Abstract
Introduction
Chloroquine and hydroxychloroquine are widely used in the long-term treatment of connective tissue disease and usually considered safe. However, chloroquine- or hydroxychloroquine-related cardiac disorder is a rare but severe adverse event, which can lead to death. This systematic review investigates cardiac complications attributed to chloroquine and hydroxychloroquine.
Methods
PubMED, EMBASE, and Cochrane database searches were conducted using keywords derived from MeSH terms. Reports published prior to 31 July, 2017 were eligible for inclusion, without restriction to study design. Searches were also conducted on reference lists of included studies.
Results
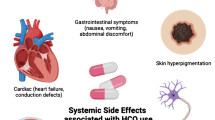
Eighty-six articles were identified, reporting individual cases or short series, providing information on 127 patients (65.4% female). A majority of patients were treated with chloroquine (58.3%), with the remaining treated with hydroxychloroquine (39.4%), or both in succession. Most patients had been treated for a long time (median 7 years, minimum 3 days; maximum 35 years) and with a high cumulative dose (median 1235 g for hydroxychloroquine and 803 g for chloroquine). Conduction disorders were the main side effect reported, affecting 85% of patients. Other non-specific adverse cardiac events included ventricular hypertrophy (22%), hypokinesia (9.4%), heart failure (26.8%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%). For 78 patients reported to have been withdrawn from treatment, some recovered normal heart function (44.9%), while for others progression was unfavorable, resulting in irreversible damage (12.9%) or death (30.8%).
Limitations
The risk of cardiac complications attributed to chloroquine/hydroxychloroquine was not quantified because of the lack of randomized controlled trials and observational studies investigating the association.
Conclusions
Clinicians should be warned that chloroquine- or hydroxychloroquine-related cardiac manifestations, even conduction disorders without repercussion, may be initial manifestations of toxicity, and are potentially irreversible. Therefore, treatment withdrawal is required when cardiac manifestations are present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chloroquine/hydroxychloroquine-related cardiac disorder is a rare, but severe adverse event (only 45% of patients recovered after drug withdrawal). |
Although some studies suggest a vascular protective role of hydroxychloroquine in the context of inflammatory diseases, long-term hydroxychloroquine administration may induce severe cardiac disorders, the early signs of which may be important to detect. |
Cardiac monitoring of patients using chloroquine/hydroxychloroquine drugs seems necessary. |
1 Introduction
The antimalarial drugs chloroquine (CQ) and hydroxychloroquine (HCQ) are widely used in the long-term treatment of connective tissue disease and are usually considered safe. In systemic lupus erythematosus (SLE), HCQ improves cutaneous and arthritis symptoms, prevents renal involvement and thrombosis, and prevents osteoporosis by limiting corticosteroid consumption. For all these reasons, HCQ is recommended by both the European League Against Rheumatism and the American College of Rheumatology, as the standard treatment in SLE to prevent relapse [1, 2]. Moreover, CQ/HCQ have been used for many years to treat rheumatoid arthritis (RA), with poor efficacy in monotherapy but demonstrated added value in combination therapy [3].
Hydroxychloroquine differs from CQ by the presence of a hydroxyl group on one of the ethyl groups attached to nitrogen in the side chain. Both drugs are well absorbed, with tissue concentrations increasing rapidly to reach steady-state after five half-lives, i.e., 1–3 months (β-half-lives are in the range of 10–30 days for CQ and 3–20 days for HCQ). After withdrawal, the decrease in tissue concentration is about 90–95% in 15 days [4]. Comparison between CQ and HCQ is difficult. While not a controlled study, Scherbel et al. suggest a similar profile between both drugs in their direct comparison of effectiveness and toxicity [5]. Chloroquine more often appears associated with retinopathy, an observation probably accounting for the increasingly frequent use of HCQ [6]. Other adverse events, such as skin changes, and neuro-myopathy, [7, 8] are also associated with the long-term use of CQ/HCQ therapy. Recently, the American Academy of Ophthalmology outlined risk factors associated with retinopathy following therapeutic use of HCQ and CQ, and recommendations for screening [6]. These antimalarial drugs seem to have a cumulative effect, as treatment duration longer than 5 years, cumulative doses higher than 1000 g for HCQ and 460 g for CQ, and a high daily dose, were all shown to increase the risk of retinopathy. It was recommended that following a baseline examination when the drug is started, annual screenings should begin after 5 years.
Several recent studies have highlighted the usefulness of HCQ in preventing the occurrence of cardiovascular disorders, particularly in inflammatory diseases such as SLE or RA [9, 10]. In addition to its anti-inflammatory properties, HCQ prevents the occurrence of thromboses, limits atherosclerosis, reduces cholesterol levels, and reduces the risk of type 2 diabetes mellitus [11]. Conversely, through poorly understood mechanisms, perhaps involving the lysosomal pathway, [12] these drugs may also induce cardiac toxicity. Long-term treatment with CQ and HCQ provokes dysfunction of lysosomal enzymes, [13] leading to impairment of intracellular degradation processes and subsequent accumulation of metabolic products (glycogen and phospholipids) [14]. Chloroquine- or HCQ-related cardiac disorder is a rare but severe adverse event, which can lead to death. Presently, there are no guidelines regarding cardiac care with the long-term use of CQ or HCQ. Here, we present a thorough systematic review of case series concerning cardiac complications attributed to CQ and HCQ drugs, and provide recommendations for clinicians.
