72–80. https://doi.org/10.1111/j.1365-2222.2008.03104.x.
Blanca M, Romano A, Torres MJ, Fernandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–93. https://doi.org/10.1111/j.1398-9995.2008.01916.x.
Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–7.
• Phillips EJ. New strategies to predict and prevent serious immunologically mediated adverse drug reactions. Trans Am Clin Climatol Assoc. 2018;129:74–87 This paper explains why not all patients with an HLA risk allele will develop an immunologically mediated adverse drug reaction.
Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. Clinical perspectives. J Am Acad Dermatol. 2013;68(5):693 e1–14; quiz 706-8. https://doi.org/10.1016/j.jaad.2013.01.033.
Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209(2):123–9. https://doi.org/10.1016/j.tox.2004.12.022.
Roujeau JC, Bioulac-Sage P, Bourseau C, Guillaume JC, Bernard P, Lok C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127(9):1333–8.
Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002 Aug;2(4):325–31. https://doi.org/10.1097/00130832-200208000-00006.
Ju C. Immunological mechanisms of drug-induced liver injury. Curr Opin Drug Discov Devel. 2005;8(1):38–43.
Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4(6):489–99. https://doi.org/10.1038/nrd1750.
Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43(3):618–31. https://doi.org/10.1002/hep.21095.
El-Ghaiesh S, Sanderson JP, Farrell J, Lavergne SN, Syn WK, Pirmohamed M, et al. Characterization of drug-specific lymphocyte responses in a patient with drug-induced liver injury. J Allergy Clin Immunol. 2011;128(3):680–3. https://doi.org/10.1016/j.jaci.2011.04.031.
Tomioka R, King TE Jr. Gold-induced pulmonary disease: clinical features, outcome, and differentiation from rheumatoid lung disease. Am J Respir Crit Care Med. 1997;155(3):1011–20.
Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13:39. https://doi.org/10.1186/1465-9921-13-39.
Spanou Z, Keller M, Britschgi M, Yawalkar N, Fehr T, Neuweiler J, et al. Involvement of drug-specific T cells in acute drug-induced interstitial nephritis. J Am Soc Nephrol. 2006;17(10):2919–27. https://doi.org/10.1681/ASN.2006050418.
Zanni MP, von Greyerz S, Schnyder B, Brander KA, Frutig K, Hari Y, et al. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human alpha beta T lymphocytes. J Clin Invest. 1998;102(8):1591–8.
Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: mechanisms and cross-reactivity. Clin Exp Allergy. 2006;36(1):59–69. https://doi.org/10.1111/j.1365-2222.2006.02402.x.
Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, et al. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55(1):17–25. https://doi.org/10.2332/allergolint.55.17.
Wuillemin N, Terracciano L, Beltraminelli H, Schlapbach C, Fontana S, Krahenbuhl S, et al. T cells infiltrate the liver and kill hepatocytes in HLA-B(*)57:01-associated floxacillin-induced liver injury. Am J Pathol. 2014;184(6):1677–82. https://doi.org/10.1016/j.ajpath.2014.02.018.
Yun J, Marcaida MJ, Eriksson KK, Jamin H, Fontana S, Pichler WJ, et al. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. J Immunol. 2014;192(7):2984–93. https://doi.org/10.4049/jimmunol.1302306.
Watkins S, Pichler WJ. Sulfamethoxazole induces a switch mechanism in T cell receptors containing TCRVbeta20-1, altering pHLA recognition. PLoS One. 2013;8(10):e76211. https://doi.org/10.1371/journal.pone.0076211.
Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486(7404):554–8. https://doi.org/10.1038/nature11147.
Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci U S A. 2012;109(25):9959–64. https://doi.org/10.1073/pnas.1207934109.
Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57:01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. 2012;26(11):F21–9. https://doi.org/10.1097/QAD.0b013e328355fe8f.
Yun J, Cai F, Lee FJ, Pichler WJ. T-cell-mediated drug hypersensitivity: immune mechanisms and their clinical relevance. Asia Pac Allergy. 2016;6(2):77–89. https://doi.org/10.5415/apallergy.2016.6.2.77.
Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129(6):1562–9 e5. https://doi.org/10.1016/j.jaci.2011.12.990.
Schnyder B, Brockow K. Pathogenesis of drug allergy--current concepts and recent insights. Clin Exp Allergy. 2015;45(9):1376–83. https://doi.org/10.1111/cea.12591.
Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, et al. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125(2):411–8 e4. https://doi.org/10.1016/j.jaci.2009.10.031.
Elsheikh A, Castrejon L, Lavergne SN, Whitaker P, Monshi M, Callan H, et al. Enhanced antigenicity leads to altered immunogenicity in sulfamethoxazole-hypersensitive patients with cystic fibrosis. J Allergy Clin Immunol. 2011;127(6):1543–51 e3. https://doi.org/10.1016/j.jaci.2010.12.1119.
Gerber BO, Pichler WJ. Cellular mechanisms of T cell mediated drug hypersensitivity. Curr Opin Immunol. 2004;16(6):732–7. https://doi.org/10.1016/j.coi.2004.09.016.
Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001;107(11):1433–41. https://doi.org/10.1172/JCI12118.
Wuillemin N, Adam J, Fontana S, Krahenbuhl S, Pichler WJ, Yerly D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. J Immunol. 2013;190(10):4956–64. https://doi.org/10.4049/jimmunol.1202949.
Yang CW, Hung SI, Juo CG, Lin YP, Fang WH, Lu IH, et al. HLA-B*1502-bound peptides: implications for the pathogenesis of carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2007;120(4):870–7. https://doi.org/10.1016/j.jaci.2007.06.017.
Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–9. https://doi.org/10.1073/pnas.0409500102.
Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J Immunol. 1995;155(1):462–72.
Naisbitt DJ, Britschgi M, Wong G, Farrell J, Depta JP, Chadwick DW, et al. Hypersensitivity reactions to carbamazepine: characterization of the specificity, phenotype, and cytokine profile of drug-specific T cell clones. Mol Pharmacol. 2003;63(3):732–41. https://doi.org/10.1124/mol.63.3.732.
Naisbitt DJ, Farrell J, Wong G, Depta JP, Dodd CC, Hopkins JE, et al. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol. 2003;111(6):1393–403. https://doi.org/10.1067/mai.2003.1507.
McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015 Mar 12;519(7542):237–41. https://doi.org/10.1038/nature14022.
Schlumberger HD. Pseudo-allergic reactions to drugs and chemicals. Ann Allergy. 1983;51(2 Pt 2):317–24.
Lieberman P, Garvey LH. Mast cells and anaphylaxis. Curr Allergy Asthma Rep. 2016;16(3):20. https://doi.org/10.1007/s11882-016-0598-5.
Subramanian H, Gupta K, Ali H. Roles of MAS-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138(3):700–10. https://doi.org/10.1016/j.jaci.2016.04.051.
•• Kowalski ML, Asero R, Bavbek S, Blanca M, Blanca-Lopez N, Bochenek G, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68(10):1219–32. https://doi.org/10.1111/all.12260 This paper proposes consistent definitions for the classification of hypersensitivity reactions to NSAIDs.
Choi JH, Kim MA, Park HS. An update on the pathogenesis of the upper airways in aspirin-exacerbated respiratory disease. Curr Opin Allergy Clin Immunol. 2014;14(1):1–6. https://doi.org/10.1097/ACI.0000000000000021.
Arroyo-Mercado F, Khudyakov A, Chawla GS, Cantres-Fonseca O, McFarlane IM. Red man syndrome with oral vancomycin: a case report. Am J Med Case Rep. 2019;7(1):16–7. https://doi.org/10.12691/ajmcr-7-1-5.
Fruth K, Pogorzelski B, Schmidtmann I, Springer J, Fennan N, Fraessdorf N, et al. Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy. 2013;68(5):659–65. https://doi.org/10.1111/all.12131.
Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of MAS-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014 Sep;134(3):622–33 e9. https://doi.org/10.1016/j.jaci.2014.05.004.
Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A, Gonzalez-Aveledo L. Aspirin-exacerbated cutaneous disease (AECD) is a distinct subphenotype of chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2015;29(4):698–701. https://doi.org/10.1111/jdv.12658.
Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111(5):913–21; quiz 22. https://doi.org/10.1067/mai.2003.1487.
Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID-exacerbated respiratory disease: a meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy. 2015;70(7):828–35. https://doi.org/10.1111/all.12629.
Blanca-Lopez N, Ariza A, Dona I, Mayorga C, Montanez MI, Garcia-Campos J, et al. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin Exp Allergy. 2013;43(5):560–7. https://doi.org/10.1111/cea.12099.
Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S126–37. https://doi.org/10.1016/j.jaci.2009.10.028.
Schnyder B. Approach to the patient with drug allergy. Immunol Allergy Clin N Am. 2009;29(3):405–18. https://doi.org/10.1016/j.iac.2009.04.005.
Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions: S2K-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Dermatological Society (DDG) in collaboration with the Association of German Allergologists (AeDA), the German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Swiss Society for Allergy and Immunology (SGAI), the Austrian Society for Allergology and Immunology (OGAI), the German Academy of Allergology and Environmental Medicine (DAAU), the German Center for Documentation of Severe Skin Reactions and the German Federal Institute for Drugs and Medical Products (BfArM). Allergo J Int. 2015;24(3):94–105. https://doi.org/10.1007/s40629-015-0052-6.
Dona I, Blanca-Lopez N, Torres MJ, Garcia-Campos J, Garcia-Nunez I, Gomez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012;22(5):363–71.
Torres MJ, Blanca M, Fernandez J, Romano A, de Weck A, Aberer W, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58(10):961–72. https://doi.org/10.1034/j.1398-9995.2003.00280.x.
Atanaskovic-Markovic M, Gaeta F, Gavrovic-Jankulovic M, Velickovic TC, Valluzzi RL, Romano A. Tolerability of imipenem in children with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol. 2009;124(1):167–9. https://doi.org/10.1016/j.jaci.2009.02.031.
Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother. 2009;43(2):304–15. https://doi.org/10.1345/aph.1L486.
Saxon A, Adelman DC, Patel A, Hajdu R, Calandra GB. Imipenem cross-reactivity with penicillin in humans. J Allergy Clin Immunol. 1988;82(2):213–7. https://doi.org/10.1016/0091-6749(88)91001-9.
Saxon A, Hassner A, Swabb EA, Wheeler B, Adkinson NF Jr. Lack of cross-reactivity between aztreonam, a monobactam antibiotic, and penicillin in penicillin-allergic subjects. J Infect Dis. 1984;149(1):16–22. https://doi.org/10.1093/infdis/149.1.16.
Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126(5):994–9. https://doi.org/10.1016/j.jaci.2010.06.052.
Romano A, Gaeta F, Valluzzi RL, Caruso C, Rumi G, Bousquet PJ. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126(5):994–9. https://doi.org/10.1016/j.jaci.2010.06.052.
Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med. 2001;345(11):804–9. https://doi.org/10.1056/NEJMra993637.
Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Zaffiro A, Caruso C, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of alternative cephalosporins. J Allergy Clin Immunol. 2015;136(3):685–91 e3. https://doi.org/10.1016/j.jaci.2015.03.012.
• Dona I, Caubet JC, Brockow K, Doyle M, Moreno E, Terreehorst I, et al. An EAACI task force report: recognising the potential of the primary care physician in the diagnosis and management of drug hypersensitivity. Clin Transl Allergy. 2018;8:16. https://doi.org/10.1186/s13601-018-0202-2 This task force remarks how the diagnostic process for drug hypersensitivity reactions often depends on the drug involve and the type of hypersensitivity reaction.
Naisbitt DJ, Yang EL, Alhaidari M, Berry NG, Lawrenson AS, Farrell J, et al. Towards depersonalized abacavir therapy: chemical modification eliminates HLA-B*57:01-restricted CD8+ T-cell activation. AIDS. 2015;29(18):2385–95. https://doi.org/10.1097/QAD.0000000000000867.
Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11(3):349–56. https://doi.org/10.2217/pgs.09.162.
