Abstract
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) has gained popularity as a very attractive target for diabetic therapies due to its role in lipid and glucose metabolism. Pharmacological activation of PGC-1α is thought to elicit health benefits. However, this notion has been questioned by increasing evidence, which suggests that insulin resistant is exacerbated when PGC-1α expression is far beyond normal physiological limits and is prevented under the condition of PGC-1α deficiency. This narrative review suggests that PGC-1α, as a master metabolic regulator, exerts roles in insulin sensitivity in a tissue-specific manner and in a physical activity/age-dependent fashion. When using PGC-1α as a target for therapeutic strategies against insulin resistance and T2DM, we should take these factors into consideration.
Level of evidence: Level V, narrative review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most common metabolic diseases in the world. The pathogenesis has been shown to be associated with insulin resistance in insulin-responsive tissues (e.g., skeletal muscle, liver, and adipose tissues) and reduced insulin secretion by β-cells [1, 2]. Furthermore, T2DM may lead to lots of comorbidities, such as cardiovascular disease and certain cancers [3]. Therefore, there is an increasing need for more effective treatments than medications currently available to help manage T2DM.
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), originally discovered in brown adipose tissue (BAT) [4], is mainly expressed at high levels in energy-demanding tissues such as skeletal muscle, BAT, pancreas, and liver [5]. It interacts with a wide range of transcription factors (TFs, reviewed in [6]) to modulate multiple cellular processes, including muscle fiber-type switching [7], hepatic gluconeogenesis [8], mitochondrial biogenesis [9], and insulin secretion [10]. It is highly inducible in response to physiological stimuli, including cold exposure, fasting, and exercise [11, 12]. It is dysregulated in pathological states, such as hypergluconeogenesis in the liver and mitochondrial dysfunction in skeletal muscle. Dysregulation of PGC-1α has been implicated in the pathogenesis of insulin resistance and T2DM [6]. On this basis, PGC-1α has been regarded as a promising target for anti-diabetic therapy [12].
However, mounting evidence has recently indicated that when using PGC-1α as a target for therapeutic strategies against insulin resistance and T2DM, we should take the following factors into consideration: PGC-1α’s expression level, the target tissues, the patient’s age, and the patient’s exercise [13, 14]. More importantly, several studies have highlighted the importance of PGC-1α deficiency, but not PGC-1α activation, in the prevention and treatment of insulin resistance and diabetes [15, 16]. In this narrative review, we summarize the major findings on the function of PGC-1α in multiple cellular processes and in different tissues, and then discuss its unlikely therapeutic applications for T2DM.
Regulation of PGC-1α
PGC-1α expression and activity are modulated by different stimuli in distinct tissues. More especially, PGC-1α expression is markedly induced by exercise and calorie restriction in muscle [17, 18], by fasting in the liver [19], and by cold temperatures in BAT [4], by exercise in white adipose tissue (WAT) [20]. In addition, PGC-1α expression is modulated on both transcriptional and post-translational levels. At the level of gene expression, several signaling cascades (e.g., the cAMP pathway) and proteins (e.g., cAMP response element binding protein (CREB) and activating transcription factor 2 (ATF2)) have been involved in the modulation of PGC-1α. In particular, the cAMP pathway plays central roles in activating PGC-1α transcription via promoting the binding of ATF-2 or CREB to the PGC-1α promoter, hence activating its transcription in many tissues. PGC-1α transcription is activated by β-adrenergic agonists through ATF-2 in BAT. The activation of PGC-1α in the liver by glucagon is mediated by CREB [8, 21], whereas calcium signaling cascades in combination with CREB are involved in the activation of PGC-1α in skeletal muscle by exercise [22, 23]. In addition, well-established post-translational modifications of PGC-1α include methylation [24], acetylation (mediated by the sirtuin (SIRT) family and the histone acetyltransferase GCN5) [25,26,27,28], and phosphorylation (by p38 MAPK, AMPK, Akt) [29]. The dynamic modifications of PGC-1α makes it to be implicated in the cellular adaptation to environmental conditions [30].
On the other hand, PGC-1α drives a pleiotropic transcriptional response through binding to and co-activating TFs and nuclear receptors, leading to improved lipid, glucose, and energy homeostasis [6, 31]. These TFs and nuclear receptors include myocyte enhance factor 2 [32], SIRT3 [33], hepatocyte nuclear factor 4α (HNF4α) [8], forkhead box O 1a (FOXO1a) [19], estrogen receptor-related α (ERRα) [34], peroxisome proliferator-activated receptor (PPAR) γ [4], PPARα [35], transcription factor A (TFAM), nuclear respiratory factor 1 (NRF1) [36], NF-κB [37], X-box binding protein 1 (XBP1) [38]. Accordingly, this makes regulation of PGC-1α a promising target for the treatment of insulin resistance and T2DM (Fig. 1).