2 Methods
2.1 Data Sources and Literature Searches
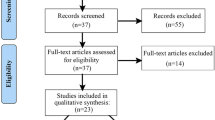
We conducted a systematic review following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. On 1 August, 2017, we performed a systematic electronic search in MEDLINE of PubMed, EMBASE, and Cochrane databases, including all articles published prior to 31 July, 2017. Our search strategy used a combination of relevant keywords chosen according to the MeSH terms for heart diseases, with subcategories such as arrhythmias, cardiomegaly, cardiomyopathies, heart arrest, heart failure, heart valve diseases, myocardial ischemia, and ventricular dysfunction (“hydroxychloroquine”[MeSH] OR “chloroquine”[MeSH]) AND (“heart failure”[MeSH] OR “atrioventricular block”[MeSH] OR “cardiomyopathy”[MeSH] OR “cardiac toxicity”[MeSH] OR “cardiovascular disease”[MeSH]). One author (YMP) conducted an initial screening of potentially relevant records, based on titles and abstracts, with the final selection of articles performed independently by another author (CC) based on a full-text evaluation. Consensus between the two reviewers was used to resolve any disagreement. The full search strings used for the electronic database queries, including all the search terms, are listed in Electronic Supplementary Material (ESM) 1. The reference lists of publications identified in our electronic search were searched by hand to find any additional studies. The PRISMA flow chart figure summarize the different steps leading to the final data selection and analysis.

2.2 Study Selection
We included all studies reporting patients experiencing cardiac complications related to long-term CQ/HCQ treatment. We used the Mendeley citation manager to automatically identify and delete duplicates among imported references. The resulting list was first reduced by reading the title and abstract and then, for those remaining articles, further reduced by reading the text. This second careful reading was performed to avoid inclusion of multiple reports for the same patient. Studies that were excluded include reports of patients with acute self-poisoning attributed to CQ/HCQ, basic science studies explaining the pathophysiologic mechanisms, and reports of patients with skeletal muscle disorders but without heart involvement. Animal studies were also excluded. The remaining list, found in English, French, German, and Spanish publications, was then reviewed and summarized by the authors.
2.3 Data Extraction
Using a standardized form, two reviewers (CC and YMP) independently extracted study characteristics (details of participants, interventions, and outcomes) from the included case reports. The data collected for each patient included: age, sex, treatment, daily dose, cumulative dose (i.e., the total dose taken by the patient, specified either by authors or calculated from the daily dose and duration of treatment), underlying disease, cardiac disorders (clinic, electrocardiogram, echocardiography), extra-cardiac toxicity, pathologic findings, heart magnetic resonance imaging (MRI), evolution, and Fabry disease (FD) screening. We did not contact study authors for missing data. Cardiac disorders were described according to the Medical Dictionary for Regulatory Activities, Version 20.1, which allows a systematic classification of medical information on adverse drug reactions [15]. The Medical Dictionary for Regulatory Activities is organized into five hierarchical levels: medical discipline ‘System Organ Class’, ‘High Level Group Term’, ‘High Level Term’, ‘Preferred Term’, and ‘Lowest Level Term’ (see ESM 2). Moreover, the probability of drug adverse events has been assessed using the Naranjo scale [16]. We did not exclude randomized clinical trials or observational studies. However, none of the randomized clinical trials or observational studies investigated a possible role of CQ/HCQ in the onset of cardiac disorders. Indeed, we only found individual or case series with relevant data.
2.4 Statistical Analysis
A comprehensive description of the sample population provides frequencies of different categories for qualitative variables, and the mean and standard deviation, as well as median and minimum/maximum values, of quantitative variables. Differences in the frequency of adverse events between CQ and HCQ treatment were analyzed using Fisher’s exact test. The significance level was set at 5%. Statistical analysis was performed using Prism version 7.0c (GraphPad Software Inc., La Jolla, CA, USA).
3 Results
3.1 Study Characteristics and General Data
Our search identified 86 articles for review, in which 127 cases of cardiac disorders attributed to CQ or HCQ drugs were identified [7, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. The articles are descriptions of individual cases or short series. Periods of publication were 1963–79 (n = 6), 1980–9 (n = 10), 1990–9 (n = 12), 2000–9 (n = 23), and since 2010 (n = 35). Patient data are summarized in Table 1. Articles reviewed are described in ESM 3. Patients with cardiac side effects were mainly women (n = 83; 65.4%), and had a median age of 56 years (minimum 27 years; maximum 81 years). Seventy-four patients (58.3%) were treated with CQ, 50 (39.4%) with HCQ, and three patients had received treatment with both drugs in succession. Patients experienced various inflammatory or infectious diseases: SLE (n = 49), RA (n = 28), discoid lupus erythematosus (n = 22), Sjögren syndrome (n = 4), juvenile idiopathic arthritis (n = 1), scleroderma (n = 2), mixed connective tissue disease (n = 4), psoriasis (n = 1), malaria (repeated episode or long-term use both by self-medication) (n = 19), Whipple disease (n = 1), primary pulmonary hemosiderosis (n = 1), sarcoidosis (n = 1), and overlap syndrome with autoimmune hepatitis and primary cirrhosis (n = 1). The median duration of treatment was 7 years (minimum 3 days; maximum 35 years) for CQ and 8 years (minimum 10 days; maximum 30 years) for HCQ, and the median cumulative dose was 803 g for CQ (minimum 1.5 g; maximum 9125 g) and 1235 g for HCQ (minimum 1.9 g; maximum 4380 g).