Szczeklik A. Aspirin-induced asthma: new insights into pathogenesis and clinical presentation of drug intolerance. Int Arch Allergy Appl Immunol. 1989;90(Suppl 1):70–5. https://doi.org/10.1159/000235079.
• Tai YH, Tai YJ, Hsu HC, Lee SP, Chen YY, Chiang YC, et al. Risk factors of hypersensitivity to carboplatin in patients with gynecologic malignancies. Front Pharmacol. 2017;8:800. https://doi.org/10.3389/fphar.2017.00800 This paper suggests different risk factors involved in carboplatin hypersensitivity reactions and how these reactions could be prevented.
• Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140(2):321–33. https://doi.org/10.1016/j.jaci.2017.06.012 This paper summarizes how allergists have to provide the management tools to improve the quality of life of patients with anaphylaxis.
• Chung SJ, Kang SY, Kang RY, Kim YC, Lee KH, Kim TY, et al. A new non-dilution rapid desensitization protocol successfully applied to all-grade platinum hypersensitivity. Cancer Chemother Pharmacol. 2018;82(5):777–85. https://doi.org/10.1007/s00280-018-3662-0 This paper focuses on the application of a new, safe, and effective desensitization protocol with platinum agents.
•Madrigal-Burgaleta R, Bernal-Rubio L, Berges-Gimeno MP, Carpio-Escalona LV, Gehlhaar P, Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7(2):618–32. https://doi.org/10.1016/j.jaip.2018.07.031 This paper describes general management procedures and risk factors that have to be considered in platins, taxanes, and biological hypersensitivity reactions.
Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity—a consensus statement. Allergy. 2010;65(11):1357–66. https://doi.org/10.1111/j.1398-9995.2010.02441.x.
de Las Vecillas Sanchez L, Alenazy LA, Garcia-Neuer M, Castells MC. Drug hypersensitivity and desensitizations: mechanisms and new approaches. Int J Mol Sci. 2017;18(6). https://doi.org/10.3390/ijms18061316.
Perez-Rodriguez E, Martinez-Tadeo JA, Perez-Rodriguez N, Hernandez-Santana G, Callero-Viera A, Rodriguez-Plata E, et al. Outcome of 490 desensitizations to chemotherapy drugs with a rapid one-solution protocol. J Allergy Clin Immunol Pract. 2018;6(5):1621–7 e6. https://doi.org/10.1016/j.jaip.2017.11.033.
Lopez-Gonzalez P, Madrigal-Burgaleta R, Carpio-Escalona LV, Bernal-Rubio L, Guerra E, Berges-Gimeno MP, et al. Assessment of antihistamines and corticosteroids as premedication in rapid drug desensitization to paclitaxel: outcomes in 155 procedures. J Allergy Clin Immunol Pract. 2018;6(4):1356–62. https://doi.org/10.1016/j.jaip.2017.11.013.
Santos RB, Galvao VR. Monoclonal antibodies hypersensitivity: prevalence and management. Immunol Allergy Clin N Am. 2017;37(4):695–711. https://doi.org/10.1016/j.iac.2017.07.003.
Gan H, Wang L, Fu W, Zhang J, Yu M, Liu G. Rapid subcutaneous desensitization for the management of delayed hypersensitivity reactions to omalizumab. A case report. J Clin Pharm Ther. 2019;44(3):486–8. https://doi.org/10.1111/jcpt.12827.
Kuyucu S, Caubet JC. Hypersensitivity reactions to antiepileptic drugs in children: epidemiologic, pathogenetic, clinical, and diagnostic aspects. J Allergy Clin Immunol Pract 2018;6(6):1879–1891 e1. https://doi.org/10.1016/j.jaip.2018.07.003.
Siripassorn K, Ruxrungtham K, Manosuthi W. Successful drug desensitization in patients with delayed-type allergic reactions to anti-tuberculosis drugs. Int J Infect Dis. 2018;68:61–8. https://doi.org/10.1016/j.ijid.2018.01.006.
Dilley MA, Lee JP, Broyles AD. Methotrexate hypersensitivity reactions in pediatrics: evaluation and management. Pediatr Blood Cancer. 2017;64(5). https://doi.org/10.1002/pbc.26306.
