Abstract
With universal HIV testing prior to or early in pregnancy, early and effective antiretroviral therapy for all pregnant women, scheduled cesarean delivery if indicated, and antiretroviral therapy for the infant, as well as effective pre- and postconception counseling, HIV-infected women can have healthy and successful pregnancies.
Similarly, women chronically infected with hepatitis B or C without severe liver damage can have successful pregnancy. The risk of vertical transmission was greatly reduced after implementation of active and passive immunoprophylaxis for hepatitis B and universal precaution and is estimated at <3 % for both viruses.
Acute viral hepatitis continues to be an important cause of maternal death worldwide. Herpes simplex hepatitis and hepatitis E can be extremely severe during the gestation, and these infections should always be excluded in a pregnant patient with acute hepatic failure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Perinatal HIV transmission
- Antiretrovirals
- ARVs
- HIV prevention
- Discordant couples
- Preexposure prophylaxis
- HIV testing
- Pediatric
- HIV medication
- Postexposure prophylaxis
- Viral load
- HIV
- Hepatitis
- Hepatitis B
- Hepatitis C
- HBV
- HCV
- Viral infections
- Pregnancy
HIV
Background/Definition
The human immunodeficiency virus (HIV) infection is a chronic infection caused by the retrovirus HIV infection of T cells that express CD4 receptors causing immunodeficiency. Once the CD4-positive cell count falls below a certain level, HIV infection causes increased susceptibility to infections, malignancies, and neurologic damage. The syndrome was first recognized in the United States in 1981.
Perinatal transmission of HIV was common before medical intervention and informed public health policy changes. With antenatal HIV testing to diagnose unknown maternal HIV infection, antiretroviral medication administered antepartum and peripartum to the mother, infant treatment, scheduled operative delivery when indicated, and avoidance of breastfeeding when there is a safe alternative, the transmission rate from mother to child can be less than 1 % [1]. With these strategies, there has been a 90 % decline in the numbers of perinatally infected infants in the United States, and a vertical transmission rate of less than 1 % in the United States and Europe [2, 3].
Epidemiology
Women represent about 49 % of all adults living with HIV. In some areas such as sub-Saharan Africa and the Caribbean, 60 % of those living with HIV are women. The most common mode of infection among women is heterosexual transmission.
According to a data from 2009, women represented 24 % of all diagnoses of HIV infection among US adults and adolescents, and in that year, there were approximately 11,200 new HIV infections among US women. In 2008, an estimated 25 % of adults and adolescents living with HIV were women. HIV disproportionately affects black and Hispanic/Latina women with the rate of new HIV infection among black women 15 times that of the white women and over three times the rate among Hispanic/Latina women. The most common mode of transmission is heterosexual sex [3].
As women are living longer and well with HIV, the number of women with HIV giving birth in the United States has increased by about 30 %. Despite that fact, the number of perinatal infections has continued to decline, yet they still occur [3]. Challenges to eliminating perinatal transmission altogether are missed opportunities for prenatal HIV testing and therefore diagnosis of HIV infection during pregnancy (since approximately 18 % of those with HIV are not aware of their diagnosis). Lack of preconception counseling in women with HIV, unintended pregnancies, and lack of access to or poor adherence to antiretroviral medication during pregnancy (or late initiation of antiretroviral medications often due to late prenatal care) all contribute to persistence of vertical transmission. Among mothers of HIV-infected infants reported to the CDC between 2003 and 2007, only 62 % had at least one prenatal visit, 27 % were diagnosed with HIV after delivery, and only 29 % had received some kind of antiretroviral medication during pregnancy [3].
Pathobiology
Pregnancy does not generally affect the course of HIV disease. With good prenatal care and careful monitoring, HIV-infected women can have healthy pregnancies and deliver HIV-uninfected infants. The majority of the perinatal transmission occurs during labor and delivery, when there is the most exposure of the infant to maternal blood and secretions. HIV can be transmitted to the fetus at any gestation, but most transmission occurs at >28 weeks. In areas where there is not a safe alternative to breastfeeding such as in some areas of sub-Saharan Africa, there is significant HIV transmission from mother to child during breastfeeding.
Antiretroviral therapy is the mainstay of perinatal HIV prevention, and the mechanism is likely twofold. The largest benefit is probably from decreasing the maternal viral load and therefore limiting the exposure of the infant to high levels of circulating HIV in the blood and genital secretions. However, this is not the only mechanism of prevention because vertical transmission happens across all viral loads, even in women with undetectable virus [4–6]. The other mechanism of action is likely the provision of preexposure prophylaxis to the infant while in utero by utilizing antiretroviral drugs that cross the placenta and reach adequate levels in the fetal circulation [1]. The infant also receives antiretroviral medications for 6 weeks postpartum, and this is also effective in decreasing the risk of HIV transmission, even in the absence of maternal antiretroviral therapy [4, 5].
Diagnosis
Acute infection with HIV, or acute retroviral conversion syndrome, can present as a mononucleosis-like illness, and a high level of suspicion must be maintained if a pregnant woman presents with fever because acute seroconversion is associated with a very high risk of perinatal transmission due to the very high viral load [6]. It is important to know the performance characteristics of the HIV test in use at individual institutions because early in HIV infection, the HIV antibody (the basis of most HIV tests) can be negative. The viral load must be checked in order to diagnose acute infection during the early window period before the HIV antibody forms. The new fourth-generation HIV tests have shortened this window period between infection and positive antibody test to 1–2 weeks and may be particularly useful in pregnancy [7].
