Abstract
Aims/hypothesis
Recent studies have identified the involvement of inhibitor IκB kinase (IKK) in the pathogenesis of insulin resistance. To investigate the mechanism involved, we examined the role of nuclear factor κB (NF-κB), the distal target of IKK, in hepatic glucose metabolism.
Methods
To inhibit NF-κB activity, db/db mice were infected with adenovirus expressing the IκBα super-repressor.
Results
The IκBα super-repressor adenovirus infection caused a moderate reduction of NF-κB activity in liver. The treatment was associated with improved glucose tolerance, reduction in the serum insulin level, and increased hepatic triacylglycerol and glycogen contents, but had no effect on insulin-stimulated phosphorylation of Akt. On the other hand, quantification of mRNA in the liver revealed marked reduction of expression of gluconeogenic genes, such as those encoding phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase, concurrent with reduced expression of gene encoding peroxisome proliferator-activated receptor gamma coactivator-1α (PPARGC1A, also known as PGC-1α). Furthermore, the production of super-repressor IκBα suppressed the increase in blood glucose level after pyruvate injection.
Conclusions/interpretation
Our results indicate that moderate inhibition of NF-κB improved glucose tolerance through decreased gluconeogenesis associated with reduced PGC-1α gene expression in db/db mice, and suggest that inhibition of NF-κB activity in liver is a potentially suitable strategy for the normalisation of blood glucose concentration in type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin resistance is the hallmark of obesity and type 2 diabetes [1–3]. Although the exact mechanism of insulin resistance is not fully understood, recent data implicate the inhibitor κB (IκB) kinase (IKK) β in the deterioration of insulin action. It has been reported that mice with heterozygous disruption of the IKBKB (also known as IKKβ) gene are resistant to the development of peripheral insulin resistance caused by a high-fat diet and lipid infusion, and also when crossed with ob/ob mice, a genetic model of obesity [4, 5]. In addition, inhibition of IKKβ activity by salicylates prevents the development of skeletal muscle insulin resistance induced by acute lipid infusion [5]. Therefore, IKKβ represents a new target for the treatment of type 2 diabetes. In fact, Hundal et al. reported that administration of high-dose aspirin effectively improves insulin resistance associated with the improvement of peripheral and hepatic insulin resistance in human type 2 diabetes [6].
Recently, IRS-1 was identified as a novel direct substrate for IKK [7]. IKK phosphorylates IRS-1 at the Ser312 residue and impairs the insulin signalling pathway. On the other hand, IKK was identified originally as the proximal element of the IKK-IκB-nuclear factor κB (NF-κB) signalling pathway, which regulates inflammatory and anti-apoptotic effects. Without the signal, IκB binds directly to NF-κB to inhibit nuclear translocation. Once IKK is activated, it phosphorylates IκBα at Ser32 and Ser36 residues. Subsequently, phosphorylated IκBα is ubiquitinated and degraded by the 26S proteasome complex, thus releasing NF-κB to translocate into the nucleus, and it functions as a transcriptional activator of proinflammatory and anti-apoptotic genes. Because several studies have suggested the importance of chronic low-grade inflammation in the pathogenesis of insulin resistance [8, 9], in addition to IRS-1, NF-κB might be involved in the pathogenesis of insulin resistance as a direct target of IKK. In fact, deletion IKKβ in hepatocytes as well as in myeloid cells ameliorates hepatic insulin resistance concurrently with the decrease in inflammatory cytokines such as IL-1, IL-6 and TNF-α [10]. More directly, another recent in vivo study using transgenic mice demonstrated that inhibition of NF-κB ameliorates hepatic insulin resistance induced by a high-fat diet [11].
The present study was designed to explore the role of NF-κB in hepatic insulin resistance. For this purpose, we injected an adenovirus encoding the IκBα super-repressor (SR-IκBα) [12] into db/db mice, a genetic model of type 2 diabetes mellitus. The results demonstrated that moderate suppression of NF-κB activity in the liver is enough to improve glucose tolerance caused by reduced expression of gluconeogenesis genes. The reduced expression of peroxisome proliferator-activated receptor γ co-activator-1α (PPARGC1, also known as PGC-1α), independently of the insulin-stimulated phosphorylation of Akt, seems to be involved in this mechanism.
Materials and methods
Recombinant adenoviral vectors
The recombinant replication-deficient adenovirus Ad5IκB, encoding haemagglutinin (HA)-tagged mutated IκBα (substitution of Ala32 and Ala36 for two Ser residues), was kindly provided by D. A. Brenner (University of North Carolina) [12]. The control adenovirus expressing β-galactosidase (LacZ) (Ad-LacZ) was kindly provided by M. S. German (University of California, San Francisco). Both adenoviruses were produced based on human adenovirus type 5. Both adenoviruses were amplified in HEK-293 cells and purified by CsCl density centrifugation. Viral titres were determined by the 50% tissue culture infective dose (TCID50) method.