3.2 Overview of the Clinical Cardiac Complications
Individual presentation of cardiac manifestations attributed to CQ or HCQ is provided in ESM 3. Conduction disorders were the main side effect reported and affected 85% of patients. Other non-specific cardiac events included ventricular hypertrophy (22%), hypokinesia (9.4%), heart failure (26.8%), pulmonary arterial hypertension (3.9%), or valvular dysfunction (7.1%). In the 78 cases of patients removed from treatment by the drugs, recovery of heart function subsequent to withdraw was observed in 44.9% of cases, but with unfavorable progression in others, with irreversible damage (10.3% with pacemakers), deaths (30.8%), or heart transplant (2.6%). According to the Naranjo scale, we classified the cases into definite (n = 0), probable (n = 38), and possible (n = 69). In a few cases, the score could not be evaluated because of missing data (n = 20) [see ESM 3].
3.2.1 Drug Toxicity from a Low Cumulative Dose (< 100 g)
Cardiac toxicity was induced by a high cumulative dose of CQ or HCQ in most patients. However, a few papers surprisingly mentioned complications in patients with a very low cumulative dose [39, 53, 65, 90, 92, 99, 100]. Most of these patients presented with unusual heart failure, with a severe depressed left ventricular ejection fraction [65, 90, 92, 99, 100]. In one case, the patient had occasionally taken low doses, but over a long period (15 g over 8 years), which may have led to myocyte accumulation [65]. Finally, we can assume that some observations of low-dose toxicity were caused by a quinidine-like effect arising from a severe valvular insufficiency in the patient [39, 53]. One patient with a normal electrocardiogram 2 years prior to receiving 1 year of HCQ treatment (total cumulative dose around 65 g) presented with corrected QT interval lengthening and ventricular arrhythmia (torsade de pointe), which is more an acute mechanism than a complication related to long-term drug accumulation. Moreover, this patient had cirrhosis, which may have contributed to toxicity even if HCQ liver metabolism is low [53].
3.2.2 Drug Toxicity from a Large Cumulative Dose
Long-term administration of CQ and HCQ has been associated with cardiac events, including myocardial thickening, restrictive cardiomyopathy, conduction disorders, and heart failure. The frequency of heart complications associated with CQ or HCQ treatment, organized according to Medical Dictionary for Regulatory Activities classification, is summarized in Table 2. Conduction disorders are the main cardiac side effect described under CQ/HCQ therapy. Indeed, 53 of 127 patients presented with an atrioventricular block and 20 patients presented with a complete heart block, some leading to cardiac arrest [21, 24, 51, 58]. Complete atrioventricular block and heart block are more frequently associated with CQ treatment (Table 2). Sick sinus syndrome was reported [67, 68, 77]. We also found four studies reporting a prolonged corrected QT interval with a high accumulated dose [61, 82, 84, 93]. Clinically, cardiac events could have occurred in an acute or chronic way, with dyspnea, congestive heart failure, chest pain, or syncope.
Regarding transthoracic ultrasound abnormalities, 22 patients presented with left ventricular hypertrophy (more frequently with HCQ) and six patients presented with biventricular hypertrophy. Valvular regurgitation was found in nine patients and could involve the tricuspid, mitral, or aortic valve [45, 55, 59, 65, 67, 69, 72, 74, 79]. Left ventricular hypokinesia was present in 12 patients, most often with HCQ. The left ventricular ejection fraction was specified in 46 patients, 21 of whom had a left ventricular ejection fraction lower than 40%, indicating mild-to-severe heart failure, and only ten had a normal left ventricular ejection fraction (> 60%). Elevated troponin levels were reported in eight cases [52, 54, 57, 74, 77, 79, 85, 96].
3.2.3 Extra-Cardiac Toxicity
Combined toxicity could exist in nearly half of patients (n = 63; 49.6%) with extra-cardiac symptoms (Table 1). Skin changes were described for seven patients. Chloroquine/hydroxychloroquine-related retinopathy was present in 30 patients and was more frequently associated with CQ treatment (66.7% vs. 9.5%; p < 0.05). Cardiomyopathy was associated with symptomatic myopathy in 24 cases and with neurologic side effects in six cases. Cardiac events could occur in the absence of other signs of toxicity.
3.3 Morphological Abnormalities
Diagnosing morphologic abnormalities in heart tissue as a potential side effect of long-term treatment with HCQ or CQ is difficult, and can require invasive methods such as endomyocardial or peripheral muscle biopsy, although MRI could also provide useful information.