Dysregulation of PGC-1α in animals and humans with T2DM
Over the past years, people have realized that PGC-1α expression is dysregulated in key metabolic tissues (e.g., skeletal muscle, adipose tissue, and liver) of animals and humans with insulin resistance and T2DM. In particular, in skeletal muscle of humans with T2DM and prediabetic individuals, PGC-1α expression and its co-transcription activity were reduced, in parallel with the reduction of the expression of PGC-1α-responsive genes implicated in mitochondrial biogenesis and oxidative phosphorylation (OXPHOS) [8, 10, 39, 40]. Similarly, in adipose tissue of insulin-resistant subjects, PGC-1α mRNA and protein expression were markedly downregulated [41]. In line with this observation, an high-fat diet (HFD)-fed mice with an adipose tissue-specific deletion of PGC-1α exhibited systemic deregulation of glucose homeostasis, as manifested by the reduction of hepatic insulin sensitivity and elevated levels of blood triglycerides and cholesterol [42]. Moreover, the obesity-associated reduction of adipose PGC-1α has been correlated with obesity-associated inflammation [43]. Therefore, obesity-associated inflammation and the development of systemic insulin resistance may be linked by the down-regulation of adipose PGC-1α. Furthermore, pre-diabetic individuals also exhibit decreased PGC-1α expression in muscle, indicating that PGC-1α inactivation is an early event in the development of this disease [39, 40].
Interestingly, ectopic expression of PGC-1α in muscle cells recovered expression of insulin-sensitive glucose transporter 4 (GLUT4) by coordinating the transcriptional MEF2C on the promoter [32]. Consistent with this in vitro study, muscle overexpression of PGC-1α showed improvement in metabolic responses, as evidenced by increased insulin sensitivity and insulin signaling in aged mice [44]. These results highlight the importance of targeting PGC-1α modulators to specific tissues and its efficacy in metabolic disease models. As a consequence, increasing PGC-1α expression/activity has great potential in the prevention and treatment of insulin resistance and diabetes, which has been extensively reviewed [12, 45, 46].
Paradoxical effects of increased PGC-1α activation on T2DM
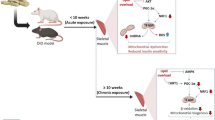
These foregoing studies have highlighted the therapeutic potential of increasing PGC-1α activation for T2DM treatment. However, data from tissue-specific transgenic or knockout animal models of PGC-1α have yielded disappointing results. In fact, the effects of PGC-1α activation on insulin sensitivity depend on several key factors, including tissue, physical activity, age, and the level of PGC-1α expression (Fig. 2).
Tissue
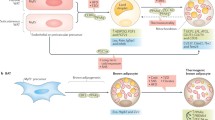
Skeletal muscle is a primary site for the utilization of glucose and fatty acids. Defects of these factors in combination with chronic low-grade inflammation, mitochondrial dysfunction, and oxidative stress contribute to the development of T2DM [2]. Evidence is emerging that PGC-1α can regulate the metabolic profile of muscle. First, PGC-1α can modulate the muscle fiber-type switch. Increases in the proportion of type I fibers, which contain more GLUT4 and mitochondria, have been observed in muscle of transgenic mice and pigs as well as exercised humans [7, 17, 47]. Second, PGC-1α regulates glucose metabolism. Increased GLUT4 expression and glucose uptake were observed in the ex vivo muscle with electrotransfection of PGC-1α [48] and in C2C12 and L6 muscle cells that overexpressed PGC-1α [32]. Apart from increasing glucose uptake, PGC-1α may increase fatty acid oxidation and glycogen synthesis and decrease glycolysis and glucose oxidation, resulting in enhanced muscle glycogen stores [49, 50]. These findings are in accordance with other studies showing that mice with muscle-specific PGC-1α overexpression displayed elevated muscle glycogen stores [51]. Third, muscle PGC-1α overexpression inhibits the development of inflammation, which has been demonstrated by both gain- and loss-of-function studies [52,53,54]. Last but not least, PGC-1α mediates OXPHOS and mitochondrial biogenesis in skeletal muscle. For example, forced expression of PGC-1α in cultured cardiac myocytes increased the cellular mitochondrial number and stimulated coupled respiration, as evidenced by the upregulated expression of nuclear and mitochondrial genes including NRF-1/2 and TFAM [55]. Likewise, cardiac-specific induction of PGC-1α resulted in a large increase in cardiac mitochondrial number and size during the neonatal stages [56]. In contrast, PGC-1α knockout mice exhibit a reduction in the expression of genes involved in OXPHOS and impaired mitochondrial function [52, 57, 58]. In cell cultures of human myotubes, PGC-1α overexpression has been demonstrated to increase fatty acid oxidative capacity by improving mitochondrial function [59]. In accord with these findings, recent in vitro and in vivo studies have also reported that the expression of PGC-1α in myotubes and skeletal muscle was elevated by a novel small molecule (ZLN005), exerting promising therapeutic effects for treating T2DM [60]. Overall, these findings highlight the importance of PGC-1α activation in the treatment of T2DM.
Adipose tissue is mainly composed of WAT and BAT. WAT mainly stores energy in form of lipid droplets, whereas BAT possesses non-shivering thermogenic properties due to increased mitochondrial content and expression of uncoupling protein-1 (UCP-1) [2, 61]. In vivo and in vitro studies have shown that PGC-1α increases adaptive thermogenesis [15, 16, 62] and mitochondrial biogenesis in BAT, as evidenced by the activation of transcription of mitochondrial UCP-1 [4]. In contrast, PGC-1α knockout in BAT resulted in dysregulation in lipid turnover, as manifested by a reduced level of lipid metabolizing enzymes and fatty acid transporters [63]. A recent study has identified four compounds with the ability to stabilize PGC-1α1 protein in BAT, setting the foundation for a novel generation of therapeutics based on the activation of PGC-1α1 that could be of use in metabolic disease [64]. Moreover, PGC-1α is implicated in the conversion of white fat into a brown fat-like phenotype. Ectopic expression of PGC-1α in WAT leads to its “browning” while suppression of PGC-1α may favor a white adipocyte phenotype [65,66,67]. Intriguingly, a brown-like adipose tissue gene programme can also be induced by muscle-specific overexpression of PGC-1α, as evidenced by increased UCP1 and Cidea expression in the subcutaneous fat layer (inguinal). This browning of WAT is stimulated by muscle-derived FNDC5 (a membrane protein that is cleaved and secreted as a myokine, irisin), whose expression is induced by increased muscle PGC-1α [68]. Unlike BAT, PGC-1α mRNA expression in WAT can be induced by exercise [20]. PGC-1α overexpression in WAT promotes mitochondrial biogenesis [69], whereas its deficiency gives rise to reductions in mitochondria [16]. Taken together, these observations highlight the beneficial effects of PGC-1α activation in BAT and WAT.