Animals and administration of recombinant adenovirus
The study was reviewed and approved by the Animal Care and Use Committee of Juntendo University. Specific-pathogen-free female C57BL/KsJ-db/db mice and their lean littermates, C57BL/KsJ-db/m, were purchased from Japan Clea (Tokyo, Japan). All mice were housed in stainless steel wire cages in a temperature-controlled clean room with a 12 h light–dark cycle. The animals were provided with standard chow and autoclaved tap water ad libitum. Ad5IκB or Ad-LacZ (5 × 109 plaque-forming units) diluted in PBS buffer or the same volume of PBS alone was injected into 13-week-old db/db mice through the tail vein. Food intake and body weight were measured before treatment and 2 and 4 days after treatment. To measure blood glucose and insulin concentrations, blood samples were collected from the tail vein. Mice were killed under anaesthesia induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg; Nembutal, Abbott Laboratories, Abbott Park, IL, USA).
Laboratory tests
The methods used for the laboratory tests are described in the Electronic supplementary material (ESM).
Intraperitoneal glucose tolerance and pyruvate challenge tests
Five days after adenovirus infection, mice were injected intraperitoneally with glucose (0.5 g/kg body weight) or pyruvate (1.5 g/kg body weight) [13–15] after overnight fasting. Blood samples were taken at various time points (0–120 min) from the tail vein and blood glucose levels were measured as described above. Serum insulin levels were measured 0 and 60 min after glucose administration.
Measurement of DNA binding activity
Livers from control and adenovirus-treated mice were harvested 5 days after the injection. Then, nuclear extracts were prepared as described previously [16]. The electrophoretic mobility shift assay (EMSA) was undertaken with 32P-labelled DNA probes for the NF-κB consensus binding site of the IκBα gene promoter [17]. The labelled oligonucleotide was purified on a column and annealed to an excess of the complementary strand. The EMSA buffers and electrophoresis conditions have been described previously [18]. Five micrograms of the nuclear extract was used for each 20 μl binding reaction. When using antibody, reactions were performed with 1 μl of horse serum (control), anti-p50, p65, p52 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). The mixture was incubated for 15 min at room temperature before polyacrylamide gel electrophoresis, and radioactive bands were visualised using Fuji BAS2500 (Fujifilm, Tokyo, Japan). DNA binding activity of NF-κB was quantified by EMSA-based NF-κB-specific ELISA (TransBinding NF-κB Assay Kit; Panomics, Fremont, CA, USA).
Hepatic glycogen and triacylglycerol measurements
Hepatic glycogen and triacylglycerol were measured as described previously [19, 20] (the methods used are also briefly described in the ESM).
Immunoprecipitation assay and western blotting analysis
To investigate the tissue distribution of SR-IκBα production, various tissues were isolated 24 h after infection with the adenovirus. To investigate the effect of adenovirus injection, after overnight fasting or refeeding for 12 h, the livers were isolated, weighed and snap-frozen in liquid nitrogen. Some livers were collected 5 min after injection of 10 mIU/body weight (g) into the inferior vena cava or 1 min after portal injection of 5 IU of insulin (Humulin) (Eli Lilly, Indianapolis, IN, USA). The isolated tissues were then homogenised with a hand-held homogeniser in RIPA buffer [21]. The samples were sonicated on ice and centrifuged at 15,000 g at 4°C for 20 min. The supernatant fractions were collected and western blotting analysis or immunoprecipitation–western blotting was performed as described previously [21, 22]. The sources of antibodies used for western blotting are described in the ESM.
Isolation of tissue RNA and real-time quantitative RT-PCR
Total RNA was extracted from livers by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Then cDNAs were synthesised using Superscript II RNase H Reverse Transcriptase (Invitrogen) and oligo-dT primers. The resulting cDNAs were amplified using the SYBR Green PCR kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed with an ABI Prism 7700 sequence detection system (Perkin Elmer Life Sciences, Boston, MA, USA). The relative abundances of mRNAs were calculated by the comparative cycle of threshold (CT) method with β-actin mRNA as the invariant control. The primers used in this study are listed in ESM, Table 1.