Shah SR, Millan T, Alamzaib SM, Luu SW. Desensitization therapy using ‘Mariana Castells’ protocol in a patient with multiple autoimmune disorders—does it work? J Community Hosp Intern Med Perspect. 2019;9(1):53–4. https://doi.org/10.1080/20009666.2018.1528107.
Rial Prado MJ, Rico Diaz MA, Cosgaya Ceballos A, Cuesta HJ. A new rush schedule for cotrimoxazole desensitization: a report of 2 cases. J Investig Allergol Clin Immunol. 2018;28(4):267–9. https://doi.org/10.18176/jiaci.0256.
• Cortellini G, Romano A, Santucci A, Barbaud A, Bavbek S, Bignardi D, et al. Clinical approach on challenge and desensitization procedures with aspirin in patients with ischemic heart disease and nonsteroidal anti-inflammatory drug hypersensitivity. Allergy. 2017;72(3):498–506. https://doi.org/10.1111/all.13068 This paper focuses in the establishment of clinical criteria in the choice of challenge and/or desensitization in patients with hypersensitivity to acetylsalicylic acid.
Heath JL, Heath RD, Tamboli C, Johnson L, Wilson AS, Chervinskiy S, et al. Mesalamine desensitization in a patient with treatment refractory ulcerative colitis and aspirin and nonsteroidal anti-inflammatory drug hypersensitivity. Ann Allergy Asthma Immunol. 2017;118(4):518–20. https://doi.org/10.1016/j.anai.2017.01.026.
•• Kidon M, Blanca-Lopez N, Gomes E, Terreehorst I, Tanno L, Ponvert C, et al. EAACI/ENDA Position Paper: diagnosis and management of hypersensitivity reactions to non-steroidal anti-inflammatory drugs (NSAIDs) in children and adolescents. Pediatr Allergy Immunol. 2018;29(5):469–80. https://doi.org/10.1111/pai.12915 This position paper summarizes diagnostic and management guidelines for children with non-steroidal anti-inflammtory drugs.
Rosa JS, Vuong VB, Haskin O, Liu AY. A novel outpatient desensitization protocol for recombinant human erythropoietin allergy in a pediatric patient. Allergy, Asthma Clin Immunol. 2018;14:8. https://doi.org/10.1186/s13223-018-0233-1.
Bahloul N, Ben Mahmoud L, Ghozzi H, Hadjkacem F, Zeghal K, Abid M, et al. Hypersensitivity to levothyroxine: a case report of a successful oral desensitization. Therapie. 2018;73(4):349–50. https://doi.org/10.1016/j.therap.2017.10.009.
Castells Guitart MC. Rapid drug desensitization for hypersensitivity reactions to chemotherapy and monoclonal antibodies in the 21st century. J Investig Allergol Clin Immunol. 2014;24(2):72–9 quiz 2 p following 9.
Kim S, Choi IS, Kim YJ, Kim CS, Han ER, Park DJ, et al. Airway responsiveness to inhaled aspirin is influenced by airway hyperresponsiveness in asthmatic patients. Korean J Intern Med. 2010;25(3):309–16. https://doi.org/10.3904/kjim.2010.25.3.309.
Fajt ML, Petrov AA. Outpatient aspirin desensitization for patients with aspirin hypersensitivity and cardiac disease. Crit Pathw Cardiol. 2011;10(1):17–21. https://doi.org/10.1097/HPC.0b013e318213d5a6.
Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–33. https://doi.org/10.1056/NEJMoa1009717.
Pavlos R, Mallal S, Ostrov D, Buus S, Metushi I, Peters B, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–54. https://doi.org/10.1146/annurev-med-050913-022745.
Gueant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-Lopez N, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135(1):253–9. https://doi.org/10.1016/j.jaci.2014.07.047.
Wei CY, Lee MT, Chen YT. Pharmacogenomics of adverse drug reactions: implementing personalized medicine. Hum Mol Genet. 2012;21(R1):R58–65. https://doi.org/10.1093/hmg/dds341.