The liver is an organ that exerts important roles in controlling glucose homeostasis. In both fasting and well-fed states, the glucose concentration in the blood is stably maintained within a narrow range under normal conditions. Gluconeogenesis by the liver, together with glucose absorption by the intestine and glucose utilization by skeletal muscle, determines blood glucose levels [12]. The liver of mice with T2DM exhibits elevated gluconeogenesis, which is the main source of endogenous glucose production [8, 70]. The gluconeogenic enzymes such as phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6P) control rates of hepatic glucose production [71]. Interestingly, these gluconeogenic enzymes (PEPCK and G6P) are transcriptionally modulated by PGC-1α. It functions as a co-activator of FOXO1 and HNF-4α and as a co-suppressor of XBP1s, leading to impaired glucose homeostasis in obese and diabetic mice [19, 38, 71]. Furthermore, PGC-1α may decrease inhibition of hepatic glucose production by insulin, contributing to the onset of insulin resistance in liver through PPARα-dependent induction of Tribbles homologue 3 (an Akt inhibitor) [72]. Thus, PGC-1α expression is positively associated with hepatic glucose production [8, 73]. Increased expression of hepatic PGC-1α, as seen in several insulin-resistant animal models, including liver-specific insulin receptor-knockout, ob/ob [8], db/db [74], and HFD-fed [75] mice, has been demonstrated to cause hepatic insulin resistance [73]. Conversely, obese mice with genetically decreased levels of hepatic PGC-1α produced less glucose and were protected against insulin resistance [76]. Thus, in contrast to the induction sought after in tissues like skeletal muscle and adipose tissues, an inhibition of hepatic PGC-1α activity can reduce gluconeogenic activity and hence hold more promise for treating diabetes [77].
The main function of pancreatic β cells is to synthesize and secrete insulin, which contributes to maintain circulating glucose concentrations within a normal range. Normal mitochondrial function and ATP production are required for insulin secretion from pancreatic β cells [78]. The reduction of insulin secretion from β cells have been demonstrated to exacerbate T2DM. Like in the liver, PGC-1α is expressed at abnormally high levels in islets from diabetic rodents. In vitro studies have shown that overexpressing PGC-1α in isolated pancreatic rat islets blocks membrane polarization and induces G6P, thus decreasing insulin secretion [10]. PGC-1α impairs the ability of β-cells to secrete insulin through multiple mechanisms: (1) by impairing β-cell mass and function [79]; (2) by promoting β-cell apoptosis [80]; (3) by increasing mitochondrial biogenesis, oxidative stress and AMPK activation [81]. Of note, the onset of β-cell dysfunction may be associated with elevated expression of UCP2 [82], whose expression can be increased by PGC-1α in rat pancreatic islets and in INS-1 cells [83, 84]. In contrast, antisense oligonucleotide-induced suppression of rat islet PGC-1α corrects UCP2 expression level and to some extent normalizes insulin secretion by β-cells [83]. Intriguingly, the function of pancreatic islets is also regulated by IL-6, a myokine that is produced and secreted from muscle. In glucose intolerant and type 2 diabetic patients, reduced muscle PGC-1α expression elevated levels of circulating IL-6, leading to a reduction in insulin secretion by β-cells [53]. Therefore, similar to liver, a suppression of PGC-1α activity in pancreatic islets is beneficial for the treatment of diabetes.
Overall, PGC-1α affects lipid and glucose metabolism in a tissue-specific manner. In particular, increased PGC-1α expression is beneficial in skeletal muscle and adipose tissues, where it induces a change of muscle fiber phenotype towards oxidative metabolism and promotes thermogenesis and fat browning in adipose tissue, changes that inhibit diabetes. In contrast, its role is obviously deleterious in the liver and pancreas, in which it increases hepatic glucose production and suppresses insulin secretion, leading to the development of diabetes. Of note, the health benefits of increased PGC-1α expression in muscle go beyond the muscle tissue itself. PGC-1α induces the production and secretion of myokines (such as IL-6 and irisin) from skeletal muscle that influence the function of other tissues such as adipose tissue and pancreatic β cells (Fig. 3).
Physical activity
In response to exercise training, insulin sensitivity is enhanced and circulating levels of free fatty acids and insulin are reduced [85, 86]. PGC-1α expression can be increased in skeletal muscle (especially, the high-oxidative fast type) after prolonged exercise in humans [87, 88] and rodents [11, 86, 89]. Increased muscle PGC-1α promotes the production and secretion of certain myokines, mediating many of the beneficial effects of exercise locally and systemically. Once exposed to these myokines, muscle resident macrophages are polarized towards anti-inflammatory M2 phenotype, leading to increased secretion of anti-inflammatory cytokines (Fig. 2) [90]. Moreover, increased PGC-1α induces a coordinated program of increased fatty acid uptake, mitochondrial biogenesis, and fatty acid oxidation to meet the increased energy demands of working skeletal muscle [14, 91]. The activation and upregulation of PGC-1α have been demonstrated to be partially responsible for the beneficial effects exerted by exercise on skeletal muscle oxidative metabolism and insulin sensitivity, making it an attractive target for the development of antiobesity and/or antidiabetic drugs [11, 92,93,94,95]. Of note, increased physical activity does not upregulate the PGC-1α protein expression in the liver, suggesting that PGC-1α may be not involved in the action of exercise in improving hepatic insulin sensitivity [86].
