Abstract
The proper management of pregnant women infected with hepatitis B virus (HBV) is necessary to prevent maternal and fetal morbidity and mortality and to protect the baby from HBV infection. In the majority of cases, vertical transmission can be prevented with a universal screening program, HBV vaccine immunoprophylaxis, and administration of hepatitis B immunoglobulin (HBIg) for babies born to mothers with HBV. However, in mothers with a high viral load (>200,000 or >1,000,000 IU/ml, depending on the guideline), the chance of immunoprophylaxis failure remains high. The standard recommendation is to give an antiviral agent during the third trimester in these patients. US FDA pregnancy category B agents such as tenofovir and telbivudine are allowed through all trimesters of pregnancy. Breastfeeding for patients who receive antiviral agents can be allowed after a risk–benefit discussion with the patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Universal screening for hepatitis B surface antigen (HBsAg) in all pregnancy groups is cost effective for the prevention of vertical transmission. |
Immunoprophylaxis with hepatitis B virus (HBV) vaccine and hepatitis B immunoglobulin (HBIg) administration remains the standard strategy to reduce the risk of mother-to-child transmission in HBV-infected mothers. |
In mothers with a high viral load (>200,000 or >1,000,000 IU/ml), the standard recommendation is to add antiviral therapy during the third trimester of pregnancy. |
For pregnant women with HBV who were already receiving treatment before the pregnancy, switching to a pregnancy category B agent is recommended. |
1 Introduction
Chronic hepatitis B (CHB) is a global public health issue associated with cirrhosis and hepatocellular carcinoma (HCC), especially in endemic areas. An estimated 240 million people worldwide are chronically infected with hepatitis B virus (HBV), and more than 686,000 CHB-related deaths occur every year [1]. Sub-Saharan Africa and East Asia have the highest prevalence of CHB, with rates of 5–10% of the adult populations. High rates of chronic infection are also found in the Amazon and the southern parts of eastern and central Europe. In the Middle East and the Indian subcontinent, an estimated 2–5% of the general population are chronically infected. Less than 1% of the population of Western Europe and North America are chronically infected [2].
Despite a worldwide HBV vaccination program over the last 3 decades, the numbers of chronically infected people have decreased only slightly because of rapid increases in the populations in endemic areas, especially Africa and Asia [3]. More than 50% of the CHB populations in endemic areas acquired their infection perinatally and contribute to 80% of the overall HCC burden in those areas [4, 5]. A total of 85–95% of infected babies develop chronic infection compared with <5% of those who become infected during adulthood [6,7,8,9]. According to all evidence, the prevention of mother-to-child transmission (MTCT) is the most efficient way to decrease the global disease burden of CHB and HCC. Taiwan demonstrates the best model of a successful MTCT program, showing not only a lower hepatitis B surface antigen (HBsAg) seroprevalence among children born after initiation of a universal vaccination program than among those born in prior but also a reduced incidence of HCC in children after two decades of the universal vaccination program [10]. The incidence of HCC among children born after the universal HBV vaccination program was reduced to 30–32% of that among those born before the program [11].
Furthermore, the complex relationship between the physiological changes of pregnancy and the host immunological responses to HBV and concerns about fetal and maternal safety lead to different management strategies for CHB in pregnancy. This review suggests an optimal approach to HBV management in pregnant women, including screening strategies, the treatment of CHB in pregnancy, and the prevention of perinatal transmission.
2 Screening Strategies for Hepatitis B Virus (HBV) in Pregnant Women
The universal screening for HBV in pregnant women has had a major impact in decreasing the risk of neonatal infection by identifying HBV-infected mothers whose babies can benefit from HBV vaccinations in combination with passive immunization with hepatitis B immunoglobulins (HBIgs). However, HBIg is a limited resource in some countries, such as Thailand, China, and many low-income countries. In countries where HBIg is unavailable, administering a universal HBV vaccination to every neonate is still the national preventive strategy against HBV MTCT. In these circumstances, universal screening for HBV is unnecessary because it does not provide additional management for HBsAg-positive mothers.
Over the last few years, the universal screening program has introduced international policies to identify HBV-infected women with exceptionally high viral loads who would benefit from receiving antiviral therapy in the third trimester of pregnancy. These policies have been recommended by all international guidelines, including the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Asian Pacific Association for the Study of the Liver (APASL) [12,13,14].