Results
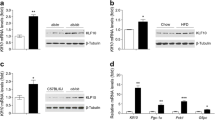
Ad5IκB treatment reduces hepatic NF-κB activity in db/db mice
Previous data showed that intravenous injection of Ad5IκB efficiently inhibited NF-κB activity in the rat liver [12]. To confirm the efficiency of Ad5IκB expression, the production of SR-IκBα was investigated in various tissues 5 days after tail vein injection in db/db mice. Mice infected with either type of adenovirus were apparently healthy. As shown in Fig. 1a and b, the production of SR-IκBα was identified in the liver but not in other tissues investigated. Production of SR-IκBα was not observed after injection of AdLacZ. In addition, the production of SR-IκBα reduced the production of the native form of IκBα, probably because IκBα is a direct downstream target of NF-κB. Next, we evaluated the DNA binding activity of NF-κB in liver (Fig. 1c and d). The basal DNA binding activity of NF-κB in db/db mice was increased compared with db/m mice. The injection of AdLacZ significantly increased the binding activity of NF-κB, probably as a result of the modest inflammation caused by adenovirus infection. The production of SR-IκBα significantly reduced the NF-κB binding activity compared with control and AdLacZ-treated db/db mice. To further confirm the effect of SR-IκB, we investigated the levels of the known downstream targets of NF-κB, such as the glucocorticoid receptor [23], manganese superoxide dismutase, C-reactive protein, IL-1β, IL-6 and TNF-α 5 days after the treatment. As shown in Fig. 1e, the expression level of C-reactive protein mRNA was notably decreased, and glucocorticoid receptor and manganese superoxide dismutase tended to be decreased by the production of SR-IκBα. However, we did not find a significant decrease in the expression of some genes, such as those encoding IL-1β, IL-6 and TNF-α, agreeing with previous data [12]. This might be partly because of incomplete suppression of NF-κB activity. In addition, because the production of these cytokines is not regulated only by NF-κB, it is possible that the modest inflammation induced by adenovirus infection increases the production of the cytokines independently of NF-κB activity.
Production and effects of SR-IκBα after injection of Ad5 IκB in db/db mice. a After intravenous injection of Ad5 IκB or AdLacZ (5 × 109 plaque-forming units per mouse) into db/db mice, 20 μg of tissue extract was immunoblotted using anti-HA antibody. b Liver extract (20 μg) after each treatment was immunoblotted using anti-IκBα and anti-HA antibodies. c Liver nuclear extracts were obtained from control db/m mice (lane 1), PBS-injected db/db mice (lanes 2, 5, 6, 7, 8 and 9), AdLacZ-treated db/db mice (lane 3) and Ad5IκB-treated db/db mice (lane 4). The DNA binding activity of NF-κB was evaluated by EMSA. One microlitre of horse serum (lane 5), anti-p50 antibody (lane 6), anti-p65 antibody (lane 7), anti-p52 antibody (lane 8) or a tenfold excess of unlabelled oligonucleotide (Comp.) was added to the reaction mixture. Arrow indicates major NF-κB complex. d DNA binding activity of NF-κB was quantified by ELISA. Data are expressed by setting the activity in the liver after PBS injection to 1. e Expression level of NF-κB-regulated genes was quantified by RT-PCR 6 days after treatment with PBS (grey bars), AdLacZ (open bars) or Ad5IκB (filled bars) (n = 5–7 for each group). Data are expressed relative to the β-actin mRNA level. The relative expression level was calculated by setting the expression level in the liver after PBS injection to 1. GR glucocorticoid receptor; MnSOD manganese superoxide dismutase; CRP C-reactive protein. Values are mean ± SEM. *p < 0.05 vs PBS group, # p < 0.05 vs LacZ group
SR-IκBα production ameliorates glucose tolerance in db/db mice
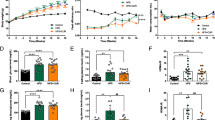
Table 1 lists the results of laboratory tests 5 days after each treatment. Serum triacylglycerol concentration was increased after both types of adenovirus treatment, as previously reported after adenoviral treatment [20]. Total cholesterol, NEFA and lactate levels did not change after any of the treatments. On the other hand, the production of SR-IκBα significantly reduced non-fasting blood glucose level, at least from 2 days to 10 days after the treatment (Fig. 2a). The fasting blood glucose level was also improved 5 days after Ad5IκB treatment (Fig. 2b). The results of an intraperitoneal glucose tolerance test 5 days after treatment indicated that the hepatic production of SR-IκBα improved glucose tolerance, with lower serum insulin 60 min after the glucose load (Fig. 2c and d). In addition, the homeostasis model assessment–insulin resistance (HOMA-IR) level was significantly lower in SR-IκBα-treated mice (HOMA-IR, LacZ group, 85.4 ± 17.7; PBS group, 83.3±19.9; SR-IκB, 41.0 ± 6.1, p < 0.05 vs LacZ and PBS group). To rule out the possibility that the improvement in glucose tolerance was due to loss of appetite, we measured the food intake and body weight. By 4 days after treatment, food intake was slightly, but similarly, decreased in the SR-IκBα and LacZ groups, whereas body weights were comparable among the groups (Fig. 2e and f).