In newly diagnosed HIV infection during pregnancy, a CD4 with percentage and a quantitative HIV RNA (viral load) level should be obtained and an HIV genotype to evaluate for transmitted drug resistance. Testing should also be done for hepatitis B, C, and A infection past or present, in order to vaccinate for hepatitis A (if indicated) and B if not immune from prior infection or vaccination. Other sexually transmitted infections such as syphilis, Chlamydia, gonorrhea, as well as consideration of herpes serology, should be obtained. Serology for toxoplasmosis should be obtained, and if negative, the patient should be counseled to avoid exposure that can lead to infection. Screening for tuberculosis with a TST skin test or serum quantiFERON gold assay should be performed [8]. The newly diagnosed pregnant patient should be comanaged with a specialist in treating patients with HIV infection and ideally with experience in pregnancy.
Management/Treatment
HIV care in pregnancy begins with careful preconception counseling in all women of childbearing age independent of reproductive plans. Ideally, the goal of therapy is a stable, suppressed viral load prior to conception. This can be addressed prior to conception and will increase the chances of an HIV-uninfected infant. The importance of safe contraception methods in the case of a serodiscordant couple can also be addressed as part of preconception counseling, as well as the availability of effective contraception in order to prevent unintended pregnancy. In the United States, the Centers for Disease Control and Prevention, the American College of Obstetricians and Gynecologists, and other national organizations have formal recommendations for preconception care [2].
Antepartum management focuses on maintaining a suppressed viral load throughout pregnancy and especially later in gestation when the majority of perinatal transmission can occur [1]. The consensus guidelines for antiretroviral therapy in the nonpregnant patient in the United States now recommend treatment for everyone regardless of CD4 cell count, although the strength of that recommendation varies by CD4 count [9]. This means that many HIV-infected pregnant patients will already be on antiretroviral therapy. If that therapy is effective in maintaining a suppressed viral load, then the recommendation is not to change drugs, regardless of the regimen, as it is thought that switching regimens increases the risk of viral escape and therefore increases the risk of perinatal HIV transmission in that setting [2]. The drug efavirenz warrants mention since until recently, cessation of this drug was recommended upon pregnancy; however, data shows that the small increased risk of neural tube defects with this drug is isolated to very early pregnancy, 4–5 weeks gestation, prior to the usual recognition of pregnancy. Recently, the US Health and Human Services Panel on Prevention of Perinatal HIV Transmission updated their treatment guidelines to recommend continuing efavirenz in women who are on an efavirenz-containing regimen and present for antenatal care in the first trimester, as long as the regimen is effectively suppressing the viral load [2]. Additional fetal monitoring should be considered for efavirenz exposure in the first trimester.
Choice of antiretroviral medication for the pregnant woman should take into consideration her prior treatment history and resistance mutations and should be chosen with the assistance of a practitioner specializing in the HIV care of the pregnant woman. In general, a recommended regimen would consist of two nucleoside reverse transcriptase inhibitors (NRTIs) (and currently those recommended are zidovudine and lamivudine) with the addition of a protease inhibitor (currently recommended are lopinavir or atazanavir, each boosted with ritonavir) [2]. Because one mechanism of perinatal HIV transmission prevention is preexposure prophylaxis in the fetus, at least two agents that cross the placenta (such as the NRTIs) should be chosen [2]. Viral load should be monitored every 2–4 weeks until viral suppression and then every 3 months or at least once per trimester during pregnancy. A viral load should be checked close to delivery so that mode of delivery can be planned.
Women who are on an effective antiretroviral regimen during pregnancy with a suppressed viral load should continue this regimen throughout labor and delivery. If the viral load is >1000 copies/ml, then intravenous zidovudine is recommended at the start of labor or 3–4 h prior to a cesarean section and continued until the cord is clamped [2]. If the viral load is <1000 copies/ml, then intravenous zidovudine is not necessary.
In terms of mode of delivery, if the HIV viral load is >1,000, then data have shown a reduction in the rate of perinatal transmission with a cesarean section [10, 11], and this is the current recommendation [2, 12].
The continuation of maternal antiretroviral therapy after delivery will largely be based on the indications for the nonpregnant adult. In general, antiretroviral medication is recommended for all HIV-infected adults regardless of CD4 count or viral load, although the strength of that recommendation varies based on CD4 count or other details such as HIV-negative partner, need for hepatitis B treatment, renal disease, and history of opportunistic infection [9]. Because there is significant risk of HIV transmission to the infant, breastfeeding is contraindicated in the developed world where there are safe and acceptable alternatives such as formula feeding. Avoidance of breastfeeding should be discussed with the patient over the course of her antepartum visits and again postpartum. The infant will receive 6 weeks of oral antiretroviral medication (usually zidovudine as a single agent), and the importance of adherence to the infant’s medications should be stressed as well as the need for regular HIV testing of the infant over the course of the first year of life.
Adherence to antiretroviral medication in the postpartum period can be poor, and support should be provided if possible, as poor adherence can have lifelong implications for drug resistance, future options for antiretroviral therapy, and opportunistic infections [13, 14]. Comprehensive family planning including choices for effective contraception should be discussed in the antepartum as well as the postpartum period. There may be some pharmacologic interactions between antiretroviral medication and hormonal contraception so these should be taken into consideration when choosing a contraceptive method or methods [15–18].