3.3.1 Magnetic Resonance Imaging
Results from a cardiac MRI were reported for 19 patients, [51, 52, 54, 56, 59, 64, 67, 79, 80, 83,84,85, 88,89,90, 95, 96, 100] and may reveal left ventricular or biventricular hypertrophy with increased wall thickness, hypokinesia, and systolic dysfunction. There was delayed contrast enhancement in a patchy non-ischemic zone in six patients [51, 54, 67, 80, 85, 96]. Such abnormalities may also be found in myocarditis, which calls into question diagnosis in patients with systemic disease [96]. These six patients with late gadolinium enhancement had left ventricular hypertrophia. Therefore, no specific sign of CQ/HCQ cardiotoxicity is available with MRI. Nevertheless, MRI remains necessary to eliminate differential diagnoses and sometimes to guide biopsy sampling.
3.3.2 Endomyocardial Biopsy
To diagnose CQ/HCQ toxicity, 62 patients had an endomyocardial biopsy (EMB). Light microscopic study found vacuolization in 100% of myocytes. Positive periodic acid–Schiff staining of enlarged granule-containing myocytes suggested an accumulation of polysaccharides. Transmission electron microscopy was performed for 47 biopsies and showed myelin figures in 83% and curvilinear bodies in 68%. None of these biopsies were normal. Furthermore, mega-mitochondria were described. Frustaci et al. explored the clinico-pathologic pathway of lysosomal hydrolase activity in heart biopsies collected before and after recovery, from a patient experiencing cardiomyopathy induced by CQ/HCQ therapy [80]. They found that activity of alpha-galactosidase A, beta-galactosidase, and arylsulfatase was inhibited in affected cardiomyocytes. This inhibition was reversible, as enzyme activity was higher in the EMB after recovery. Importantly, this reversibility was reflected by lysosomal enzyme activity in peripheral blood lymphocytes. In contrast, alpha-galactosidase A activity in peripheral blood was normal for nine patients [46, 55, 73, 74, 79, 80, 89, 91, 100]. Regardless, a decrease in lysosomal enzyme activity could be a means of diagnosing side effects related to long-term CQ/HCQ drug intoxication.
3.3.3 Skeletal Findings
As the heart is usually regarded as striated muscle, authors have analyzed biopsies of peripheral skeletal muscle, especially in patients presenting with myopathy. Skeletal muscle biopsies were performed in 17 patients [7, 20, 25, 27, 33,34,35, 40, 42, 45, 50, 57, 64, 66, 68, 77, 89]. Similar to the analysis of heart tissue, analysis of skeletal muscle with light microscopy revealed enlarged vacuolated myocytes and the presence of curvilinear bodies and myelin figures in transmission electronic microscopy. One case reported a pathologic quadriceps femoris muscle biopsy in a patient who did not exhibit muscular symptoms [34]. These abnormalities have also been described on nerve biopsy, which means that HCQ and CQ accumulation is divided among several organ compartments [25]. Only one patient with specific pathologic features on heart biopsy had a normal skeletal muscle biopsy [64]. Skeletal muscle biopsy could therefore be an important diagnostic tool.
3.4 Prognosis after Withdrawal
Outcomes after withdrawal of CQ/HCQ therapy were reported for 78 patients. Treatment withdrawal resulted in complete recovery of heart function in 44.9% of patients (n = 35). Improvement was histologically documented for five patients [20, 25, 34, 56, 80]. At the control biopsy, cardiomyocytes were reduced in size and free of intracellular vacuoles, transmission electron microscopy did not show myelin or curvilinear bodies, and mitochondria were normalized. As in the heart, Magnussen and de Fine Olivarius reported improvement and normalization of peripheral skeletal muscle biopsy 5 months after withdrawal [20]. For the remaining patients, the clinical course was unfavorable. Improvement of atrioventricular block was not reported. Several patients experienced irreversible damage, requiring pacemaker implantation (n = 8) or, in two patients, heart transplant [49, 57]. Death occurred in 24 patients, most often shortly after diagnosis. For 12 patients, symptoms were unchanged after CQ/HCQ therapy withdrawal. Whether patients recovered after ending CQ/HCQ therapy was highly variable, and predictive prognostic factors remain unknown.
3.5 Differential Diagnosis: Fabry Disease
Cardiac complications could be the result of an impairment related to the underlying auto-immune disease, a differential diagnosis often not considered. Indeed, several clinical and histopathologic similarities in endomyocardial biopsies are shared between CQ/HCQ-associated cardiotoxicity and FD that need to be detailed.