Paradoxically, in sedentary state, elevated PGC-1α expression was not followed by improved glucose and insulin levels, and instead contributed to insulin resistance in mice fed HFDs [13, 14]. This increased insulin resistance may be due to an elevated provision of lipid, which exceeded the energetic demand of β-oxidation, thus causing a net elevation of intramyocellular fat and diacylglycerol content and insulin resistance [13, 96, 97]. Importantly, however, these detrimental effects could be reversed when these HFD-fed mice received a continuous exercise intervention [13]. These findings suggest that the effects of PGC-1α overexpression, as a monotherapy, depend on physical activity. In particular, elevation of PGC-1α is beneficial in exercised animals and is detrimental in sedentary animals who consume a HFD.
Age
Diabetes risk elevates greatly with age and is related to lower muscle oxidative capacity. Intriguingly, PGC-1α expression/activity in skeletal muscle also decreases with age [98, 99]. Using muscle-specific PGC-1α knockout mice, recent studies have demonstrated that a decline in PGC-1α and reduced mitochondrial oxidative capacity potentiate the development of glucose intolerance and insulin resistance associated with aging [100]. More importantly, when PGC-1α is lacking, age-associated decreases in mitochondrial proteins in skeletal muscle cannot be prevented by exercise training [101]. Consistent with these observations, gain-of-function studies have suggested that mildly increased muscle PGC-1α protects against age-related obesity and diabetes [44]. Overall, these observations highlight the importance of increasing PGC-1α expression in the prevention and treatment of age-induced diabetes.
Paradoxically, muscle PGC-1α overexpression led to insulin resistance in young mice fed HFDs [14]. In contrast, young muscle-specific PGC-1α knockout mice showed modestly improved glucose homeostasis [100]. These mice are capable of elevating mitochondrial protein expression in response to exercise training [102]. Therefore, PGC-1α is mandatory for the beneficial effects of moderate exercise training in elderly but not in young subjects to maintain mitochondrial metabolic and anti-oxidant capacity.
The level of PGC-1α expression
The extent of PGC-1α increases also influences its effects. For instance, in transgenic mice that overexpressed PGC-1α mRNA 10–13-fold, the GLUT4 mRNA and whole-body insulin sensitivity were reduced [103]. Limiting PGC-1α overexpression to skeletal muscle also yielded undesirable pathological effects, exacerbating fat-induced muscle insulin resistance despite an increase in mitochondrial density and mitochondrial activity [14]. PGC-1α overexpression in these models may have been far too large to be physiologically beneficial, since the increase in PGC-1α protein (5–30-fold) in genetically altered mice is considerably higher than those in rodent muscle stimulated by exercise training (1.5–2.5 fold) [89, 104] or cold exposure (1.5–2.8 fold) [105]. Excessive PGC-1α production leads to intramuscular lipid accumulation, contributing to insulin resistance in humans and animals [14, 48]. Therefore, these findings suggest that massively overexpression of PGC-1α leads to deleterious effects, calling into question the therapeutic potential of PGC-1α activation.
Paradoxically, upregulation of PGC-1α in vivo, similar to those that can be induced by physiological stimuli (< 100%), can protect against obesity and T2DM. In rat tibialis anterior muscle, overexpression of PGC-1α within physiological limits via an electrotransfection procedure, such as is observed with exercise, led to increased mitochondrial biogenesis and insulin sensitivity [48]. Beneficial effects of a modest elevation in PGC-1α levels have also been obtained in insulin-resistant muscle of obese Zucker rats, in which intramuscular lipids were reduced [106]. Therefore, modest upregulation of PGC-1α within physiological limits may be sufficient to reprogram the metabolic capacity of skeletal muscle. However, these observations should be subjected to a clinical trial.
PGC-1α deficiency prevents the development of insulin resistance and diabetes
Several loss-of-function studies have provided evidence of the contribution of PGC-1α to the pathogenesis of insulin resistance and diabetes. For instance, data from two models of PGC-1α-null mice have shown that these mice were protected from diet-induced obesity and insulin resistance [15, 16]. Moreover, these findings show that PGC-1α is dispensable for mitochondrial biogenesis and muscle fiber-type transformation [15, 16, 107]. Like whole body PGC-1α-null mice, muscle-specific PGC-1α-null mice have preserved mitochondrial content and displayed normal peripheral insulin sensitivity [53, 108]. More importantly, when challenged with a HFD, glucose intolerance or insulin resistance was not observed in these mice with total muscle PGC-1 deficiency, although mitochondrial structural derangements and impaired muscle oxidative capacity were observed [109]. Interestingly, normal glucose tolerance was also observed in β-cell-specific PGC-1α-null mice, despite disruption of insulin secretion [110]. Further evidence comes from the finding that adipose tissue-specific PGC-1α-null mice did not exhibit impaired mitochondria biogenesis, manifested by no alterations in the expression levels of mitochondrial genes. Consistent with these in vivo observations, PGC-1α knockdown in cultured 3T3-L1 adipocytes did not impact the mitochondrial gene expression and the adipocytes’ respiratory capacity [111]. Overall, these observations present evidence that PGC-1α deficiency may exert beneficial effects on insulin resistance and consequently diabetes. Although in that loss of function models one would expect to observe worsening of the metabolic profile, in most cases these mice show no effects. This could be the results of compensatory effects occurring in the transgenic animals, masking the real functional role of the transcription factor.