Summary
With universal HIV testing prior to or early in pregnancy, early and effective antiretroviral therapy for all pregnant women, scheduled cesarean delivery if indicated, and antiretroviral therapy for the infant, as well as effective pre- and postconception counseling, HIV-infected women can have healthy and successful pregnancies. Their care requires a comprehensive, collaborative, and multidisciplinary approach.
Acute Hepatitis
Hepatitis E
Hepatitis E is caused by an RNA virus of the family of Herpesviridae [19]. Infection is more common in nonindustrialized countries, and it is spread by orofecal route: person to person and through contaminated water sources. In rare cases, transmission has been described parenterally and vertically. It affects humans but similar viruses infect pigs, wild boars, and rats [20]. Globally, HEV is the most common cause of acute viral hepatitis and an important cause of maternal and perinatal death claiming 57,000 deaths worldwide yearly. In developing counties in Asia, the Middle East, Africa, and Central America, it occurs as an epidemic disease during the monsoon season but may also be endemic in these areas. Infection in industrialized countries is mainly a sporadic infection in patients returning from endemic areas. However, its prevalence is rising and might be an important cause of unexplained severe hepatitis.
Clinical Course and Management in Pregnancy
Acute hepatitis presents following 4–8 weeks of incubation, and it is characterized by malaise, fatigue, and jaundice. In children, it follows a mild course; in adults, it can be severe and in 7 % of cases leads to hepatic failure. Pregnant mothers infected with HEV have higher rates of fulminant hepatic failure and death than the general population. Acute infection is associated with 20–40 % maternal mortality and 26 % fetal mortality including intrauterine death and perinatal mortality. It is the most common cause of acute liver failure in pregnancy in countries such as Pakistan and India [21]. Diagnosis is made by serological testing for HEV IGG and IGM antibodies. PCR assay is not yet FDA approved, but it is available through the Centers of Disease Control and Prevention (CDC). HEV testing should be included in any workup of acute elevation of LFTs.
The therapy of the affected women is supportive. Two vaccines were developed and tested in small groups of risk individuals with good results. Unfortunately, China is the only country that pursued the commercialization and distribution of the vaccine to the population. Elsewhere, the production of the vaccine that would have saved many women’s lives is stalling.
Hepatitis B
Epidemiology
The hepatitis B virus is a DNA virus of the Hepadnavirus family that is transmitted from infected individuals parenterally, sexually, and vertically. As a blood-borne pathogen, it is present in all major body fluids including saliva, semen, and maternal milk [22]. Hepatitis B virus infection is a serious global health problem, with two billion people infected worldwide and 350 million chronic carriers. In the United States, 0.1–0.2 % of the population is infected with HBV, but in certain areas of Southeast Asia, Africa, and East Europe, the infection rates are as high as 8 % of the total population [23]. In the United States, the prevalence of HBV infection varies widely in the population with the highest in individuals born in areas with high endemic prevalence.
Acute hepatitis occurs 6–12 weeks from contact, and resolution of illness with permanent immunity occurs in 90 % of adult cases. Infection acquired in children younger than age 5 years usually leads to chronic infection, as 90 % of infants infected at birth will remain chronically infected. Thus, nearly 50 % of the 350 million people chronically infected with hepatitis B acquired the infection through maternal vertical transmission.
All chronically infected individuals, defined as those with persistence of virus for longer than 6 months after an acute infection, are at risk of developing chronic active hepatitis, cirrhosis, and/or hepatocellular carcinoma (HCC). Recent therapeutic advances have modified the natural history of the disease. Appropriate antiviral therapy delays the onset of liver damage and might be protective against HCC development [24].
Due to safe and effective immunoprophylaxis, the epidemiology of the disease has changed radically. In the United States, vaccination for HBV was recommended for at-risk individuals in 1982 and then recommended for all newborns and children in 1991. In 1988, universal testing for hepatitis B early in pregnancy in all women and active and passive immunoprophylaxis of their newborn was recommended. The estimated number of infections in 2000 fell dramatically to 81,000, a 70 % decrease from the peak of the disease in the mid-1980s. However, according to the Institute of Medicine (IOM), 1,000 infants born to HBV-infected mothers are chronically infected with HBV per year, which has not declined further in the last decade. In 2010, the Institute of Medicine (IOM) renewed the efforts to eradicate HBV infection. It included enhanced immunoprophylaxis upon delivery, targeted testing of adults at risk, vaccination of adults, and public education in schools and local communities [25] (Table 9.1).
The Pregnant Patient with Hepatitis B
Screening and Diagnosis
The prevalence of HBsAg positivity in pregnant mothers mirrors the general population (Table 9.2) [26] and varies by race: 6 % Asian, 1 % Black, 0.6 % Caucasian, and 0.14 % Hispanic.
In American-born patients, the most likely source of infection is drug use or sexual transmission, whereas patients born in endemic areas have most likely acquired the infection at birth. Foreign-born individuals represent the majority of HBsAg-positive mothers. In the United States, there is universal screening for HBV infection in all pregnant women with an estimated 96 % of all pregnant women tested. Mothers who have not had antenatal testing are tested at delivery by the birthing hospital [27].