Injection of Ad5IkB improves glycaemic control and glucose tolerance. a Blood glucose level after injection of PBS (grey triangles), AdLacZ (open squares), and Ad5IκB (filled squares) into db/db mice. b Fasting blood glucose concentrations 5 days after treatment with PBS (grey bar), AdLacZ (open bar), and Ad5IκB (filled bar). c, d Blood glucose (c) and immunoreactive insulin concentrations (d) after intraperitoneal injection of glucose (0.5 g/kg) 5 days after injection after treatment with PBS (grey triangles), AdLacZ (open squares) and Ad5IκB (filled squares). e, f Food intake (e) and body weight (f) after treatment with PBS (grey triangles), AdLacZ (open squares) and Ad5IκB (filled squares). g Five days after injection of PBS (grey triangles), AdLacZ (open squares) or Ad5IκB (filled squares), blood glucose concentration was measured following intraperitoneal pyruvate injection (1.5 g/kg) in animals that fasted overnight. Values are expressed as the increase in blood glucose compared with blood glucose before pyruvate injection. Values are mean ± SEM of six to ten (a–c, e, f) or four or five (d, g) experiments. *p < 0.05 vs PBS group, # p < 0.05 vs LacZ group, § p < 0.05 vs baseline of LacZ group, § p < 0.05 vs baseline in SR-IκBα group
Considering the finding that the production of SR-IκBα was observed mainly in the liver, it is likely that the amelioration of glucose tolerance by inhibition of NF-κB was mainly due to the alteration of hepatic glucose metabolism. Thus, we examined blood glucose levels after intraperitoneal injection of pyruvate, which is known to be a precursor of gluconeogenesis [13–15]. As shown in Fig. 2g, the increase in blood glucose level 30 min after pyruvate injection was significantly lower in the SR-IκBα-treated group than in other groups, suggesting that the production of SR-IκBα reduces hepatic gluconeogenesis.
Effect of SR-IκBα production on hepatic glycogen and triacylglycerol
To investigate the metabolic changes in the liver, we examined liver weight, hepatic triacylglycerol and glycogen contents. The liver weight of the SR-IκBα group was 60% higher than that of the PBS control group, whereas that of the LacZ-treated group was 30% higher than that of the PBS control group. A previous study [20] reported that infection with adenovirus increases liver weight, probably as a result of the increase in glycogen and triacylglycerol contents. Also in the present study, we observed a significant increase in glycogen and triacylglycerol contents after treatment with LacZ adenovirus. The increase in lipid accumulation in liver might be related to the increase in serum triacylglycerol level in both adenovirus treatment groups (Table 1). On the other hand, we definitely observed a difference in liver weight between the SR-IκBα treatment and the LacZ treatment. These changes were associated with 50% higher triacylglycerol content and 230% higher glycogen content in the liver in the SR-IκBα group compared with the LacZ group (Table 2).
Treatment with SR-IκBα does not alter insulin signalling
The changes observed in liver after SR-IκBα treatment were similar to those in mice with hepatic over-production of constitutively active Akt [24], although the phenotypic changes were very modest in our model. Thus, the improvement in blood glucose level after treatment with SR-IκBα might have been due to improvement in the insulin signal. However, we found no significant increase in the tyrosine phosphorylation state of IR-β, IRS-1 and IRS-2 and the association between IRSs and p85 subunit of phosphoinositide 3-kinase after insulin injection in the mice treated with SR-IκBα (Fig. 3a). Furthermore, the decreased insulin-stimulated phosphorylation of Akt observed in db/db mice was not improved by treatment with SR-IκBα (Fig. 3b). In addition, when we used a higher dose of insulin (5 IU per mouse), the phosphorylation of Akt and its downstream target, GSK3α in the liver, was not altered (Fig. 3c and d).
Insulin signal in the liver of SR-IκBα treated db/db mice. a With (+) and without (−) 10 mU of insulin injection per gram of body weight, total liver homogenates were isolated from Ad LacZ- and Ad5IκB adenovirus-injected db/db mice and used for western blotting analysis after immunoprecipitation using anti-IRβ, IRS-1 or IRS-2 antibody. Similar results were obtained in three independent experiments. b Total liver homogenates isolated from db/m mice and PBS-, Ad LacZ- and Ad5IκB adenovirus-treated db/db mice were used to investigate the insulin-stimulated phosphorylation of Akt. c, d Total liver homogenates were isolated from db/db mice injected with Ad LacZ- and Ad5IκB adenovirus with or without portal injection of 5 IU insulin to investigate the phosphorylation of Akt and GSK3. Values are mean ± SEM of four or five experiments
Effects of SR-IκBα on production of key metabolic factors
To further investigate the mechanism involved in metabolic changes induced by the production of SR-IκBα, we quantified the mRNA levels of key metabolic regulators in the liver by quantitative real-time PCR. As shown in Fig. 4a, the expression levels of gluconeogenic genes, such as those encoding phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase and glucose-6-phosphatase (G6Pase), were significantly suppressed following the production of SR-IκBα. These changes in gene expression patterns are compatible with the results of the pyruvate challenge test. On the other hand, no significant changes were noted in the expression of the genes encoding glucokinase and glycogen phosphorylase a, the rate-limiting enzymes of glycolysis and glycogen breakdown [25] respectively. Interestingly, the above changes in the gluconeogenic genes were associated with a significant reduction in expression of PGC-1α, which is the main regulator of the expression of these gluconeogenic genes [26, 27] (Fig. 4a). The reduction in PGC-1α expression was confirmed by western blotting analysis (Fig. 4b).