While HBsAg can be found in either acute or chronic infections, it is more likely to be found in chronic than in acute infections in an asymptomatic population [15]. In such settings, performing routine HBV screening with an isolated maternal HBsAg test is an adequate universal screening program. If the HBsAg result is positive, then evaluating for liver disease and the risk of MTCT is suggested to decide whether or what antiviral treatment is required in patients with significant liver disease or a high viral load (>200,000 or 1,000,000 IU/ml, depending on the guideline) indicating MTCT prophylaxis [12,13,14].
3 Influence of HBV Infection in Pregnancy
HBV does not interfere with fertility or conception except in patients with advanced liver disease [16]. However, the impact of CHB on pregnancy and fetal outcomes has not been clearly defined. Possible associations between CHB and gestational diabetes mellitus, antepartum hemorrhage, increased risk of prematurity, and lower birth weight have been described [17]. Recently, a large case–control study [18] demonstrated that HBsAg carrier mothers had minimally increased risks of preterm birth and low birth weight. In that study, a positive hepatitis B envelop antigen (HBeAg) status had a greater effect on preterm birth and low birth weight and increased risk of gestational diabetes than HBe-negative or control pregnancies [18].
Cirrhotic pregnant women have an increased danger of developing significant perinatal complications and having a poor pregnancy outcome [19]. A population-based study reported a higher spontaneous abortion rate and maternal complications, including gestational hypertension, placental abruption, and peripartum hemorrhage in cirrhotic pregnant women than in historical period-matched controls. Hepatic decompensation has been reported in 15% of pregnancies with liver disease [20] and up to 30% developed esophageal variceal hemorrhage. The risk of variceal hemorrhage increases up to 50–78% if there are pre-existing varices [21, 22].
4 Effects of Pregnancy on HBV-Associated Liver Disease
The physiological changes associated with normal pregnancy result in higher levels of adrenal corticosteroids and estrogen hormones, which can increase HBV viremia [23]. Nevertheless, worsening of pre-existing liver disease upon becoming pregnant is uncommon, primarily because the normal cell-mediated immunity (CMI) is suppressed during pregnancy. However, restoration of the CMI function occurs during the postpartum period, and studies have reported higher than expected HBeAg seroconversion rates [24] and higher than normal rates of alanine aminotransferase (ALT) during the initial postpartum period in HBV-infected women [23, 25,26,27,28,29]. While most hepatic flares have been mild and resolved spontaneously [25], a small number of case reports indicate severe hepatitis flares leading to hepatic decompensation during the peripartum period [29]. Some experts have suggested that HBV DNA and ALT should be monitored every 4–6 weeks during the first and second trimesters, every 4 weeks during the third trimester, and at postpartum months 3 and 6 in women with CHB [29]. The APASL recommends checking maternal HBeAg, HBV DNA status, and ALT levels during pregnancy to determine liver disease activity and the risk of MTCT [14].
5 Treatment of Chronic Hepatitis B (CHB) During Pregnancy
CHB treatment in pregnant women in whom HBV infection is diagnosed for the first time, or when a previously existing condition becomes exacerbated during the pregnancy, is initiated according to evidence of active liver disease in the same way as management of general CHB cases [14]. Assessment of liver disease severity should be made so as to weigh the risks and benefits for both the mother and the fetus [14, 30]. Theoretically, a mother who has advanced liver disease (advanced fibrosis, cirrhosis) will be at risk for developing liver decompensation if a hepatitis B flare occurs. These patients are the best candidates for initiating antiviral therapy, even in the first trimester. In patients with a milder form of liver disease, antiviral agents would be introduced only after thorough risk–benefit discussion.
For women who received antiviral therapy before becoming pregnant, the discontinuation of their antiviral therapy should be weighed against the possible risk of teratogenicity related to antiviral therapy exposure and the possibility of hepatitis flares. The severity of the liver disease, especially at the time of initiating the antiviral therapy, is the main factor that needs to be considered [31]. If the mother has advanced liver disease (advanced fibrosis, cirrhosis, or hepatic decompensation), treatment should be continued with oral antiviral agents because the treatment may benefit both mother and fetus. If a woman receiving interferon therapy becomes pregnant, the teratogenic risk to the fetus should be discussed with the woman and her family. For pregnant women who have received prior interferon treatment who have indications for CHB treatment, it is advisable to switch to a pregnancy category B oral antiviral agent such as telbuvidine or tenofovir [12].