3.5.1 Similarities with Chloroquine/Hydroxychloroquine Toxicity
Fabry disease heart involvement is the differential diagnosis of CQ/HCQ-associated cardiotoxicity. Fabry disease is also accompanied by left ventricular hypertrophy, valvular dysfunction, microvascular angina, and conduction abnormalities [101]. Both diseases involve lysosomal acid hydrolases. The cardiac presentation of these two diseases is similar, regarding clinical symptoms, medical imaging, and pathologic findings [46]. Fabry disease is a genetic storage disorder, which results from a deficiency of the lysosomal enzyme alpha-galactosidase A. As mentioned above, this enzyme is also involved in the pathophysiology of CQ/HCQ-related cardiotoxicity. Indeed, CQ and HCQ provoke dysfunction of lysosomal enzymes, leading to the impairment of intracellular degradation processes in conjunction with the accumulation of pathologic metabolic products [101]. Roos et al. compared clinicopathologic features of CQ cardiotoxicity with those of FD. In their study, patients with cardiac dysfunction due to CQ cardiotoxicity or FD had similar ages and presenting clinical symptoms, and similar features in cardiac biopsies examined under light microscopy and transmission electron microscopy, including the presence of myelinoid bodies [46]. The personal or family history of some patients with FD was not known. Similarly, a history of CQ use may not have been known by the pathologist. Nevertheless, the presence of curvilinear bodies in electron microscopy was only found in CQ cardiotoxicity. This finding may be useful for the diagnosis of CQ cardiotoxicity, emphasizing that in the case of suspected toxicity, or by contrast a suspected case of FD, electronic microscopy remains the cornerstone. In addition, because FD is a genetic disease, genotyping should be performed in patients treated with CQ/HCQ presenting with FD-like symptoms.
3.5.2 Underlying Fabry Disease
There are documented cases of combined FD and autoimmunity, including SLE, [102, 103] as well as a high prevalence (57%) of autoimmune markers in patients with FD [104]. Moreover, rheumatologic diseases are often a misdiagnosis of FD (39% of cases) [105]. Among 127 patients in this review presenting with pathologic features corresponding to FD, screens for FD were performed in only 11 [46, 55, 73, 74, 79, 80, 87, 89, 91, 96, 100]: nine of them were negative, leaving two reported cases of connective tissue disease treated with HCQ, in patients presenting with heart involvement associated with FD. In the first case, the patient had SLE and antiphospholipid syndrome and had been previously diagnosed with FD [87]. The patient presented with cardiac dysfunction. The resulting heart failure was described as FD associated, in combination with SLE and antiphospholipid syndrome. Her EMB showed only myelin figures. She was treated by HCQ withdrawal and enzyme replacement therapy. Despite improvement, she continued to have FD-associated vascular complications, with myocardial infarction, and stroke. However, the majority of patients have shown clinical improvement after enzyme replacement therapy [106]. In the second case, the patient was a woman with a family history of cardiac sudden death in two brothers and a personal history of SLE treated with HCQ. She presented with a recent stroke and dyspnea. Her EMB showed curvilinear bodies and myelin figures, and the screen for FD was positive. Heart failure was described as cardiomyopathy due to FD, probably promoted by long-term use of HCQ [96]. Because the two diseases are similar, FD must be precluded by screening especially if there is a family history or neurologic or kidney involvement.
4 Discussion
We performed the first systematic review related to cardiac disorders attributed to treatment with HCQ or CQ. In the last decade, we found an increase in the number of reports of cardiac disorders, but the prevalence of HCQ/CQ-associated cardiotoxicity is undetermined because of a high number of diagnostic failures.
Our findings show that CQ and HCQ cardiac disorder is very severe and may provoke potentially irreversible damage and death. The two main clinical manifestations reported are conduction disorders (bundle or atrioventricular block) and myocardial hypertrophy. More specifically, conduction disorders should be considered a side effect of CQ/HCQ therapy and alert clinicians for termination of treatment. Myocardial toxicity associated with CQ/HCQ remains a difficult diagnosis, in particular because of the multiplicity of clinical presentations, potential confounding effects of underlying auto-immune disease in some patients, and the need for invasive tests for definitive diagnosis; it is often overlooked because of its unspecificity. In association with recommendations for screening toxic retinopathy, clinicians should be aware of possible iatrogenic effects and perform regular electrocardiograms to detect conduction disorders due to HCQ or CQ. Indeed, quinidine-like acute cardiac toxicity has been well documented following intravenous CQ administration or in overdoses from self-poisoning. Chloroquine provokes sodium and calcium channel blockades, which lead to membrane-stabilizing effects, negative inotropic effects, and peripheral vasodilatation. Membrane stabilization results in conduction disturbances with atrioventricular block, and QRS interval widening [107]. A dangerous QT prolongation induced by CQ could eventually happen [107]. Hydroxychloroquine is known to be safer than CQ. However, this is more frequent than in the general population, and is independent of previous myocardial disease [108]. Generally, treatment with HCQ or CQ should be reconsidered in the case of conduction abnormalities. We summarize the pathophysiology of CQ/HCQ toxicity in Fig. 1.