Expression of genes for key glucose metabolic regulators in the liver after production of SR-IκBα. a After adenovirus injection, mRNA levels of several genes in liver were measured by real-time RT-PCR. To quantify PGC-1α, and the expression of genes encoding PEPCK, F1,6-BPase and G6Pase, total RNA was isolated from the liver of mice in the fasted state. For the quantification of other genes, total RNA isolated from refed mice was used. Data are expressed relative to β-actin mRNA levels. The relative expression level was calculated by setting to 1 the expression level in the liver of mice injected with AdLacZ. Open bars Ad LacZ group; filled bars Ad5IkB group. b Total liver homogenates isolated from db/m mice from db/db mice treated with PBS, Ad LacZ or Ad5IκB adenovirus were used to investigate the production of PGC-1α by western blotting. Values are mean ± SEM of four to six experiments. Data were compared between the SR-IκBα group and the LacZ group. *p < 0.05 vs LacZ group; # p < 0.05 vs PBS, LacZ, db/m mice; † p < 0.05 vs db/m mice
Next, we measured the expression levels of lipogenic genes in the liver. As shown in Fig. 5a, the mRNA levels of the genes encoding fatty acid synthase, glycerol-3-phosphate acyltransferase and acetyl CoA carboxylase were modestly but significantly higher in the SR-IκBα group than in the control group. These increases were accompanied by a modest increase in sterol regulatory element binding protein 1 (SREBP1), which regulates the expression of these lipogenic genes. On the other hand, the expression levels of lipoxidative genes, such as those encoding acylCoA oxidase, carnitine palmitoyltransferase 1, medium-chain acyl-CoA dehydrogenase and peroxisome proliferator-activated receptor alpha (PPAR-α), an activator of lipoxidative genes, were not altered by the production of SR-IκBα (Fig. 5b).
Expression of genes for key lipid metabolic regulators in the liver after production of SR-IκBα. After adenovirus injection, mRNA levels of several genes in liver were measured by real-time RT-PCR. Total RNA isolated from refed mice was used for quantification. Data are expressed relative to β-actin mRNA levels. The relative expression level was calculated by setting to 1 the expression level in the liver of mice injected with AdLacZ. Open bars Ad LacZ group; filled bars Ad5IkB group. Values are mean ± SEM of four to six experiments. *p < 0.05 vs LacZ group
Effects of SR-IκBα production on STAT3, CREB and AMPK phosphorylation
The improvement of glucose tolerance by inhibition of NF-κB seems to be due to reduced expression of gluconeogenic genes and their regulator, PGC-1α. To further understand the mechanism, we investigated several factors that are known to regulate the expression levels of PGC-1α. STAT3 overexpression in primary hepatocytes is known to decrease the expression levels of PGC-1α and gluconeogenic genes [28]. However, the phosphorylation level of STAT3 was not altered by the production of SR-IκBα (Fig. 6a).
Effects of SR-IκBα production on protein phosphorylation of CREB, STAT3 and AMPK. Effects of adenovirus treatment on the phosphorylation levels of CREB, STAT3 and AMPK were investigated. Total liver homogenates were immunoblotted with antibodies to phospho-STAT3 (p-STAT3), STAT3, phospho-CREB (p-CREB), CREB, phospho-AMPK (p-AMPK) and AMPK. Values are mean ± SEM of four experiments
The expression levels of PGC-1α and gluconeogenic genes are positively regulated by the protein kinase A (PKA)–CREB axis [26, 27]. On the other hand, AMPK is known to suppress the expression levels of gluconeogenic genes [29]. However, the production of SR-IκBα did not significantly change the phosphorylation levels of CREB and AMPK (Fig. 6b and c).
Discussion
In this study, we investigated the role of NF-κB in hepatic glucose metabolism in db/db mice. There were two major findings of the present study. First, moderate inhibition of NF-κB activation without a significant decrease in the hepatic expression of the genes encoding the major cytokines in liver effectively improved glucose tolerance in db/db mice. Second, this effect was not accompanied by improved activation of Akt in the liver by insulin, but was associated with reduced expression of PGC-1α and its downstream gluconeogenic genes.