Concluding remarks
The prevalence of T2DM is increasing rapidly in both developed and developing countries. Effective therapeutic measures are urgently needed to reduce the current epidemic and to control this disease. PGC-1α, a transcription co-activator, has been regarded as a potential therapeutic target of antidiabetic therapy and pharmacological activation of PGC-1α is thought to elicit health benefits. However, this notion remains contentious, with studies failing to provide consensus evidence of a benefit from PGC-1α activation. Insulin resistant occurs when PGC-1α overexpression is far beyond normal physiological limits. In addition, PGC-1α activation exacerbates insulin resistance when PGC-1α expression is increased in tissues such as liver and pancreas, in young insulin-resistant subjects, and in animals and humans in sedentary state. More importantly, increasing studies have highlighted the contribution of PGC-1α deficiency to T2DM prevention and treatment. Thus, the literature reviewed here suggests that PGC-1α is differentially expressed in different tissues and has distinct and even opposite functions in different cells. When using PGC-1α as a target for therapeutic strategies against insulin resistance and T2DM, we should take the following factors into consideration: its expression level, the target tissues, the patient’s age, and the patient’s exercise.
References
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Investig 32:14–23. https://doi.org/10.1046/j.1365-2362.32.s3.3.x
Yao K, Duan Y, Li F, Tan B, Hou Y, Wu G, Yin Y (2016) Leucine in obesity: therapeutic prospects. Trends Pharmacol Sci 37:714–727. https://doi.org/10.1016/j.tips.2016.05.004
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253. https://doi.org/10.2147/DMSO.S43731
Puigserver P, Wu ZD, Park CW, Graves R, Wright M, Spiegelman BM (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839. https://doi.org/10.1016/S0092-8674(00)81410-5
Esterbauer H, Oberkofler H, Krempler F, Patsch W (1999) Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 62:98–102. https://doi.org/10.1006/geno.1999.5977
Finck BN, Kelly DP (2006) PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Investig 116:615–622. https://doi.org/10.1172/JCI27794
Lin J, Wu H, Tarr PT, Zhang CY, Wu ZD, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801. https://doi.org/10.1038/nature00904
Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM (2001) Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138. https://doi.org/10.1038/35093050
Corona J, Duchen M (2015) PPAR gamma and PGC-1 alpha as therapeutic targets in parkinson’s. Neurochem Res 40:308–316. https://doi.org/10.1007/s11064-014-1377-0
Yoon JC, Xu G, Deeney JT, Yang S-N, Rhee J, Puigserver P, Levens AR, Yang R, Zhang C-Y, Lowell BB, Berggren P-O, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM (2003) Suppression of β cell energy metabolism and insulin release by PGC-1α. Dev Cell 5:73–83. https://doi.org/10.1016/s1534-5807(03)00170-9
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886. https://doi.org/10.1096/fj.02-0367com
Wu H, Deng X, Shi Y, Su Y, Wei J, Duan H (2016) PGC-1α, glucose metabolism and type 2 diabetes mellitus. J Endocrinol 229:R99–R115. https://doi.org/10.1530/JOE-16-0021
Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C (2013) PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 62:85–95. https://doi.org/10.2337/db12-0291/-/DC1
Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman B, Shulman GI (2008) Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105:19926–19931. https://doi.org/10.1073/pnas.0810339105
Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135. https://doi.org/10.1016/j.cell.2004.09.013
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. https://doi.org/10.1371/journal.pbio.0030101
Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O (2003) Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52:2874–2881. https://doi.org/10.2337/diabetes.52.12.2874
Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E (2007) Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4:485–494. https://doi.org/10.1371/journal.pmed.0040076
Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D (2003) Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423:550. https://doi.org/10.1038/nature01667
Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC (2009) Exercise and adrenaline increase PGC-1α mRNA expression in rat adipose tissue. J Physiol 587:1607–1617. https://doi.org/10.1113/jphysiol.2008.165464
Herzig S, Long FX, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M (2001) CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183. https://doi.org/10.1038/35093131
Wu ZD, Huang XM, Feng YJ, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC (2006) Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci USA 103:14379. https://doi.org/10.1073/pnas.0606714103
Handschin C, Rhee J, Lin JD, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor γ coactivator 1α expression in muscle. Proc Natl Acad Sci USA 100:7111–7116. https://doi.org/10.1073/pnas.1232352100
Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR (2005) Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev 19:1466–1473. https://doi.org/10.1101/gad.1295005
Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab 3:429–438. https://doi.org/10.1016/j.cmet.2006.04.013
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature 434:113–118. https://doi.org/10.1038/nature03354
Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, Hochhauser E (2018) Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol 17:111. https://doi.org/10.1186/s12933-018-0754-4
Hu N, Ren J, Zhang YM (2016) Mitochondrial aldehyde dehydrogenase obliterates insulin resistance-induced cardiac dysfunction through deacetylation of PGC-1α. Oncotarget 7:76398–76414. https://doi.org/10.18632/oncotarget.11977