The US FDA categorizes the pregnancy safety of anti-HBV agents into categories B (safe for use during pregnancy) and C (relatively contraindicated during pregnancy). Tenofovir and telbivudine are classed as category B, and lamivudine, entecavir, and adefovir are category C. The exception is lamivudine, which was accepted for use in pregnancy following extensive clinical trials and registration in the Antiretroviral Pregnancy Registry (APR) [32]. The APR is the most important source of information involving the safety of HBV therapy during pregnancy; it has been collecting birth defect data among HIV-positive women exposed to antiretroviral agents as well as all oral antiviral agents used for HBV therapy since 1 January 1989. The latest APR report, January 2016, included more than 20,000 cases (including 4589 exposed to lamivudine, 2779 exposed to tenofovir, and a small number of cases exposed to other anti-HBV agents) and reported that the birth defect rate of babies born to mothers who received an antiviral agent was similar to that of the general population, regardless of the agent used or the trimester during which the exposure occurred [32]. However, the registry includes only a small number of cases exposed to entecavir or adefovir, and both agents should be replaced by an FDA pregnancy category B anti-HBV agent. The APASL guideline recommends tenofovir as the first-line drug of choice for mothers indicated for antiviral treatment during any stage of pregnancy because of its safer FDA pregnancy category, adequate safety rating from the APR registry, and lowest chance of viral resistance [14]. Telbivudine is also a pregnancy class B drug, and is recommended by the EASL guideline for use in pregnancy [13].
6 Mother-to-Child-Transmission (MTCT) of HBV Infection
MTCT of HBV can occur at three stages of pregnancy: intrauterine, peripartum, or postpartum. The high success rate of immunoprophylaxis during the peripartum period supports the various studies suggesting that MTCT occurs predominantly during that period. The risk factors for MTCT include both viral and maternal factors. In the clinical setting, HBeAg is a strong indicator for active viral replication and infectivity. The risk of MTCT of HBV infection in HBeAg-positive mothers is as high as 70–90% and as 10–40% in HBeAg-negative mothers [33, 34]. In the era of vaccines and HBIg used for MTCT prevention, serum HBV DNA levels are the most significant independent risk factor for HBV MTCT [35, 36]. One study reported that pre-delivery maternal HBV DNA of more than 6 log IU/ml was associated with failure of immunoprophylaxis [35]. The reported maternal factors before the era of immunoprophylaxis were mainly related to placental leakage. Prolonged uterine contractions and threatened preterm delivery or abortion, which may disrupt the placenta and lead to maternal–fetal micro transfusions, are the main risk factors for MTCT [37, 38]. However, these maternal factors are not a significant risk of immunophylaxis failure [35].
The intrauterine MTCT of HBV is reported to take place in 10–16% of pregnancies and probably accounts for the low percentage of infants who do not respond to immunoprophylaxis treatment for HBV at birth [39,40,41]. As mentioned, the risk of intrauterine transmission is associated with placental leakage, which also raises the concern of the risk of transmission of HBV from amniocentesis or other invasive procedures during pregnancy. Several case–control studies have reported non-significant differences between the rates of HBV MTCT in newborns born to HBV-infected mothers who had undergone amniocentesis and those who had not [42,43,44]. However, the maternal HBeAg status and HBV DNA levels were unreported in those prior studies. The latest study enrolled 642 mothers with 63 in the amniocentesis group and demonstrated a significantly higher MTCT rate after amniocentesis in HBsAg-positive mothers with HBV DNA ≥7 log 10 copies/ml (odds ratio [OR] 21.3, 95% confidence interval [CI] 2.960–153.775) [45]. The Society for Maternal-Fetal Medicine (SMFM) states that for HBV-infected women who have an indication for invasive genetic testing (e.g., amniocentesis or chorionic villus sampling), the procedure may be offered providing there is counseling about the increased risk of maternal–fetal transmission with HBV viral load >7 log 10 copies/ml [46]. Although amniocentesis increases the risk of MTCT, the role of prophylactic antiviral therapy in a mother who has undergone amniocentesis has not yet been adequately studied.
7 Strategies to Prevent HBV-MTCT
7.1 Immunoprophylaxis
The immunoprophylaxis strategy of using HBV vaccine with HBIg has been the standard recommendation to prevent MTCT for more than two decades. The Wold Health Organization (WHO) immunoprophylaxis program for HBV infection in infants born to a mother with HBV includes a monovalent HBV vaccination within 24 h of birth with or without HBIg, followed by completion of the HBV vaccine series within 6 months [47]. In addition to the HBV vaccination, HBIg given to neonates after birth plays a significant role in reducing mother-to-infant vertical transmission, especially in HBeAg-positive women [48, 49]. Augmenting a universal HBV vaccination program with immunoglobulin treatment is likely to be cost effective, especially in settings with an already existing adequate healthcare infrastructure. In countries where the willing-to-pay section of the population is only of moderate number, targeting HBIg to neonates of higher-risk HBeAg-positive mothers may be preferred. However, universal vaccination alone is acceptable in a very resource-limited setting [50]. The universal vaccination program alone has been proven efficacious in preventing CHB infection. For example, a study from Thailand found 78% estimated protective efficacy of a three-dose series of the recombinant HBV vaccine (H-B-VAX™) for healthy newborn infants of HBeAg-positive carrier mothers [51].