Cornejo-Garcia JA, Romano A, Gueant-Rodriguez RM, Oussalah A, Blanca-Lopez N, Gaeta F, et al. A non-synonymous polymorphism in galectin-3 lectin domain is associated with allergic reactions to beta-lactam antibiotics. Pharm J. 2016;16(1):79–82. https://doi.org/10.1038/tpj.2015.24.
Kowalski ML, Woszczek G, Bienkiewicz B, Mis M. Association of pyrazolone drug hypersensitivity with HLA-DQ and DR antigens. Clin Exp Allergy. 1998;28(9):1153–8. https://doi.org/10.1046/j.1365-2222.1998.00346.x.
Garcia-Martin E, Esguevillas G, Blanca-Lopez N, Garcia-Menaya J, Blanca M, Amo G, et al. Genetic determinants of metamizole metabolism modify the risk of developing anaphylaxis. Pharmacogenet Genomics. 2015;25(9):462–4. https://doi.org/10.1097/FPC.0000000000000157.
• Perkins JR, Acosta-Herrera M, Plaza-Seron MC, Jurado-Escobar R, Dona I, Garcia-Martin E, et al. Polymorphisms in CEP68 gene associated with risk of immediate selective reactions to non-steroidal anti-inflammatory drugs. Pharm J. 2019;19(2):191–9. https://doi.org/10.1038/s41397-018-0038-0 This paper focuses on the potential use of CEP68 gene as biomarker of clinical utility in drug hypersensitivity reactions.
Yang CY, Chen CH, Deng ST, Huang CS, Lin YJ, Chen YJ, et al. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan. JAMA Intern Med. 2015 Sep;175(9):1550–7. https://doi.org/10.1001/jamainternmed.2015.3536.
Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312(5):525–34. https://doi.org/10.1001/jama.2014.7859.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–79. https://doi.org/10.1056/NEJMoa0706135.
Karlin E, Phillips E. Genotyping for severe drug hypersensitivity. Curr Allergy Asthma Rep. 2014;14(3):418. https://doi.org/10.1007/s11882-013-0418-0.
Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. https://doi.org/10.1038/428486a.
Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007 May;48(5):1015–8. https://doi.org/10.1111/j.1528-1167.2007.01022.x.
Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol. 2011 Feb;50(2):221–4. https://doi.org/10.1111/j.1365-4632.2010.04745.x.
Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49(12):2087–91. https://doi.org/10.1111/j.1528-1167.2008.01719.x.
McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134–43. https://doi.org/10.1056/NEJMoa1013297.
Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20(5):1034–41. https://doi.org/10.1093/hmg/ddq537.
Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9(11):1617–22. https://doi.org/10.2217/14622416.9.11.1617.
Tassaneeyakul W, Jantararoungtong T, Chen P, Lin PY, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19(9):704–9. https://doi.org/10.1097/FPC.0b013e328330a3b8.
Lonjou C, Borot N, Sekula P, Ledger N, Thomas L, Halevy S, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99–107. https://doi.org/10.1097/FPC.0b013e3282f3ef9c.
Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K, et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia. 2010;51(12):2461–5. https://doi.org/10.1111/j.1528-1167.2010.02766.x.
Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. https://doi.org/10.1097/01.fpc.0000199500.46842.4a.
Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359(9308):727–32. https://doi.org/10.1016/s0140-6736(02)07873-x.
Clare KE, Miller MH, Dillon JF. Genetic factors influencing drug-induced liver injury: do they have a role in prevention and diagnosis? Curr Hepatol Rep. 2017;16(3):258–64. https://doi.org/10.1007/s11901-017-0363-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Adriana Ariza Veguillas declares that she has no conflict of interest. Tahia Diana Fernández-Duarte declares that she has no conflict of interest. Gador Bogas Herrera declares that she has no conflict of interest. María José Torres Jaén declares that she has no conflict of interest. Cristobalina Mayorga declares that she has no conflict of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Drug Allergy
Rights and permissions
About this article
Cite this article
Ariza, A., Fernández, T., Bogas, G. et al. How Mechanism Knowledge Can Help to Management of Drug Hypersensitivity. Curr Treat Options Allergy 7, 14–31 (2020). https://doi.org/10.1007/s40521-020-00244-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-020-00244-0