The diagnosis of drug toxicity is made more difficult because cardiac disorders may occur independently as a result of other causes. In many cases, heart failure arising from auto-immune diseases, hypertension, or valvular diseases could be misleading. Until now, given these confounding factors, only cardiac biopsies could provide a definitive diagnosis of myocardial toxicity associated with CQ/HCQ treatment. Nevertheless, MRI is also useful and necessary for differential diagnoses. Several patients presented with late gadolinium enhancement, a marker for fibrosis, especially in hypertrophic cardiomyopathy, and a prognostic factor of cardiac death [109]. Therefore, MRI could have a leading role in cardiac evaluation and prognosis of CQ/HCQ drug side effects. A new MRI sequence (T1 mapping) has been developed and should become a sensitive method to highlight cardiac damage induced by HCQ or CQ. Indeed, T1 mapping was useful for diagnosing heart damage in FD, [110] which is similar in pathophysiology and clinical symptoms with CQ/HCQ cardiotoxicity, as mentioned below. This sequence is based on the fact that lipids are known to shorten the MRI parameter T1. Accordingly, reduced T1 values should be linked to myocardial glycosphingolipid accumulation in FD, which is also the main pathologic factor in CQ and HCQ toxicity. T1 mapping allows evaluation of reduced non-contrast T1 values and this parameter seems to be sensitive and specific to discriminate FD from other hypertrophic cardiopathies [111].
Cardiac toxicity risk is difficult to evaluate owing to a large variation in dose, duration of treatment, and clinical presentation of the disease. However, it is known that renal or liver dysfunction may increase retinopathy risk and thus the drug has to be used carefully in these cases [6]. While determination of plasma concentration is not accurate enough to diagnose metabolic dysfunction, a high increased dosage may suggest overdose and may be associated with acute toxicity (QRS widening and/or QT interval prolongation). A long half-life and a high volume of distribution characterize the pharmacokinetics of HCQ and CQ. While stable pharmacokinetics are normally obtained after five half-lives, variable concentrations may arise after standard administered doses because of inter-individual variability affecting metabolism of the drugs [112]. Cardiotoxicity could occur even if plasma dosage of HCQ or CQ is within therapeutic standards; therefore, plasma dosage does not help in diagnosis [30]. Chloroquine and hydroxychloroquine are metabolized in the liver and kidney, via different cytochrome P450 enzymes [113]. In their study, Saussine et al. reported a patient with toxic cardiomyopathy who was a heterozygous carrier of the allele cytochrome P450 2C8*3, at the cytochrome P450 2C8 locus. They suggest that this could provoke a partially deficient metabolic capacity and modified pharmacokinetics. In addition, a partial activity deficiency of the transport protein P-glycoprotein appeared linked to the genetic polymorphism, and may also be associated with variation in CQ or HCQ pharmacokinetics and potential toxicity [64]. However, both CQ and HCQ have high bioavailabilities (> 60%), suggesting that there are not good P- glycoprotein substrates. The major portion of the available drug is excreted unchanged by the kidney, suggesting that metabolism is less important than renal function for elimination.
Published case reports have significant limitations because they cannot provide conclusive evidence, they cannot provide an estimate of incidence, and are generally only useful in hypothesis generating. However, any indication that a treatment might have a significant harmful effect should not be ignored. Clinical diagnosis of cardiac toxicity may be helped by clinical history, electrocardiography, echocardiography, and MRI, but definitive evidence of cardiac toxicity is obtained by an EMB. The pathology of heart biopsies might mimic heart manifestations of FD, but curvilinear bodies appear to definitively document CQ/HCQ cardiac toxicity, and diagnosis of FD can be accomplished by genotyping. Taking into account that conduction disorders or cardiomyopathy might occur as a toxic side effect of CQ/HCQ treatment, we propose the flow chart in Fig. 2 as a guide to monitoring potential CQ/HCQ-induced cardiac disorders, including a pre-screening before drug initiation and a clinical-morphologic follow-up every 2 years as well as a guide to confounding diagnosis in patients with autoimmune disease (Fig. 2). Obviously, these guidelines should be validated but they are simple, cautious, and proposed with reduced cost principles.
5 Conclusion
Chloroquine/hydroxychloroquine drugs are generally safe and well tolerated, but cardiac toxicity remains a rare and severe iatrogenic complication. Because of its variability and non-specificity, accurate diagnosis is difficult, especially in patients with a disease known to affect the heart. Cardiac manifestations are potentially irreversible. Fabry disease must be precluded by mutation screening because the two diseases are similar in both their clinical symptoms and pathologic features.
References
Bertsias G, Ioannidis JPA, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67:195–205.
Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64:797–808.
Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology (ACR) recommendations for the use of disease-modifying anti-rheumatic drugs and biologics in the treatment of rheumatoid arthritis (RA). Arthritis Care Res (Hoboken). 2012;64:625–39.
McChesney EW, Banks WFJ, Sullivan DJ. Metabolism of chloroquine and hydroxychloroquine in albino and pigmented rats. Toxicol Appl Pharmacol. 1965;7:627–36.
Scherbel AL, Harrison JW, Atdjian M. Further observations on the use of 4-aminoquinoline compounds in patients with rheumatoid arthritis or related diseases. Cleve Clin Q. 1958;25:95–111.
Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123:1386–94.
Hughes JT, Esiri M, Oxbury JM, Whitty CW. Chloroquine myopathy. Q J Med. 1971;40:85–93.
Estes ML, Ewing-Wilson D, Chous SM, Mitsumoto H, Hanson M, Shirey E, et al. Chloroquine neuromyotoxicity: clinical and pathologic perspective. Am J Med. 1987;82:447–55.