Hyperglycaemia observed in the diabetic state depends largely on increased hepatic glucose production and reduced glucose uptake in peripheral tissues. In particular, increased hepatic gluconeogenesis plays an important role in the pathophysiology of hyperglycaemia. The regulation of gluconeogenesis predominantly depends on the expression level of gluconeogenic genes, such as those encoding PEPCK, fructose-1,6-bisphosphatase and G6Pase. In fact, increased expression levels of these genes were observed in a diabetic model [30, 31]. Furthermore, glucose intolerance was observed in animals overexpressing the genes encoding PEPCK and G6Pase in the liver [32–34]. In particular, the importance of G6Pase in the control of blood glucose level is supported by the evidence that acute inhibition of G6Pase activity decreases blood glucose level in normal rats [35]. In the present study, inhibition of NF-κB suppressed the expression of gluconeogenic genes. The pyruvate challenge test supported the reduction of gluconeogenesis in the SR-IκBα group. Further, the production of SR-IκBα was observed exclusively in liver. Thus, we speculate that the improvement in glucose level following inhibition of NF-κB in the liver is caused mainly by reduced expression of gluconeogenic genes.
Our results showed increased hepatic triacylglycerol and glycogen contents after SR-IκBα treatment, concomitant with very modest increases in the expression of lipogenic genes. These changes are smaller but similar to the recently reported results of treatment with a G6Pase inhibitor in normal rats [35]. According to that report, inhibition of G6Pase results in an increase in de novo lipogenesis with increased flux through the acetyl-CoA pool. In addition, increased fatty acid synthase and acetyl CoA carboxylase expression levels were also observed, resulting in triacylglycerol accumulation in the liver. Therefore, the changes in lipid metabolism induced by SR-IκBα production observed in the present study might be largely due to reduced expression of G6Pase. G6Pase is a key enzyme in hepatic glucose release and defective G6Pase is a well-known cause of glycogen storage disease. Therefore, any decrease in G6Pase might contribute to the accumulation of hepatic glycogen and the improvement of blood glucose level through the inhibition of glucose release from liver.
PGC-1α functions as a coactivator of several transcription factors that play a critical role in hepatic glucose metabolism by regulating the expression of gluconeogenic genes [26, 27]. Because the expression of PGC-1α is markedly increased in the liver of diabetic rodents, such an increase could be involved in the mechanism of hyperglycaemia in type 2 diabetes [26, 27]. Recent studies reported that inhibition of hepatic PGC-1α expression can result in almost complete normalisation of the fasting glucose level and glucose tolerance in db/db mice [36]. Furthermore, in non-diabetic rodents, inhibition of PGC-1α lowered hepatic glucose output together with reduction of PEPCK and G6Pase expression levels [36]. Because it has been reported that NF-κB directly suppresses the activities of PEPCK [37] and G6Pase [38, 39] promoters in vitro and in vivo, it is unlikely that inhibition of NF-κB directly reduces the expression of gluconeogenic genes. The decrease in gluconeogenic gene expression by the production of SR-IκBα seems to depend on the decreased expression of PGC-1α.
Several studies have reported that the insulin signal reduces the expression of PGC-1α. Therefore, all the phenotypes caused by inhibition of NF-κB in db/db mice are likely to be due to the amelioration of insulin action [40, 41]. However, the phosphorylation level of molecules involved in insulin signalling, including Akt, was not altered by the production of SR-IκBα. Whereas it is well known that inflammatory cytokines such as IL-1β and TNF-α reduce tyrosine phosphorylation of IRSs, in the present experiments the expression of genes encoding IL1β and TNF-α was not suppressed, probably because of incomplete suppression of NF-κB activity and/or activation of such cytokines induced by the infection with adenovirus independently of NF-κB. However, interestingly, even in such a condition, we found a remarkable decrease in PGC-1α expression. Therefore, our results clearly demonstrate that such moderate inhibition of NF-κB decreased the expression of PGC-1α independently of the deterioration in insulin signalling produced by cytokines.
In general, PGC-1α plays a crucial role in the transactivation of several mitochondrial genes involved in β-oxidation, the Krebs cycle and oxidative phosphorylation, and is thus deeply involved in energy production. Whereas the activation of mitochondrial activity increased the production of reactive oxygen species (ROS), which are often detrimental to cell survival, PGC-1α simultaneously induced the expression of genes involved in ROS detoxification, such as superoxide dismutase and catalase [42]. In addition, PGC-1α stimulates the production of uncoupling proteins, which dissipate the proton gradient, and this results in a considerable reduction in ROS production by mitochondria [42]. The physiological role of PGC-1α in ROS metabolism is strongly supported by the abnormality seen in the brain of PGC-1α knock-out mice, in which axonal degeneration, especially in the striatum, was probably caused by the impaired ROS metabolism [15, 43]. Taking these findings into account, it is possible that ROS somehow regulates the gene expression of PGC-1α. In fact, at least in vitro, it was reported that the increase in ROS induced the expression of PGC-1α (B. M. Spiegelman, unpublished data). Thus, NF-κB, a representative redox-sensitive transcription factor, could be involved in its regulation.