Li X, Monks B, Ge Q, Birnbaum MJ (2007) Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447:1012–1016. https://doi.org/10.1038/nature05861
Popov DV, Lysenko EA, Kuzmin IV, Vinogradova V, Grigoriev AI (2015) Regulation of PGC-1a isoform expression in skeletal muscles. Acta Nat 7:48–59
Wu Z, Boss O (2007) Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets 11:1329–1338. https://doi.org/10.1517/14728222.11.10.1329
Michael LF, Wu Z, Cheatham R, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman B (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 98:3820–3825. https://doi.org/10.1073/pnas.061035098
Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y (2010) Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5:e11707. https://doi.org/10.1371/journal.pone.0011707
Huss JM, Kopp RP, Kelly DP (2002) Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma: identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem 277:40265–40274. https://doi.org/10.1074/jbc.M206324200
Vega RB, Huss JM, Kelly DP (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876. https://doi.org/10.1128/Mcb.20.5.1868-1876.2000
Wu ZD, Puigserver P, Andersson U, Zhang CY, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124. https://doi.org/10.1016/S0092-8674(00)80611-X
Barroso WA, Victorino VJ, Jeremias IC, Petroni RC, Ariga SKK, Salles TA, Barbeiro DF, de Lima TM, de Souza HP (2018) High-fat diet inhibits PGC-1alpha suppressive effect on NFkappaB signaling in hepatocytes. Eur J Nutr 57:1891–1900. https://doi.org/10.1007/s00394-017-1472-5
Lee J, Salazar Hernandez MA, Auen T, Mucka P, Lee J, Ozcan U (2018) PGC-1alpha functions as a co-suppressor of XBP1s to regulate glucose metabolism. Mol Metab 7:119–131. https://doi.org/10.1016/j.molmet.2017.10.010
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC (2003) PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273. https://doi.org/10.1038/ng1180
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ (2003) Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471. https://doi.org/10.1073/pnas.1032913100
Hammarstedt A, Jansson PA, Wesslau C, Yang X, Smith U (2003) Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun 301:578–582. https://doi.org/10.1016/s0006-291x(03)00014-7
Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM (2012) Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci USA 109:9635–9640. https://doi.org/10.1073/pnas.1207287109
Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S (2006) TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Investig 116:2791–2798. https://doi.org/10.1172/JCI28570
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT (2009) Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA 106:20405–20410. https://doi.org/10.1073/pnas.1419043111
Handschin C (2009) The biology of PGC-1alpha and its therapeutic potential. Trends Pharmacol Sci 30:322–329. https://doi.org/10.1016/j.tips.2009.03.006
Besseiche A, Riveline JP, Gautier JF, Breant B, Blondeau B (2015) Metabolic roles of PGC-1alpha and its implications for type 2 diabetes. Diabetes Metab 41:347–357. https://doi.org/10.1016/j.diabet.2015.02.002
Ying F, Zhang L, Bu G, Xiong Y, Zuo B (2016) Muscle fiber-type conversion in the transgenic pigs with overexpression of PGC1alpha gene in muscle. Biochem Biophys Res Commun 480:669–674. https://doi.org/10.1016/j.bbrc.2016.10.113
Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A (2008) Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem 283:4228–4240. https://doi.org/10.1074/jbc.M704332200
Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP (2005) PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25:10684–10694. https://doi.org/10.1128/MCB.25.24.10684-10694.2005
Mormeneo E, Jimenez-Mallebrera C, Palomer X, De Nigris V, Vazquez-Carrera M, Orozco A, Nascimento A, Colomer J, Lerin C, Gomez-Foix AM (2012) PGC-1alpha induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS One 7:e29985. https://doi.org/10.1371/journal.pone.0029985
Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han DH, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP (2007) A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J Biol Chem 282:36642–36651. https://doi.org/10.1074/jbc.M707006200
Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282:30014–30021. https://doi.org/10.1074/jbc.M704817200
Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle–pancreatic beta cell crosstalk. J Clin Investig 117:3463–3474. https://doi.org/10.1172/JCI31785
Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C (2013) The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J Biol Chem 288:2246–2260. https://doi.org/10.1074/jbc.M112.375253
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Investig 106:847–856. https://doi.org/10.1172/Jci10268
Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP (2004) Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1 alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94:525–533. https://doi.org/10.1161/01.Res.0000117088.36577.Eb
Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM (2007) PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci USA 104:5223–5228. https://doi.org/10.1073/pnas.0611623104
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A (2006) Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4:e369. https://doi.org/10.1371/journal.pbio.0040369
Nikolic N, Rhedin M, Rustan AC, Storlien L, Thoresen GH, Stromstedt M (2012) Overexpression of PGC-1alpha increases fatty acid oxidative capacity of human skeletal muscle cells. Biochem Res Int 2012:714074. https://doi.org/10.1155/2012/714074
Zhang LN, Zhou HY, Fu YY, Li YY, Wu F, Gu M, Wu LY, Xia CM, Dong TC, Li JY, Shen JK, Li J (2013) Novel small-molecule PGC-1a transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes 62:1297–1307. https://doi.org/10.2337/db12-0703/-/DC1