Regrettably, recent data from 2012 indicate suboptimal implementation of the WHO Expanded Program on Immunization (EPI) recommendations, showing that only 52% of countries recommend giving the first HBV vaccine dose within 24 h of birth [52]. There are several reasons why countries could be having difficulty implementing the recommendations. First, both the HBV monovalent vaccine and HBIg are not widely available and affordable globally, particularly in resource-limited nations. A survey in 14 districts in China indicated that only 13–38% of HBV-exposed infants received HBIg after birth [53]. Second, out-of-hospital births are common in countries with high HBV endemicity. Laos is a good example of this problem, where only 37% of women give birth with a skilled attendant and only 34% of Laotian infants received a birth-dose EPI program vaccine, even as recently as 2011 [54]. Third is compliance with the universal screening policy in low-resource settings, which is still lacking. National campaigns aimed at improving accessibility to an HBV immunoprophylaxis program when indicated, especially in low-income countries, should be conducted.
7.2 Method of Delivery
Evidence surrounding the effect of the mode of delivery on the risk of HBV-MTCT is conflicting. Older studies did not show a significant difference in rates of infant HBV infection in infants born via caesarean section versus vaginal delivery. However, these studies had some notable differences. One study included a significant proportion of mothers who underwent emergency caesarean section [55]. Other studies had contradictory results regarding the benefit of elective caesarean section [56, 57]. The largest study [58], from China, investigated 1409 infants who had been born to HBsAg-positive mothers and received appropriate immunoprophylaxis at birth and reported MTCT rates of 1.4% with elective caesarean section versus 3.4% with vaginal delivery and 4.2% with urgent caesarean section. However, after stratification according to the level of viremia (low vs. high, with a cut-off of 200,000 IU/ml), the delivery modality was found to have no impact on HBV-MTCT [58]. In summary, the mode of delivery of CHB mothers is indicated by the obstetric condition. There is no role for elective caesarean section in preventing MTCT since it needs to be performed prior to the beginning of labor. Overall, other strategies, such as giving anti-HBV agents to mothers with high viral load and post-delivery immunoprophylaxis, are more practical and effective in preventing HBV-MTCT.
7.3 Antiviral Therapy in Pregnancy
The combination of passive and active immunization has been found to reduce the rate of MTCT from 90 to 10% [39, 59, 60], but immunoprophylaxis failure has been reported in 10–30% of infants born to mothers with an HBV DNA level >6 log 10 copies/ml (>200,000 IU/ml) [34,35,36]. Using antiviral therapy to reduce maternal viral load before delivery has become the standard recommendation in HBsAg-positive mothers with a high viral load, according to the most recent AASLD, APASL, and EASL guidelines [12,13,14]. However, the guidelines do not agree on the high viral load cut-off levels. AASLD recommends a lower HBV DNA cut-off of >200,000 IU/ml, whereas both the EASL and the APASL recommendations suggest >1,000,000 IU/ml [12,13,14]. Many studies have demonstrated the safety and efficacy of using lamivudine, telbivudine, and tenofovir as MTCT prophylaxis agents (summarized in Table 1) [61,62,63,64,65,66,67,68,69]. While giving an antiviral agent to mothers with a high viral load becomes a standard recommendation according to all international guidelines, exposure to a low genetic barrier agent such as lamivudine or telbivudine may theoretically raise the possibility of contributing to the development of a new drug-resistant strain. Although no data currently exist regarding drug resistance in maternal prophylaxis, this possibility should be mentioned during doctor–patient–family discussions before starting any such treatment.