The mechanism involved has not yet been clarified; however, NF-κB might alter the expression of the gene(s) that modifies signals involved in the regulation of the expression level of PGC-1α independently of CREB and STAT3. One such candidate gene is that for the glucocorticoid receptor. In the present study, we found reduced expression of glucocorticoid receptor following inhibition of NF-κB. Glucocorticoid positively regulates gluconeogenic genes, and it is therefore possible that the reduced expression of glucocorticoid receptor might have been involved in the reduced expression of gluconeogenic genes in the present study. In fact, it was reported that liver-specific gene disruption of glucocorticoid receptor resulted in hypoglycaemia [44]. In addition, treatment of db/db mice with a glucocorticoid receptor antagonist decreased plasma glucose levels by 50% and reduced the expression of genes encoding PEPCK and G6Pase to levels similar to those of non-diabetic control mice [45]. More importantly, glucocorticoid can strongly induce the expression of PGC-1α in the presence of PKA signal [26], although the glucocorticoid-responsible element has not been identified in the PGC-1α promoter. Further studies are required to clarify the role of glucocorticoid receptor expression level in the expression level of PGC-1α.
In agreement with previous data [20], the adenoviral infection in the present study induced hypertriacylglycolaemia and higher triacylglycerol accumulation in liver. In addition, the adenovirus infection induced mild liver inflammation with a modest increase in circulating asparate aminotransferase level, in general [46]. Because it is widely known that the inflammation and accumulation of triacylglycerol in liver is associated with insulin resistance, the presence of insulin resistance in the LacZ group compared with the PBS group was suspected. In the present experiments, whereas glucose tolerance was not different between the LacZ and PBS groups, the insulin level 60 min after the glucose tolerance test in the LacZ-treated group was higher than that in the PBS-treated group, although the difference was not statistically significant (Fig. 2c and d). Thus, the LacZ group might be modestly insulin resistant compared with the PBS group. However, the level of insulin resistance caused by adenoviral infection is too modest to induce the apparent metabolic changes seen in this study. It is likely that, in db/db mice, compared with insulin resistance caused by leptin receptor mutation, fatty liver and the inflammation caused by adenoviral infection might result in only a subtle effect on insulin resistance. In any case, our results were derived mainly from the comparison between the LacZ and SR-IκBα groups.
In conclusion, we have demonstrated that inactivation of NF-κB improves glycaemic control and glucose tolerance in db/db mice, in agreement with recently published data [10, 11]. The present results suggest that NF-κB is a potentially suitable therapeutic target for the treatment of type 2 diabetes.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- CREB:
-
cAMP response element-binding protein
- EMSA:
-
electrophoretic mobility shift assay
- G6Pase:
-
glucose-6-phosphatase
- GPAT:
-
glycerol-3-phosphate acyltransferase
- HA:
-
haemagglutinin
- HOMA-IR:
-
homeostasis model assessment–insulinresistance
- IKKβ:
-
IκB kinase β
- IκB:
-
inhibitor of κB
- IR:
-
insulin receptor
- NF-κB:
-
nuclear factor κB
- PEPCK:
-
phosphoenolpyruvate carboxykinase
- PGC-1α:
-
peroxisome proliferator-activated receptor γcoactivator 1α
- PKA:
-
protein kinase A
- ROS:
-
reactive oxygen species
- SR-IκBα:
-
IκBα super-repressor
- STAT:
-
signal transducer and activation of transcription
References
Perseghin G, Petersen K, Shulman GI (2003) Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord 27 (Suppl 3):S6–S11
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32 (Suppl 3):14–23
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171–176
Yuan M, Konstantopoulos N, Lee J et al (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293:1673–1677
Kim JK, Kim YJ, Fillmore JJ et al (2001) Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108:437–446
Hundal RS, Petersen KF, Mayerson AB et al (2002) Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109:1321–1326
Gao Z, Hwang D, Bataille F et al (2002) Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277:48115–48121
Gurnell M, Savage DB, Chatterjee VK, O’Rahilly S (2003) The metabolic syndrome: peroxisome proliferator-activated receptor gamma and its therapeutic modulation. J Clin Endocrinol Metab 88:2412–2421
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827
Arkan MC, Hevener AL, Greten FR et al (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198
Cai D, Yuan M, Frantz DF et al (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11:183–190
Iimuro Y, Nishiura T, Hellerbrand C et al (1998) NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest 101:802–811
Miyake K, Ogawa W, Matsumoto M, Nakamura T, Sakaue H, Kasuga M (2002) Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J Clin Invest 110:1483–1491
Banerjee RR, Rangwala SM, Shapiro JS et al (2004) Regulation of fasted blood glucose by resistin. Science 303:1195–1198