Handschin C, Spiegelman BM (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454:463–469. https://doi.org/10.1038/nature07206
Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM (2006) Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 3:333–341. https://doi.org/10.1016/j.cmet.2006.04.002
Supruniuk E, Miklosz A, Chabowski A (2017) The implication of PGC-1alpha on fatty acid transport across plasma and mitochondrial membranes in the insulin sensitive tissues. Front Physiol 8:923. https://doi.org/10.3389/fphys.2017.00923
Pettersson-Klein AT, Izadi M, Ferreira DMS, Cervenka I, Correia JC, Martinez-Redondo V, Southern M, Cameron M, Kamenecka T, Agudelo LZ, Porsmyr-Palmertz M, Martens U, Lundgren B, Otrocka M, Jenmalm-Jensen A, Griffin PR, Ruas JL (2018) Small molecule PGC-1α1 protein stabilizers induce adipocyte Ucp1 expression and uncoupled mitochondrial respiration. Mol Metab 9:28–42. https://doi.org/10.1016/j.molmet.2018.01.017
Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O (2011) Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One 6:e28290. https://doi.org/10.1371/journal.pone.0028290
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM (2012) FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281. https://doi.org/10.1101/gad.177857.111
Pan D, Fujimoto M, Lopes A, Wang YX (2009) Twist-1 is a PPARdelta-inducible, negative feedback regulator of PGC-1alpha in brown fat metabolism. Cell 137:73–86. https://doi.org/10.1016/j.cell.2009.01.051
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–472. https://doi.org/10.1038/nature10777
Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D (2003) Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278:33370–33376. https://doi.org/10.1074/jbc.M305235200
Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM (2003) PGC-1beta in the regulation of hepatic glucose and energy metabolism. J Biol Chem 278:30843–30848. https://doi.org/10.1074/jbc.M303643200
Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM (2003) Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA 100:4012–4017. https://doi.org/10.1073/pnas.0730870100
Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M (2004) PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 10:530–534. https://doi.org/10.1038/nm1044
Liang H, Balas B, Tantiwong P, Dube J, Goodpaster BH, O’Doherty RM, DeFronzo RA, Richardson A, Musi N, Ward WF (2009) Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am J Physiol Endocrinol Metab 296:E945–E954. https://doi.org/10.1152/ajpendo.90292.2008
Tamura Y, Ogihara T, Uchida T, Ikeda F, Kumashiro N, Nomiyama T, Sato F, Hirose T, Tanaka Y, Mochizuki H, Kawamori R, Watada H (2007) Amelioration of glucose tolerance by hepatic inhibition of nuclear factor kappaB in db/db mice. Diabetologia 50:131–141. https://doi.org/10.1007/s00125-006-0467-1
Xu J, Li Y, Lou M, Xia W, Liu Q, Xie G, Liu L, Liu B, Yang J, Qin M (2018) Baicalin regulates SirT1/STAT3 pathway and restrains excessive hepatic glucose production. Pharmacol Res 136:62–73. https://doi.org/10.1016/j.phrs.2018.08.018
Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM (2009) PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci USA 106:22510–22515. https://doi.org/10.1073/pnas.0912533106
Sharabi K, Lin H, Tavares CDJ, Dominy JE, Camporez JP, Perry RJ, Schilling R, Rines AK, Lee J, Hickey M, Bennion M, Palmer M, Nag PP, Bittker JA, Perez J, Jedrychowski MP, Ozcan U, Gygi SP, Kamenecka TM, Shulman GI, Schreiber SL, Griffin PR, Puigserver P (2017) Selective chemical inhibition of PGC-1alpha gluconeogenic activity ameliorates type 2 diabetes. Cell 169:148.e115–160.e115. https://doi.org/10.1016/j.cell.2017.03.001
Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S (2008) Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51:615–622. https://doi.org/10.1007/s00125-007-0916-5
Valtat B, Riveline JP, Zhang P, Singh-Estivalet A, Armanet M, Venteclef N, Besseiche A, Kelly DP, Tronche F, Ferre P, Gautier JF, Breant B, Blondeau B (2013) Fetal PGC-1alpha overexpression programs adult pancreatic beta-cell dysfunction. Diabetes 62:1206–1216. https://doi.org/10.2337/db12-0314/-/DC1
Zhang P, Liu C, Zhang C, Zhang Y, Shen P, Zhang J, Zhang CY (2005) Free fatty acids increase PGC-1alpha expression in isolated rat islets. FEBS Lett 579:1446–1452. https://doi.org/10.1016/j.febslet.2005.01.046
Besseiche A, Riveline JP, Delavallée L, Foufelle F, Gautier JF, Blondeau B (2017) Oxidative and energetic stresses mediate beta-cell dysfunction induced by PGC-1α. Diabetes Metab 44:45–54. https://doi.org/10.1016/j.diabet.2017.01.007
Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM (2011) MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pr 91:94–100. https://doi.org/10.1016/j.diabres.2010.11.006
De Souza CT, Gasparetti AL, Pereira-da-Silva M, Araujo EP, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA (2003) Peroxisome proliferator-activated receptor gamma coactivator-1-dependent uncoupling protein-2 expression in pancreatic islets of rats: a novel pathway for neural control of insulin secretion. Diabetologia 46:1522–1531. https://doi.org/10.1007/s00125-003-1222-5
Oberkofler H, Klein K, Felder TK, Krempler F, Patsch W (2006) Role of peroxisome proliferator-activated receptor-gamma coactivator-1alpha in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology 147:966–976. https://doi.org/10.1210/en.2005-0817
Santos RF, Mondon CE, Reaven GM, Azhar S (1989) Effects of exercise training on the relationship between insulin binding and insulin-stimulated tyrosine kinase-activity in rat skeletal-muscle. Metabolism 38:376–386. https://doi.org/10.1016/0026-0495(89)90128-5