7.3.1 Lamivudine
The earliest study of using lamivudine in pregnancy was published in 2003 [61]. It reported on eight women with high HBV DNA levels who were treated with lamivudine 150 mg daily beginning at 34 weeks of gestation; 25 children born to untreated women served as controls. All children received active and passive immunization at birth. At 12 months of age, 1 of 8 children (12.5%) in the treatment group remained HBsAg positive with positive HBV DNA levels compared with 7 of 25 children (28%) in the control group. No adverse events were noted [61]. In 2009, a double-blind, randomized controlled trial (RCT) examined the use of lamivudine 100 mg daily with active–passive immunization versus placebo with active–passive immunization in Chinese women at 32 weeks gestational age. At 52 weeks of age, infants born to the mothers who had received lamivudine had a significantly lower rate of HBsAg-positive and detectable HBV DNA than did those in the placebo group (18 vs. 39%). One congenital anomaly was noted in the lamivudine group [62]. A meta-analysis including 15 trials randomizing lamivudine treatment beginning at 28 weeks of pregnancy in 1693 CHB pregnancies found a risk ratio of 0.33 (95% CI 0.21–0.50; 6 RCTs; P [heterogeneity] = 0.46) for the detection of HBsAg in babies at the age of 6–12 months. Importantly, the risk reduction was significant only if HBV DNA was <6 log 10 copies/ml after taking lamivudine [63].
7.3.2 Telbivudine
Telbivudine is the first pregnancy category B agent to have data demonstrating efficacy in prevention of HBV MTCT. In an open-label study, 135 women with HBV DNA levels >7 log 10 copies/ml received talbivudine 600 mg daily from week 20 to week 32 of gestation compared with 94 untreated women. All infants received standard active-passive immunization at birth. At 7 months after delivery, HBV MTCT was significantly lower in the infants born to the telbivudine-treated mothers than in the controls (0 vs. 8%). No congenital abnormalities were reported [64]. A larger Chinese study including 648 women with high viral load were randomized to receive lamivudine, telbivudine, or placebo from 28 weeks’ gestation until 4 weeks postpartum. On-treatment analysis showed 0% HBsAg-positive infants in the treated group versus 2.84% in the placebo group. There was no difference in the MTCT rates between the lamivudine and telbivudine groups [65].
7.3.3 Tenofovir
Concerns about possible drug resistance in telbuvudine and lamivudine led to interest in the use of tenofovir as an MTCT prophylaxis agent. A Taiwanese prospective multicenter trial enrolled 118 HBsAg- and HBeAg-positive pregnant women with HBV DNA ≥7.5 log 10 IU/ml. HBV-positive pregnant women received either no medication (n = 56) or tenofovir disaproxil fumarate 300 mg daily (n = 62) from 30 to 32 weeks’ gestation until 1 month postpartum. The primary endpoint was HBsAg status at 6 months post-delivery, at which time the tenofovir group had lower rates of HBsAg positivity (1.54 vs. 10.71%; P = 0.0481) without significant adverse outcomes [67]. Additionally, a recent RCT trial confirmed the efficacy and safety of tenofovir disaproxil fumarate use for HBV-MTCT prophylaxis. HBV-positive pregnant women were randomly assigned to receive standard care (n = 100) or tenofovir (n = 97) from 30 to 32 weeks of gestation until postpartum week 4. The rate of MTCT of HBV at postpartum week 28 was significantly lower in the tenofovir group than in the control group, both in the intention-to-treat analysis (5 vs. 18%; P = 0.007) and in the per-protocol analysis (0 vs. 7%; P = 0.01) [68].
7.3.4 Other Aspects of Antiviral Treatment During Pregnancy
A number of studies have found that the use of lamivudine and telbivudine for MTCT prophylaxis in pregnant HBV-infected women with high levels of viremia is cost effective both in high-income countries such as the USA and Taiwan and in China, a middle-income country [70,71,72].
Without pre-existing advanced liver fibrosis/cirrhosis or hepatitis flare, cessation of antiviral therapy is recommended at delivery, 4–12 weeks after delivery, or when breastfeeding is begun [14]. While insufficient data are available regarding maternal ALT flare rates and severity after cessation of nucleos(t)ide analog (NA) therapy, serial follow-up liver function testing is recommended for every postpartum mother with CHB [12,13,14].
7.4 Breastfeeding
Mothers with CHB are not prevented from breastfeeding, as there is currently no evidence of any additional risk of MTCT of HBV from breastfeeding [73]. A meta-analysis including ten controlled clinical trials, involving 751 infants in the breastfeeding group and 873 infants in the non-breastfeeding group, found the OR of MTCT of HBV in the breastfeeding group compared with that in the non-breastfeeding group was 0.86 (95% CI 0.51–1.45) (from 8 controlled clinical trials; P = 0.56; I 2 = 0%; P = 0.99) [74]. Even in endemic countries where HBV vaccination is not promptly available, the WHO still recommends breastfeeding for infants of HBsAg-positive mothers [75]. However, all mothers who breastfeed should take good care of their nipples to avoid cracking and bleeding.