Fasano S, Pierro L, Pantano I, Iudici M, Valentini G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J Rheumatol. 2017;44:1032–8.
Li C, Wang XR, Ji HJ, Zhang XY, Li XF, Wang LZ, et al. Cardiovascular disease in rheumatoid arthritis: medications and risk factors in China. Clin Rheumatol. 2017;36:1023–9.
Sharma TS, Wasko MCM, Tang X, Vedamurthy D, Yan X, Cote J, et al. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc. 2016;5(1):e002867.
Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004;28:277–84.
Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235:50–2.
Thome R, Lopes SCP, Costa FTM, Verinaud L. Chloroquine: modes of action of an undervalued drug. Immunol Lett. 2013;153:50–7.
Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20:109–17.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45.
Whisnant JP, Espinosa RE, Kierland RR, Lambert EH. Chloroquine neuromyopathy. Proc Staff Meet Mayo Clin. 1963;38:501–13.
Rewcastle NB, Humphrey JG. Vacuolar myopathy: clinical, histochemical, and microscopic study. Arch Neurol. 1965;12:570–82.
Wray R, Iveson M. Complete heart block and systemic lupus erythematosus. Br Heart J. 1975;37:982–3.
Magnussen I, de Fine Olivarius B. Cardiomyopathy after chloroquine treatment. Acta Med Scand. 1977;202:429–31.
Edwards AC, Meredith TJ, Sowton E. Complete heart block due to chronic chloroquine toxicity managed with permanent pacemaker. Br Med J. 1978;1:1109–10.
Oli JM, Ihenacho HN, Talwar RS. Chronic chloroquine toxicity and heart block: a report of two cases. East Afr Med J. 1980;57:505–7.
Godeau P, Guillevin L, Fechner J, Bletry O, Herreman G. Disorders of conduction in lupus erythematosus: frequency and incidence in a group of 112 patients (author’s transl) [in French]. Ann Med Interne (Paris). 1981;132:234–40.
Ladipo GO, Essien EE, Andy JJ. Complete heart block in chronic chloroquine poisoning. Int J Cardiol. 1983;4:198–200.
Ratliff NB, Estes ML, Myles JL, Shirey EK, McMahon JT. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med. 1987;316:191–3.
Ratliff NB, Estes ML, McMahon JT, Myles JL. Chloroquine-induced cardiomyopathy. Arch Pathol Lab Med. 1988;112(6):578.
Piette JC, Guillevin L, Chapelon C, Wechsler B, Bletry O, Godeau P. Chloroquine cardiotoxicity. N Engl J Med. 1987;317(11):710–1.
McAllister HAJ, Ferrans VJ, Hall RJ, Strickman NE, Bossart MI. Chloroquine-induced cardiomyopathy. Arch Pathol Lab Med. 1987;111:953–6.
Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises: case 38-1988. A 58-year-old woman with fever, sweats, congestive heart failure, and lymphadenopathy after treatment for a diagnosis of systemic lupus erythematosus. N Engl J Med. 1988;319:768–81.
Ihenacho HN, Magulike E. Chloroquine abuse and heart block in Africans. Aust N Z J Med. 1989;19:17–21.
Verny C, de Gennes C, Sebastien P, Le Thi HD, Chapelon C, Piette JC, et al. Heart conduction disorders in long-term treatment with chloroquine: two new cases. Presse Med. 1992;21:800–4.
Ogola ES, Muita AK, Adala H. Chloroquine related complete heart block with blindness: case report. East Afr Med J. 1992;69:50–2.
Iglesias Cubero G, Rodriguez Reguero JJ, Rojo Ortega JM. Restrictive cardiomyopathy caused by chloroquine. Br Heart J. 1993;69:451–2.
Fellahi JL, Dumazer P, Delayance S, Vernier I, Conte JJ. Cardiomyopathy under treatment with hydroxychloroquine disclosed by complete auriculoventricular block [in French]. Rev Med Interne. 1993;14(4):275–6.
Schroder S, August C, Pompecki R, Schmoldt A. Fatal vacuolar cardiomyopathy in chronic chloroquine drug treatment [in German]. Pathologe. 1995;16:81–4.
August C, Holzhausen HJ, Schmoldt A, Pompecki R, Schroder S. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med (Berl). 1995;73:73–7.
Romanowicz A, Larroude M, Cotelesa S, Naftal L, Bovea G. Atrioventricular block in rheumatoid arthritis by hydroxychloroquine. Prensa Med. 1997;84:309–13.
Guedira N, Hajjaj-Hassouni N, Srairi JE, el Hassani S, Fellat R, Benomar M. Third-degree atrioventricular block in a patient under chloroquine therapy. Rev Rhum Engl Ed. 1998;65:58–62.
Siqueira-Batista R, Ramos Junior AN, Pessanha BS, Sforza-de-Almeida MP, Potsch DF. Chloroquine and cardiac arrhythmia: case report. East Afr Med J. 1998;75:117–9.
Veinot JP, Mai KT, Zarychanski R. Chloroquine related cardiac toxicity. J Rheumatol. 1998;25:1221–5.