Matiello R, Fukui RT, Silva MER, Rocha DM, Wajchenberg BL, Azhar S, Santos RF (2010) Differential regulation of PGC-1α expression in rat liver and skeletal muscle in response to voluntary running. Nutr Metab 7:36. https://doi.org/10.1186/1743-7075-7-36
Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T (2004) PGC-1 alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 96:189–194. https://doi.org/10.1152/japplphysiol.00765.2003
Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N (2007) Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes - A time-course and dose-response study. Diabetes 56:836–848. https://doi.org/10.2337/db06-1119
Taylor EB, Lamb JD, Hurst RW, Chesser DG, Ellingson WJ, Greenwood LJ, Porter BB, Herway ST, Winder WW (2005) Endurance training increases skeletal muscle LKB1 and PGC-1 alpha protein abundance: effects of time and intensity. Am J Physiol Endocrinol Metab 289:E960–E968. https://doi.org/10.1152/ajpendo.00237.2005
Eisele PS, Handschin C (2014) Functional crosstalk of PGC-1 coactivators and inflammation in skeletal muscle pathophysiology. Semin Immunopathol 36:27–53. https://doi.org/10.1007/s00281-013-0406-4
Matsukawa T, Motojima H, Sato Y, Takahashi S, Villareal MO, Isoda H (2017) Upregulation of skeletal muscle PGC-1alpha through the elevation of cyclic AMP levels by Cyanidin-3-glucoside enhances exercise performance. Sci Rep 7:44799. https://doi.org/10.1038/srep44799
Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O (2007) An increase in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to exercise is mediated by beta-adrenergic receptor activation. Endocrinology 148:3441. https://doi.org/10.1210/en.2006-1646
Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM (2008) Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol (1985) 104:1304–1312. https://doi.org/10.1152/japplphysiol.01231.2007
Bonen A (2009) PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34:307–314. https://doi.org/10.1139/H09-008
Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z (2012) PGC-1alpha is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One 7:e41817. https://doi.org/10.1371/journal.pone.0041817
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic african-american women. J Nutr 139:1073–1081. https://doi.org/10.3945/jn.108.103754
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56. https://doi.org/10.1016/j.cmet.2007.10.013
Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, Beck-Nielsen H, Groop L, Vaag A (2004) Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 114:1518–1526. https://doi.org/10.1172/JCI21889
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735. https://doi.org/10.1210/er.2006-0037
Sczelecki S, Besse-Patin A, Abboud A, Kleiner S, Laznik-Bogoslavski D, Wrann CD, Ruas JL, Haibe-Kains B, Estall JL (2014) Loss of Pgc-1alpha expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab 306:E157–E167. https://doi.org/10.1152/ajpendo.00578.2013
Leick L, Lyngby SS, Wojtaszewski JF, Pilegaard H (2010) PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol 45:336–342. https://doi.org/10.1016/j.exger.2010.01.011
Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H (2008) PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294:E463. https://doi.org/10.1152/ajpendo.00666.2007
Miura S, Kai Y, Ono M, Ezaki O (2003) Overexpression of peroxisome proliferator-activated receptor gamma coactivator-1alpha down-regulates GLUT4 mRNA in skeletal muscles. J Biol Chem 278:31385. https://doi.org/10.1074/jbc.M304312200
Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z (2005) Exercise stimulates Pgc-1 alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280:19587–19593. https://doi.org/10.1074/jbc.M408862200
Oliveira RLGS, Ueno M, de Souza CT, Pereira-da-Silva M, Gasparetti AL, Bezzera RMN, Alberici LC, Vercesi AE, Saad MJA, Velloso LA (2004) Cold-induced PGC-1 alpha expression modulates muscle glucose uptake through an insulin receptor/Akt-independent, AMPK-dependent pathway. Am J Physiol Endocrinol Metab 287:E686–E695. https://doi.org/10.1152/ajpendo.00103.2004
Benton CR, Holloway GP, Han XX, Yoshida Y, Snook LA, Lally J, Glatz JF, Luiken JJ, Chabowski A, Bonen A (2010) Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia 53:2008–2019. https://doi.org/10.1007/s00125-010-1773-1
Arany Z, He HM, Lin JD, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271. https://doi.org/10.1016/j.cmet.2005.03.002
Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S, Koulisis N, Farrell C, Hirshman MF, Yan Z, Goodyear LJ, Rustin P, Arany Z (2013) Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle. Cell Rep 3:1449–1456. https://doi.org/10.1016/j.celrep.2013.04.023
Zechner C, Lai L, Zechner JF, Geng TY, Yan Z, Rumsey JW, Collia D, Chen ZJ, Wozniak DF, Leone TC, Kelly DP (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 12:633–642. https://doi.org/10.1016/j.cmet.2010.11.008
Oropeza D, Jouvet N, Bouyakdan K, Perron G, Ringuette LJ, Philipson LH, Kiss RS, Poitout V, Alquier T, Estall JL (2015) PGC-1 coactivators in beta-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Mol Metab 4:811–822. https://doi.org/10.1016/j.molmet.2015.08.001
Pardo R, Enguix N, Lasheras J, Feliu JE, Kralli A, Villena JA (2011) Rosiglitazone-induced mitochondrial biogenesis in white adipose tissue is independent of peroxisome proliferator-activated receptor γ coactivator-1α. PLoS One 6:e26989. https://doi.org/10.1371/journal.pone.0026989
Funding
This study was funded by the Project of Science and Technology of Jiangxi Province (20151BBF60008) and the Major Project of Education Department in Hunan (16A096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yuan, D., Xiao, D., Gao, Q. et al. PGC-1α activation: a therapeutic target for type 2 diabetes?. Eat Weight Disord 24, 385–395 (2019). https://doi.org/10.1007/s40519-018-0622-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-018-0622-y