Counseling
For many patients, prenatal screening coincides with the time of initial diagnosis. The role of the physician in this instance is of increased importance given the emotional implications of the diagnosis (Table 9.3). Moreover, given the multicultural background of the patients, the counseling must be provided in a context that is sensitive to this diverse and vulnerable population.
Reassurance regarding the likelihood of good outcomes in pregnancy and education regarding transmission inside the household and to the unborn child must be provided. Moreover, pregnancy is a unique and in many cases the only opportunity for these patients to be educated about hepatitis B, its long-term effects, and the available therapies.
Pregnancy Outcomes
Effect of HBV Infection on Pregnancy
It is thought that hepatitis B virus infection does not adversely affect the course of pregnancy, nor does pregnancy adversely affect the natural history of the disease. Indeed large retrospective cohorts have shown no difference in maternal or fetal outcomes compared to healthy non-HBV-infected controls. However, acute HBV infection is associated with high vertical transmission rates when occurring in the third trimester and increased incidence of low birth weight and prematurity [28]. Moreover recent studies suggested an increased risk of preterm birth, antepartum hemorrhage, and gestational diabetes in mothers chronically infected with hepatitis B [29, 30]. However, it is possible that the underlying advanced liver disease itself contributed to the high complication rate rather than the HBV infection itself.
Effect of Pregnancy on Hepatitis B Virus-Related Liver Disease
HBV-related liver disease typically does not worsen during pregnancy. However, a small percentage of patients may develop cholestasis, chronic hepatitis B flare, or liver failure [28, 31]. During normal pregnancy, increased HBV viral load has been observed, leading to minor fluctuations in liver function tests and thought to be secondary to high levels of adrenal corticosteroids and estrogen hormones [32].
In one study, HBV virus levels remained stable, but ALT levels increased particularly in late pregnancy and postpartum periods. Further evidence supports the postpartum period as the most vulnerable time for HBV exacerbations. In a Japanese study, of 269 pregnant patients with chronic HBV infection, alteration of liver function was found in 43 % during the first month postpartum with some exacerbations leading to HBV seroconversion. Given the uncertainty of the natural history of HBV infection during and after pregnancy, patients should be monitored closely during pregnancy and postpartum.
Evaluation the Underlying Liver Disease
Although pregnancy is not contraindicated in HBV-infected individuals or for patients with severe liver disease, a comprehensive assessment of the underlying chronic hepatitis B is advised for all HBsAg-positive patients. This will serve two purposes: first to detect cirrhosis and potential liver insufficiency that will complicate the pregnancy, second to inform the patient about her disease and the available therapy, and third to set up appropriate follow-up care.
The recommendation endorsed by The American College of Obstetrics and Gynecology is that all HBsAg-positive mothers should be referred during pregnancy to a physician familiar with evaluation and treatment of viral hepatitis. Unfortunately, in a recent survey, only a third of patients were seen for consultation [33].
HBsAg positivity is associated with advanced liver disease in 30 % of all patients. Assessing the extent of the liver disease is particularly challenging because it usually requires liver biopsy and histological analysis. Liver biopsy may be performed in this population, because the risks are same as in a nonpregnant patient; however, invasive testing is usually avoided during pregnancy unless absolutely necessary. The use of ultrasound and other newer noninvasive predictor of advanced liver damage such as MRI, FibroScan, and FibroSure have not been validated in pregnancy. The diagnosis thus relies on clinical and laboratory data (Table 9.4).
The immunoglobulin M class of hepatitis B core antibody (HBcAb) is the first to appear during acute infection, and its presence is used as the serologic marker of acute HBV infection. The presence of HBeAg positivity is associated with high infectivity and presence of high number of viral particles in the circulating blood (viral load). The presence of HBeAb positivity is associated with low infectivity, low replicative state, and low or undetectable viral load. Viral load is considered the most reliable indication of viral replication. HBV serological markers and their significance are reviewed in Table 9.5.
HBV viral load range is wide going from undetectable in inactive carriers to billions of viral copies in highly infective individuals. The definition of “high viral load” is not standardized. In general, a viral load exceeding 10 to the sixth copies/mL or >200,000 IU/ml is considered high, but several scientific publications and guideline use the terminology of “high viral load” for values from 10 to the fifth to 10 to the eighth.
HBsAg-positive patients with normal LFT’s, platelets, and liver synthetic functions and low or undetectable viral load are inactive carriers. It is important to remember that they still need to be followed yearly since they are at risk for reactivation and hepatocellular carcinoma.
Vertical Transmission
Overall, the virus infects 75 % of all babies born from HBsAg-positive mothers in the absence of immunoprophylaxis. If the mother has a high viral load, as is the case in hepatitis B eAg-positive patients, this number is more than 90 %. However, newborns from mothers who are healthy carriers with minimal viral replication and hepatitis BeAb positivity are infected in less than 10 % of cases [34]. In the United States, the vertical transmission of hepatitis B has been reduced dramatically by the implementation of a nationally funded Perinatal Hepatitis Prevention Programs. Table 9.6 summarizes the steps.
Currently, available vaccines are safe in pregnancy, and mothers who are at risk for acquiring infection during pregnancy (injection drug users, patients with multiple sexual partners or who are infected with other sexually transmitted diseases] should be vaccinated and retested for HBsAg again in the third trimester close to the time of delivery [35]. Passive immunoprophylaxis with HBIG is also safe in pregnancy and should be given to women with documented exposure. Management of babies born from HBsAg + mothers (including preterm infants) is outlined above. This schedule has achieved nearly a 97 % success rate in preventing overall vertical transmission [36].