Lin J, Wu PH, Tarr PT et al (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135
FitzGerald MJ, Webber EM, Donovan JR, Fausto N (1995) Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ 6:417–427
Bradham CA, Stachlewitz RF, Gao W et al (1997) Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology 25:1128–1135
Watada H, Mirmira RG, Kalamaras J, German MS (2000) Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins. Proc Natl Acad Sci USA 97:9443–9448
Passonneau JV, Lauderdale VR (1974) A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60:405–412
Desai UJ, Slosberg ED, Boettcher BR et al (2001) Phenotypic correction of diabetic mice by adenovirus-mediated glucokinase expression. Diabetes 50:2287–2295
Nomiyama T, Igarashi Y, Taka H et al (2004) Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem Biophys Res Commun 320:639–647
Ogihara T, Watada H, Kanno R et al (2003) p38 MAPK is involved in activin A- and hepatocyte growth factor-mediated expression of pro-endocrine gene neurogenin 3 in AR42J-B13 cells. J Biol Chem 278:21693–21700
Webster JC, Oakley RH, Jewell CM, Cidlowski JA (2001) Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA 98:6865–6870
Ono H, Shimano H, Katagiri H et al (2003) Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes 52:2905–2913
Martin WH, Hoover DJ, Armento SJ et al (1998) Discovery of a human liver glycogen phosphorylase inhibitor that lowers blood glucose in vivo. Proc Natl Acad Sci USA 95:1776–1781
Yoon JC, Puigserver P, Chen G et al (2001) Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138
Herzig S, Long F, Jhala US et al (2001) CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183
Inoue H, Ogawa W, Ozaki M et al (2004) Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10:168–174
Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C (2000) 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes 49:896–903
Michael MD, Kulkarni RN, Postic C et al (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 6:87–97
Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6:77–86
Valera A, Pujol A, Pelegrin M, Bosch F (1994) Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA 91:9151–9154
Sun Y, Liu S, Ferguson S, Wang L et al (2002) Phosphoenolpyruvate carboxykinase overexpression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J Biol Chem 277:23301–23307
Trinh KY, O’Doherty RM, Anderson P, Lange AJ, Newgard CB (1998) Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J Biol Chem 273:31615–31620
Bandsma RH, Wiegman CH, Herling AW et al (2001) Acute inhibition of glucose-6-phosphate translocator activity leads to increased de novo lipogenesis and development of hepatic steatosis without affecting VLDL production in rats. Diabetes 50:2591–2597
Koo SH, Satoh H, Herzig S et al (2004) PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med 10:530–534
Waltner-Law M, Daniels MC, Sutherland C, Granner DK (2000) NF-kappa B inhibits glucocorticoid and cAMP-mediated expression of the phosphoenolpyruvate carboxykinase gene. J Biol Chem 275:31847–31856
Grempler R, Kienitz A, Werner T et al (2004) Tumour necrosis factor alpha decreases glucose-6-phosphatase gene expression by activation of nuclear factor kappaB. Biochem J 382:471–479
Metzger S, Begleibter N, Barash V et al (1997) Tumor necrosis factor inhibits the transcriptional rate of glucose-6-phosphatase in vivo and in vitro. Metabolism 46:579–583
Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A (2003) Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649
Zhou XY, Shibusawa N, Naik K et al (2004) Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat Med 10:633–637
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370
Leone TC, Lehman JJ, Finck BN et al (2005) PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101
Opherk C, Tronche F, Kellendonk C et al (2004) Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol Endocrinol 18:1346–1353
Friedman JE, Sun Y, Ishizuka T et al (1997) Phosphoenolpyruvate carboxykinase (GTP) gene transcription and hyperglycemia are regulated by glucocorticoids in genetically obese db/db transgenic mice. J Biol Chem 272:31475–31481
Lieber A, He CY, Kirillova I, Kay MA (1996) Recombinant adenoviruses with large deletions generated by Cre-mediated excision exhibit different biological properties compared with first-generation vectors in vitro and in vivo. J Virol 70:8944–8960
Acknowledgements
We thank D. A. Brenner (University of North Carolina) and M. S. German (University of California San Francisco) for providing the materials described in the text, K. Tamayose, M. Yamada, T. Furuya (Juntendo University), R. Suzuki (Tokyo University), Y. Nakatani (Osaka University), A. Lieber (University of Washington), and E. Hatano (Kyoto University) for providing technical information and N. Daimaru and Y. Toyofuku for their excellent technical assistance.
Duality of interest
The authors declare that no duality of interest exists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tamura, Y., Ogihara, T., Uchida, T. et al. Amelioration of glucose tolerance by hepatic inhibition of nuclear factor κB in db/db mice. Diabetologia 50, 131–141 (2007). https://doi.org/10.1007/s00125-006-0467-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-006-0467-1