Information is currently lacking about the effects on the infant of exposure to NA during breastfeeding. Guidelines from EASL and other organizations state that the safety of NA therapy during lactation is uncertain [12,13,14]. Although the large registry data report that the birth defect rate of babies born to mothers receiving an antiviral agent was similar to that in the general population, many experts remain concerned about long-term consequences of prolonged antiviral agent exposure in the neonate, as it may have an impact on growth and development. They recommend discouraging breastfeeding in mother with HBV who receive an antiviral agent. However, breastfeeding is advantageous on many issues, especially in low-income countries where formula feeding is not widely available. Breastfeeding may not be considered a contraindication in HBsAg-positive mothers who receive an antiviral agent. In fact, although the risk of in utero exposure to drugs is likely higher than for infants through breast milk, these drugs are recommended for use during pregnancy [76]. Furthermore, antiretroviral treatment could continue during the breastfeeding period in HIV-infected women [76]. In summary, the risks and benefits of breastfeeding during NA therapy should be discussed with the woman and her family.
8 Conclusion
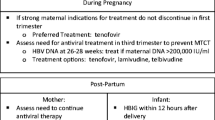
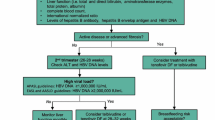
On the basis of currently available data, the optimal management regimen that we recommend for pregnant women with CHB is shown in Fig. 1. The universal HBV screening program in pregnancy plays the most important role in identifying the target population who need proper management. Thus, all countries with endemic HBV should make these strategies a priority.
Algorithm for management of hepatitis B virus during pregnancy. Asterisk Viral load ≥200,000 IU/ml can be used as a high viral load cut-off according to AASLD guidelines. LFT includes total bilirubin, direct bilirubin, aminotransferase enzyme (AST, ALT), total protein, and albumin. AASLD American Association for the Study of Liver Diseases, ALT alanine aminotransferase, AST aspartate aminotransferase, CBC complete blood count, HBeAb hepatitis Be antibody, HBeAg hepatitis B envelop antigen, HBIg hepatitis B immunoglobulin, HBsAg+ve hepatitis B surface antigen-positive, HBV hepatitis B virus, INR international normalized ratio, LFT liver function test
References
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71.
World Health Organization. Hepatitis B fact sheet. Geneva: WHO. 2016. http://www.who.int/mediacentre/factsheets/fs204/en/. Accessed 15 July 2016.
Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–9.
Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl. 1):S1–3.
Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29(Suppl. 1):133–9.
Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–4.
Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol. 2000;15:E16–9.
Tassopoulos NC, Papaevangelou GJ, Sjogren MH, Roumeliotou-Karayannis A, Gerin JL, Purcell RH. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology. 1987;92:1844–50.
McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603.
Kao JH. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2015;29(6):907–17.
Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348–55.
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–83.
European Association for the Study of the Liver, et al. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Dragosics B, Ferenci P, Hitchman E, Denk H. Long-term follow-up study of asymptomatic HBsAg-positive voluntary blood donors in Austria: a clinical and histologic evaluation of 242 cases. Hepatology. 1987;7(2):302–6.
Pirutvisuth T. Optimal management of HBV infection during pregnancy. Liver Int. 2013;33(1):188–94.
Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Semin Liver Dis. 2013;33(2):138–46.
Sirilert S, Traisrisilp K, Sirivatanapa P, Tongsong T. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int J Gynaecol Obstet. 2014;126(2):106–10.
Tan J, Surti B, Saab S. Pregnancy and cirrhosis. Liver Transpl. 2008;14:1081–91.
Shaheen AAM, Myers RP. The outcomes of pregnancy in patients with cirrhosis: a population-based study. Liver Int. 2010;30:275–83.
Russell MA, Craigo SD. Cirrhosis and portal hypertension in pregnancy. Semin Perinatol. 1998;22:156–65.
Britton RC. Pregnancy and esophageal varices. Am J Surg. 1982;143:421–5.
ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37–41.
Lin HH, Chen PJ, Chen DS, Sung JL, Yang KH, Young YC, et al. Postpartum subsidence of hepatitis B viral replication in HBeAg-positive carrier mothers. J Med Virol. 1989;29:1–6.
Dyson JK, Waller J, Turley A, Michael E, Moses S, Valappil M, et al. Hepatitis B in pregnancy. Frontline Gastroenterol. 2014;5:111–7.
Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut. 2015;64:1810–5.
Tan HH, Lui HF, Chow WC. Chronic hepatitis B virus (HBV) infection in pregnancy. Hepatol Int. 2008;2:370.
Nguyen V, Tan PK, Greenup AJ, Glass A, Davison S, Samarasinghe D, et al. Anti-viral therapy for prevention of perinatal HBV transmission: extending therapy beyond birth does not protect against post-partum flare. Aliment Pharmacol Ther. 2014;39:1225–34.
Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau D, Nguyen MH. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am J Gastroenterol. 2016;111(10):1410–5.
He T, Jia J. Chronic HBV: which pregnant women should be treated? Liver Int. 2016;36(Suppl. S1):105–8.
Patton H, Tran TT. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol. 2014;11:402–9.
Antiretroviral Pregnancy Registry Steering Committee. The Antiretroviral Pregnancy Registry interim report. 1 January 1989 through 31 January 2016. http://www.apregistry.com/forms/interim_report.pdf. Accessed 26 Nov 2016.
DegliEsposti S, Shah D. Hepatitis B in pregnancy: challenges and treatment. Gastroenterol Clin North Am. 2011;40:355–72.
Pan CQ, Duan ZP, Bhamidimarri KR, Zou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol. 2012;10(5):452–9.
Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489–92.
Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HbsAg positive mothers. J Viral Hepat. 2012;19:e18–25.
Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case–control study. J Med Virol. 2002;67:20–6.
Wong VC, Lee AK, Ip HM. Transmission of hepatitis B antigens from symptom free carrier mothers to the fetus and the infant. Br J Obstet Gynaecol. 1980;87:958–65.
Beasley RP, Hwang LY, Stevens CE, Lin CC, Hsieh FJ, Wang KY, et al. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3(2):135–41.
Schalm SW, Mazel JA, de Gast GC, Heijtink RA, Botman MJ, Banffer JR, et al. Prevention of hepatitis B infection in newborns through mass screening and delayed vaccination of all infants of mothers with hepatitis B surface antigen. Pediatrics. 1989;83(6):1041–8.
Stevens CE, Toy PT, Tong MJ, Taylor PE, Vyas GN, Nair PV, et al. Perinatal hepatitis B virus transmission in the United States. Prevention by passive-active immunization. JAMA. 1985;253(12):1740–5.
Ko TM, Tseng LH, Chang MH, Chen DS, Hsieh FJ, Chuang SM, et al. Amniocentesis in mothers who are hepatitis B virus carriers does not expose the infant to an increased risk of hepatitis B virus infection. Arch Gynecol Obstet. 1994;255:25–30.
Alexander JM, Ramus R, Jackson G, Sercely B, Wendel GD Jr. Risk of hepatitis B transmission after amniocentesis in chronic hepatitis B carriers. Infect Dis Obstet Gynecol. 1999;7:283–6.
Towers CV, Asrat T, Rumney P. The presence of hepatitis B surface antigen and deoxyribonucleic acid in amniotic fluid and cord blood. Am J Obstet Gynecol. 2001;184:1514–8.
Yi W, Pan CQ, Hao J, Hu Y, Liu M, Li L, Liang D. Risk of vertical transmission of hepatitis B after amniocentesis in HBs antigen-positive mothers. J Hepatol. 2014;60(3):523–9.
Dionne-Odom J, Tita AT, Silverman NS. # 38: Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214(1):6–14.
Centers for Disease Control and Prevention (CDC). Global routine vaccination coverage—2012. Morb Mortal Wkly Rep. 2013;62:858–61.
Wong VC, Ip HM, Reesink HW, Lelie PN, Reerink-Brongers EE, Yeung CY, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis B immunoglobulin: double-blind randomised placebo-controlled study. Lancet. 1984;1(8383):921–6.
Lo KJ, Tsai YT, Lee SD, Wu JC, Wu TC, Yang ZL, et al. Combined passive and active immunization for interruption of perinatal transmission of hepatitis B virus in Taiwan. Hepatogastroenterology. 1985;32(2):65–8.
Chen SC, Toy M, Yeh JM, Wang JD, Resch S. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobin treatment [erratum appears in Pediatrics 2014;133(2):346]. Pediatrics. 2013;131(4):e1135–43. doi:10.1542/peds.2012-1262.
Lolekha S, Warachit B, Hirunyachote A, Bowonkiratikachorn P, West DJ, Poerschke G. Protective efficacy of hepatitis B vaccine without HBIG in infants of HBeAg-positive carrier mothers in Thailand. Vaccine. 2002;20(31–32):3739–43.