Immunization Failure
Despite HBIG administration and vaccination, the rate of HBV infection in the newborn may still reach 8.5 % in mothers with high viral load [37]. CDC estimated that 1,000 newborns are infected vertically each year in the United States. In a recent study, all cases of immunoprophylaxis failure occurred in mothers with high viral load (defined as >106 copies/mL) [38]. The reason for immunoprophylaxis failure is that multifactorial prophylaxis regimens have been implemented in only 62 % [39] of cases. Vertical transmission of HBV occurs during delivery from blood-to-blood contact and occasionally in uteri by transplacental translocation of the virus [40]. Amniocentesis risk is controversial and not well established, but the test should be performed only if absolutely necessary [41].
Delivery and Breastfeeding
Elective cesarean session has been found in some studies to be protective from immunization failure, and it is advocated for mothers with high viral load and risk of vertical transmission [42]. However, this recommendation is not generally accepted, and conflicting data still exist in the literature. Specific guidelines do not exist regarding the mode of delivery [43, 44].
Breastfeeding has been a major concern for source of transmission; however, several studies have refuted this and shown that breastfeeding carries no additional risk to transmission of the virus in the setting of prompt newborn immunoprophylaxis and vaccination [45].
Antiviral Therapy in Pregnancy
Antiviral agents are used in pregnancy both to treat the expectant mother and to reduce vertical transmission.
Antiviral for Maternal Benefit
Decisions regarding therapy initiation, continuation, and termination should be the same for pregnant patients and general population. Antiviral therapy in pregnancy, however, is in general postponed until after birth unless necessary for treatment of acute flares or advanced liver disease. Successful treatment of liver failure in pregnancy has been described with antiviral therapy.
The discontinuation of treatment will result in rebound increasing viremia and subsequent disease flares. Continuation of the therapy in pregnancy is prudent especially in view of their relatively safe profile and the risk of fulminant hepatic failure associated with disease flares in pregnancy and postpartum [24] (Table 9.7).
Antiviral Therapy to Reduce Vertical Transmission
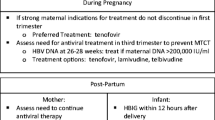
Immunization failure has been prevented by short courses of antiviral therapy during the second and third trimester of gestation in a few studies [46, 47]. In this patient population of high viral load mothers with otherwise limited underlying liver damage, therapy was initiated solely to prevent immunization failure.
Current use of antiviral to decrease vertical transmission is endorsed by many experts and was incorporated in the 2012 EASL Guidelines. This strategy is not uniformly accepted. Substantial debate still exists regarding the patient population to treat, choice of drug, length of treatment, and appropriate follow-up [48].
Two meta-analyses concluded that lamivudine treatment from 24 to 32 weeks of gestation until 1 month postpartum in those mothers who had high viral loads (>106) was not only safe but more effective than HBIG and vaccination of the newborn alone [43, 49]. Most recently, a meta-analysis included four randomized controlled trials with a total of 306 mothers showing that telbivudine given late in pregnancy was safe as well as effective in reducing vertical transmission [50].
Safety of Antiviral Therapy
The safety data for antiviral therapy is derived from the antiretroviral pregnancy registry (APR) and the Development of Antiretroviral Therapy Study (DART). No significant differences in rates of adverse outcomes have been reported for HB antiviral drugs initiated throughout the three trimesters of pregnancy [51]. These rates were comparable to the defect rates in the general population (reported by the CDC).
Lamivudine and tenofovir have the largest data set because they have been used in HIV-infected patients. Tenofovir and entecavir are the most potent drugs with less likelihood of developing resistance and the ones recommended to treat nonpregnant patients. Tenofovir has been associated with skeletal abnormalities [52], and further studies assessing newborn bone density are planned. Limited data are available for entecavir and adefovir use in pregnancy Table 9.8.
Hepatitis C in Pregnancy
Previously known as non-A, non-B hepatitis, hepatitis C was discovered only in 1989. Hepatitis C virus is an RNA single-stranded virus of the Filoviridae family. As a blood-borne pathogen, the main causes of infection are intravenous drug use and contaminated blood products. More than 80 % of drug users are positive for HCV. Sexual transmission is thought to occur, but so rarely that monogamous couples with only one partner infected are not advised to use barrier contraception. In 10 % to 20 % of patients, the source of infection is unknown. Interestingly, baby boomers (those born between 1945 and 1965) are five times more likely to have HCV infection. The CDC now recommends one time testing in this population given the increased risk.
Unfortunately, a vaccine is not available. Hepatitis C infection affects 3 % of the world population. In the United States 4 million people are infected. Although the number of new HCV infections is declining, the number of patients suffering from HCV-related chronic liver disease is still mounting. Prevalence of HCV infection in the pregnant population is 0.5 %. Despite its prevalence, the American College of Gynecology does not recommend routine screening for HCV infection.