Hu Y, Zhang S, Luo C, Liu Q, Zhou YH. Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: a provincial population-based study in China. BMC Infect Dis. 2012;12:221.
Huang Y, Li L, Sun X, Lu M, Liu H, Tang G, et al. Screening of pregnant women for hepatitis B virus surface antigen (HBsAg) and subsequent management, Qiandongnan prefecture, Guizhou, China, 2010. Vaccine. 2013;31(Suppl. 9):S62–5.
Centers for Disease Control and Prevention (CDC). Hepatitis B vaccine birthdose practices in a country where hepatitis B is endemic—Laos, December 2011–February 2012. Morb Mortal Wkly Rep. 2013;62(29):587–90.
Wang J, Zhu Q, Zhang X. Effect delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J. 2002;115:1510–2.
Yang J, Zeng X, Men Y, Zhao L. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus—a systematic review. Virol J. 2008;5:100.
Zou H, Chen Y, Duan Z, Zhang H, Pan C. A retrospective study for clinical outcome of caesarean section on perinatal transmission of hepatitis B virus in infants born to HBeAg positive mothers with chronic hepatitis B (CHB). Hepatology. 2010;52(Suppl(1)):441A.
Pan CQ, Zou HB, Chen Y, Zhang X, Zhang H, Li J, Duan Z. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol. 2013;11(10):1349–55.
Luo ZB, Li LJ, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis. 2012;16:e82–8.
Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunization in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–36.
van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL, et al. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–7.
Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16(2):94–103.
Han L, Zhang H-W, Xie J-X, Zhang Q, Wang HY, Cao GW, et al. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J Gastroenterol. 2011;17:4321–33.
Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55(6):1215–21.
Zhang H, Pan CQ, Pang Q, Tian R, Yan M, Liu X. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60(2):468–76.
Wu Q, Huang H, Sun X, Pan M, He Y, Tan S, et al. Telbivudine prevents vertical transmission of hepatitis B virus from women with high viral loads: a prospective long-term study. Clin Gastroenterol Hepatol. 2015;13(6):1170–6.
Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al.; Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV (PreMIT Study); Taiwan Study Group for the Prevention of Mother-to-Infant Transmission of HBV PreMIT Study. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62(2):375–86.
Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al.; China Study Group for the Mother-to-Child Transmission of Hepatitis B. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324–34.
Greenup AJ, Tan PK, Nguyen V, Glass A, Davison S, Chatterjee U, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol. 2014;61(3):502–7.
Fan L, Owusu-Edusei K Jr, Schillie SF, Murphy TV. Cost-effectiveness of testing hepatitis B-positive pregnant women for hepatitis B e antigen or viral load. Obstet Gynecol. 2014;123(5):929–37.
Hung HF, Chen HH. Cost-effectiveness analysis of prophylactic lamivudine use in preventing vertical transmission of hepatitis B virus infection. Pharmacoeconomics. 2011;29(12):1063–73.
Wang W, Wang J, Dang S, Zhuang G. Cost-effectiveness of antiviral therapy during late pregnancy to prevent perinatal transmission of hepatitis B virus. PeerJ. 2016;24(4):e1709.
Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99:1049–52.
Shi Z, Yang Y, Wang H, Ma L, Schreiber A, Li X, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165(9):837–46.
World Health Organization. Hepatitis B and breastfeeding. No. 22. Geneva: WHO; 1996. http://www.who.int/child_adolescent_health/documents/pdfs/hepatitis_b_and_breastfeeding.pdf. Accessed 27 Nov 2016.
Benaboud S, Pruvost A, Coffie PA, Ekouévi DK, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d’Ivoire, in the ANRS12109 TEmAA Study, step 2. Antimicrob Agents Chemother. 2011;55:1315–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Naichaya Chamroonkul has no conflicts of interest. Teerha Piratvisuth has received research grants from Geliad, MSD, and BMS and consulting fees or speakers’ honoraria from Geliad, MSD, BMS, Roche, GSK, Novatis, and Bayers.
Funding
No sources of funding were used to support the writing of this manuscript.
Rights and permissions
About this article
Cite this article
Chamroonkul, N., Piratvisuth, T. Hepatitis B During Pregnancy in Endemic Areas: Screening, Treatment, and Prevention of Mother-to-Child Transmission. Pediatr Drugs 19, 173–181 (2017). https://doi.org/10.1007/s40272-017-0229-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0229-1