Acute infection with HCV is usually asymptomatic, and clearance of virus occurs in 25–30 % of cases. In children and young women, this percentage is even higher. Chronic infection is common, with patients remaining asymptomatic for decades. Only 20–30 % of those chronically infected will develop significant liver damage, i.e., cirrhosis, and 30 % of those who develop cirrhosis will consequently develop HCC 20–30 years after the original infection. Alcohol consumption, male gender, and obesity are predisposing factors associated with development of liver damage. Hepatitis C is a systemic disease associated with the development of autoimmune diseases such as thyroiditis, autoimmune hepatitis, and rheumatoid arthritis. In addition, HCV infection may cause insulin resistance and depressive symptoms associated with mild cognitive impairment.
Effect of Pregnancy on Hepatitis C Virus Disease
Through unclear mechanisms, female gender has a positive effect on HCV disease course and response to therapy. After menopause, this benefit seems to disappear. Derivatives of estrogen, such as estradiol, are thought to have powerful antioxidant effects likely leading to decreased inflammatory by-products as well as decreased fibrosis [54]. In pregnancy, both exacerbation and improvement in liver histology and clinical parameters have been found. Normalization of liver function tests, decrease of the viral load, and even spontaneous viral clearance have been reported [54]. Pregnancy is generally well tolerated by patients without severe liver damage. Postpartum, however, may be a time of increased replication associated with fatigue, body aches, and elevation of liver function tests.
Effect of Hepatitis C Virus on Pregnancy
Complications of pregnancy caused by HCV infection are probably rare. However, retrospective studies suggest an increased incidence of fetal/maternal complications including low birth weight and requirement for assisted ventilation [55]. Prospective studies are lacking. Women with HCV are at increased risk of developing gestational diabetes as well as cholestasis of pregnancy [56].
Preconception Counseling
HCV identification during pregnancy is a time to discuss the normal progression of HCV infection as well as reduce future HCV transmission. Pregnancy is not discouraged in patients infected with HCV, but the potential for vertical transmission to the baby must be discussed as well as its effect on the course of pregnancy. Ribavirin, one of the mainstays of HCV therapy, should be avoided within 6 months of pregnancy, given its long half-life. Patients with stigmata of liver disease should be referred to obstetric medicine and gastroenterology specialists for careful monitoring during pregnancy.
Children born infected with HCV through vertical transmission may be at increased risk for low birth weight and small for gestational age. It has been observed, however, that these children generally have minimal progression of fibrosis.
Risk Factors and Screening
Currently, universal screening for HCV is not recommended. Every effort should be made to identify risks factors in order to test high-risk patients with HCV antibody. Risk factors that should trigger HCV testing in pregnancy are given in Table 9.9.
Pregnancy is a time to provide counseling and is an important step in the control of the HCV epidemic.
To confirm HCV infection, it is necessary to check HCV PCR for direct viral particle detection in the blood, as well as genotype determination. Those individuals whose HCV PCR is undetectable are aviremic but still considered infectious because further characterization of their status is not possible. HCV antibodies develop up to 6 months from the initial infection, and HCV PCR should be used to diagnose HCV acute hepatitis.
Management
Exciting drug development is changing the landscape of HCV therapy. In addition to pegylated interferon and ribavirin, protease inhibitors such as boceprevir and telaprevir have been used for treatment of HCV genotype 1 in nonpregnant patients with evidence of liver damage. Treatment for 24–48 weeks is effective in up to 60–80 % of all patients in decreasing the viral load to undetectable levels (sustained viral response). New direct antivirals (DDA) Sofosbuvir and Simeprevir were approved in December 2013 including an interferon free therapy for genotype 2 and 3 [57]. Historically with ribavirin- and interferon-based treatment, women have a much better response rate than men to therapy. Ribavirin is teratogenic and should not be administered during pregnancy.
Immunoprophylaxis for Prevention of Vertical Transmission
Vertical transmission of HCV from mother to fetus occurs but much less frequently than for HBV virus [58]. As with HBV, amniocentesis should be performed only if absolutely necessary [41]. The rate of transmission varies widely from as low as 3 % to as high as 35 %. This rate depends in part on the viral load: high viral load leads to increased transmission to the child and is very unlikely in aviremic mothers. In mothers coinfected with HIV, HCV viral loads are higher, leading to an observed higher rate of transmission (nearly 30–35 %), yet it is unclear if HIV is an independent risk factor for vertical transmission [59]. Newborn girls are twice as likely to be infected as are males for unknown reasons.
There is no specific immunoprophylaxis for HCV. Recommendations from two recent prospective studies to reduce the risk of transmission include delivery within 6 h of membrane rupture and avoidance of invasive monitoring during delivery. Cesarean section does not decrease the rate of transmission. Although HCV virus may be found in breast milk, breastfeeding is safe in the absence of breast sores [60].
Children born from HCV-positive mothers should be tested for the infection. Hepatitis C virus and HCV maternal antibodies may be detected up to 12–18 months in the neonate. The AASLD recommends checking for HCV antibody after 18 months. However, in practice, most physicians perform two HCV tests by PCR between 2 and 6 months followed by HCV antibody testing at age 18 months. Infected children should be referred to a pediatric hepatologist for treatment.
References
Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the [internet]. 2012 (cited 4 Feb 2012).
Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the united states. 2012 (cited 28 Sept 2014).
HIV in women: Centers for disease control and prevention [internet]. Available from: http://www.cdc.gov/hiv/pdf/risk_women.pdf.
Nielsen-Saines K, Watts DH, Veloso VG, Bryson YJ, Joao EC, Pilotto JH, Gray G, Theron G, Santos B, Fonseca R, Kreitchmann R, Pinto J, Mussi-Pinhata MM, Ceriotto M, Machado D, Bethel J, Morgado MG, Dickover R, Camarca M, Mirochnick M, Siberry G, Grinsztejn B, Moreira RI, Bastos FI, Xu J, Moye J, Mofenson LM, Team NHPP. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368–79.
Gray GE, Urban M, Chersich MF, Bolton C, van Niekerk R, Violari A, Stevens W, McIntyre JA, Group PEPS. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS. 2005;19:1289–97.
Morrison CS, Demers K, Kwok C, Bulime S, Rinaldi A, Munjoma M, Dunbar M, Chipato T, Byamugisha J, Van Der Pol B, Arts E, Salata RA. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS. 2010;24:573–82.
Chetty V, Moodley D, Chuturgoon A. Evaluation of a 4th generation rapid HIV test for earlier and reliable detection of HIV infection in pregnancy. J Clin Virol. 2012;54:180–4.
Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the infectious diseases society of America. Clin Infect Dis. 2014;58:e1–34.
Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Panel on clinical practices for treatment of HIV. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381–433.
The International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1–a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977–87.
European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035–9.
Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. Acog committee opinion scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. Number 219, August 1999. Int J Gynaecol Obstet. 1999;66:305–6.
Kreitchmann R, Harris DR, Kakehasi F, Haberer JE, Cahn P, Losso M, Teles E, Pilotto JH, Hofer CB, Read JS, Team NLS. Antiretroviral adherence during pregnancy and postpartum in Latin America. AIDS Patient Care STDs. 2012;26:486–95.
Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, Higgins A, Zorrilla C, Landesman S, Serchuck L, Larussa P. Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care. 2008;20:958–68.
Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, Yu S, Lertora JJ, Team AAP. Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther. 2007;81:222–7.
Vogler MA, Patterson K, Kamemoto L, Park JG, Watts H, Aweeka F, Klingman KL, Cohn SE. Contraceptive efficacy of oral and transdermal hormones when co-administered with protease inhibitors in HIV-1-infected women: pharmacokinetic results of actg trial a5188. J Acquir Immune Defic Syndr. 2010;55:473–82.
Womack J, Williams A. Hormonal contraception in HIV-positive women. AIDS Read. 2008;18:372–7. 381.
World Health Organization. Review of priorities in research on hormonal contraception and IUDs and HIV infection. Geneve: WHO; 2010.
Reyes GR, Purdy MA, Kim JP, Luk KC, Young LM, Fry KE, Bradley DW. Isolation of a cdna from the virus responsible for enterically transmitted non-a, non-b hepatitis. Science. 1990;247:1335–9.
Scobie L, Dalton HR. Hepatitis e: source and route of infection, clinical manifestations and new developments. J Viral Hepat. 2013;20:1–11.
Aggarwal R. Hepatitis E: clinical presentation in disease-endemic areas and diagnosis. Semin Liver Dis. 2013;33:30–40.
Wright TL. Introduction to chronic hepatitis b infection. Am J Gastroenterol. 2006;101 Suppl 1:S1–6.
Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis b disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39.
Kim HY, Choi JY, Park CH, Jang JW, Kim CW, Bae SH, Yoon SK, Yang JM, Lee CD, Lee YS. Outcome after discontinuing antiviral agents during pregnancy in women infected with hepatitis b virus. J Clin Virol. 2013;56:299–305.
US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care, and treatment of viral hepatitis. Washington, DC: HHS; 2011:1–76.
Euler GL, Wooten KG, Baughman AL, Williams WW. Hepatitis b surface antigen prevalence among pregnant women in urban areas: implications for testing, reporting, and preventing perinatal transmission. Pediatrics. 2003;111:1192–7.
Fischer G, Wang S, Ahring S, Fowler K, Hainline S, Chinglong M, Jacques-Carroll L, Bell B, Williams I. An investigation of perinatal hepatitis b virus infections among a high risk population: the delivery hospital as a safety net. Pediatr Infect Dis J. 2009;28:593–7.
Nguyen G, Garcia RT, Nguyen N, Trinh H, Keeffe EB, Nguyen MH. Clinical course of hepatitis b virus infection during pregnancy. Aliment Pharmacol Ther. 2009;29:755–64.
Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis b and hepatitis c carrier status and perinatal outcomes. Liver Int. 2011;31:1163–70.
Safir A, Levy A, Sikuler E, Sheiner E. Maternal hepatitis b virus or hepatitis c virus carrier status as an independent risk factor for adverse perinatal outcome. Liver Int. 2010;30:765–70.
Potthoff A, Berg T, Wedemeyer H, Group H-NBCCS. Late hepatitis b virus relapse in patients co-infected with hepatitis b virus and hepatitis c virus after antiviral treatment with pegylated interferon-a2b and ribavirin. Scand J Gastroenterol. 2009;44:1487–90.
Tan HH, Lui HF, Chow WC. Chronic hepatitis b virus (hbv) infection in pregnancy. Hepatol Int. 2008;2:370–5.
Godbole G, Irish D, Basarab M, Mahungu T, Fox-Lewis A, Thorne C, Jacobs M, Dusheiko G, Rosenberg WM, Suri D, Millar AD, Nastouli E. Management of hepatitis b in pregnant women and infants: a multicentre audit from four london hospitals. BMC Pregnancy Childbirth. 2013;13:222.
Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. E antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis b virus to their infants. N Engl J Med. 1976;294:746–9.
CDC. General recommendations on immunization: recommendations of the advisory committee on immunization practices(acip). MMWR Morb Mortal Wkly Rep. 2011;60:26.
Centers for Disease Control and Prevention. Postvaccination serologic testing results for infants aged </=24 months exposed to hepatitis b virus at birth: United States, 2008–2011. MMWR Morb Mortal Wkly Rep. 2012;61:768–71.
Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, Maley MW, Ayres A, Locarnini SA, Levy MT. Perinatal transmission of hepatitis b virus: an Australian experience. Med J Aust. 2009;190:489–92.
Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to hbsag-positive mothers. J Viral Hepat. 2012;19:e18–25.
Willis BC, Wortley P, Wang SA, Jacques-Carroll L, Zhang F. Gaps in hospital policies and practices to prevent perinatal transmission of hepatitis b virus. Pediatrics. 2010;125:704–11.
Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis b virus. World J Gastroenterol. 2004;10:437–8.
Davies G, Wilson RD, Desilets V, Reid GJ, Shaw D, Summers A, Wyatt P, Young D, Society of Obstetricians and Gynaecologists of Canada. Amniocentesis and women with hepatitis b, hepatitis c, or human immunodeficiency virus. J Obstet Gynaecol Can. 2003;25(145–148):149–52.
Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis b virus–a systematic review. Virol J. 2008;5:100.
Xu H, Zeng T, Liu JY, Lei Y, Zhong S, Sheng YJ, Zhou Z, Ren H. Measures to reduce mother-to-child transmission of hepatitis b virus in china: a meta-analysis. Dig Dis Sci. 2014;59:242–58.
Hu Y, Chen J, Wen J, Xu C, Zhang S, Xu B, Zhou YH. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis b virus. BMC Pregnancy Childbirth. 2013;13:119.
Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. Risk of hepatitis b transmission in breast-fed infants of chronic hepatitis b carriers. Obstet Gynecol. 2002;99:1049–52.
Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, Qiao FY, Campbell F, Chang CN, Gardner S, Atkins M. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis b virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103.
Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, Yue X, Wang GJ, Tang X, Fang ZX. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis b virus infection. J Hepatol. 2011;55:1215–21.
Ayres A, Yuen L, Jackson KM, Manoharan S, Glass A, Maley M, Yoo W, Hong SP, Kim SO, Luciani F, Bowden DS, Bayliss J, Levy MT, Locarnini SA. Short duration of lamivudine for the prevention of hepatitis b virus transmission in pregnancy: lack of potency and selection of resistance mutations. J Viral Hepat. 2013;21(11):809–817.
Han L, Zhang HW, Xie JX, Zhang Q, Wang HY, Cao GW. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis b virus. World J Gastroenterol. 2011;17:4321–33.
Deng M, Zhou X, Gao S, Yang SG, Wang B, Chen HZ, Ruan B. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis b virus: a systematic review and meta-analysis. Virol J. 2012;9:185.
Brown Jr RS, Verna EC, Pereira MR, Tilson HH, Aguilar C, Leu CS, Buti M, Fagan EA. Hepatitis b virus and human immunodeficiency virus drugs in pregnancy: findings from the antiretroviral pregnancy registry. J Hepatol. 2012;57:953–9.
Gafni RI, Hazra R, Reynolds JC, Maldarelli F, Tullio AN, DeCarlo E, Worrell CJ, Flaherty JF, Yale K, Kearney BP, Zeichner SL. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–718.
Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International iterim report for 1 January 1989 through 31 January 2013. Wilmington, NC: Registry Coordinating Center; 2014. Available from http://www.apregistry.com.
Floreani A. Hepatitis c and pregnancy. World J Gastroenterol. 2013;19:6714–20.
Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis c: data from a 2003–2005 Washington state birth cohort. Am J Obstet Gynecol. 2008;199:38e31–39.
Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis c virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498–500.
Thomas DL. Cure of hepatitis c virus infection without interferon alfa: scientific basis and current clinical evidence. Top Antivir Med. 2013;21:152–6.
Ohto H, Terazawa S, Sasaki N, Sasaki N, Hino K, Ishiwata C, Kako M, Ujiie N, Endo C, Matsui A, et al. Transmission of hepatitis c virus from mothers to infants. The vertical transmission of hepatitis c virus collaborative study group. N Engl J Med. 1994;330:744–50.
Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, Alter MJ. Risk factors for perinatal transmission of hepatitis c virus (hcv) and the natural history of hcv infection acquired in infancy. J Infect Dis. 2005;192:1880–9.
Tovo PA, Palomba E, Ferraris G, Principi N, Ruga E, Dallacasa P, Maccabruni A. Increased risk of maternal-infant hepatitis c virus transmission for women coinfected with human immunodeficiency virus type 1. Italian study group for hcv infection in children. Clin Infect Dis. 1997;25:1121–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Hardy, E.J., Esposti, S.D., Nee, J. (2015). Viral Infection in Pregnancy: HIV and Viral Hepatitis. In: Rosene-Montella, K. (eds) Medical Management of the Pregnant Patient. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-1244-1_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-1244-1_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-1243-4
Online ISBN: 978-1-4614-1244-1
eBook Packages: MedicineMedicine (R0)