Reuss-Borst M, Berner B, Wulf G, Muller GA. Complete heart block as a rare complication of treatment with chloroquine. J Rheumatol. 1999;26:1394–5.
Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart. 1999;81:221–3.
Stein M, Bell M, Ang L. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27:2927–31.
Duvic C, Pats B, Rouvier B. Complete heart block following chronic chloroquine treatment [in French]. Rev Med Interne. 2000;21(5):462–3.
Cervera A, Espinosa G, Font J, Ingelmo M. Cardiac toxicity secondary to long term treatment with chloroquine. Ann Rheum Dis. 2001;60(3):301.
Roos JM, Aubry M-C, Edwards WD. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol. 2002;11:277–83.
Teixeira RA, Martinelli Filho M, Benvenuti LA, Costa R, Pedrosa AA, Nishioka SAD. Cardiac damage from chronic use of chloroquine: a case report and review of the literature. Arq Bras Cardiol. 2002;79:85–8.
Charlier P, Cochand-Priollet B, Polivka M, Goldgran-Toledano D, Leenhardt A. Chloroquine cardiomyopathy revealed by complete atrio-ventricular block: a case report [in French]. Arch Mal Coeur Vaiss. 2002;95:833–7.
Freihage JH, Patel NC, Jacobs WR, Picken M, Fresco R, Malinowska K, et al. Heart transplantation in a patient with chloroquine-induced cardiomyopathy. J Heart Lung Transplant. 2004;23:252–5.
Nord JE, Shah PK, Rinaldi RZ, Weisman MH. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum. 2004;33:336–51.
Naqvi TZ, Luthringer D, Marchevsky A, Saouf R, Gul K, Buchbinder NA. Chloroquine-induced cardiomyopathy-echocardiographic features. J Am Soc Echocardiogr. 2005;18:383–7.
Keating RJ, Bhatia S, Amin S, Williams A, Sinak LJ, Edwards WD. Hydroxychloroquine-induced cardiotoxicity in a 39-year-old woman with systemic lupus erythematosus and systolic dysfunction. J Am Soc Echocardiogr. 2005;18:981.
Chen C-Y, Wang F-L, Lin C-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44:173–5.
Reffelmann T. Contrast-enhanced magnetic resonance imaging of a patient with chloroquine-induced cardiomyopathy confirmed by endomyocardial biopsy. Circulation. 2006;114:e357–8.
Soong TR, Barouch LA, Champion HC, Wigley FM, Halushka MK. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy: a report of 2 cases. Hum Pathol. 2007;38:1858–63.
Cotroneo J, Sleik KM, Rene Rodriguez E, Klein AL. Hydroxychloroquine-induced restrictive cardiomyopathy. Eur J Echocardiogr. 2007;8:247–51.
Costedoat-Chalumeau N, Hulot J-S, Amoura Z, Delcourt A, Maisonobe T, Dorent R, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology. 2007;107:73–80.
Costedoat-Chalumeau N, Hulot J-S, Amoura Z, Leroux G, Lechat P, Funck-Brentano C, et al. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology (Oxford). 2007;46:808–10.
Hernandez Jimenez V, Saavedra Falero J, Navas R. Reversible restrictive cardiomyopathy secondary to chloroquine [in Spanish]. Med Clin (Barc). 2007;129(4):157.
Elaichaoui S, Amine B, Saoud B, Guedira N, Allali F, Hajjaj-Hassouni N. Complete auriculoventricular block during chloroquine treatment [in French]. Rev Med Interne. 2007;28:134–6.
Stas P, Faes D, Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008;127(2):e80–2.
Puymirat E, Douard H, Roudaut R. Complete atrioventricular block following long term treatment with chloroquine [in French]. Rev Med Interne. 2008;29:741–3.
Manohar VA, Moder KG, Edwards WD, Klarich K. Restrictive cardiomyopathy secondary to hydroxychloroquine therapy. J Rheumatol. 2009;36:440–1.
Saussine A, Loriot M-A, Picard C, Lecerf V, Landry J, Scheer I, et al. Chloroquine cardiotoxicity in long-term lupus therapy in two patients. Ann Dermatol Venereol. 2009;136:530–5.
Fragasso G, Sanvito F, Baratto F, Martinenghi S, Doglioni C, Margonato A. Cardiotoxicity after low-dose chloroquine antimalarial therapy. Heart Vessels. 2009;24:385–7.
Kwon J-B, Kleiner A, Ishida K, Godown J, Ciafaloni E, Looney RJ Jr. Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16:28–31.
Lee JH, Chung W-B, Kang JH, Kim HW, Kim JJ, Kim JH, et al. A case of chloroquine-induced cardiomyopathy that presented as sick sinus syndrome. Korean Circ J. 2010;40:604–8.
Mobley B, Azimian M, Atkinson J. Fatal hydroxychloroquine-induced skeletal and cardiac myopathy. J Neuropathol Exp Neurol. 2011;70:533.
Urbine D, et al. Hydroxychloroquine-induced restrictive cardiomyopathy in a patient with systemic lupus erythematosus. In: 77th Annual meeting of the American college of chest physicians: chest 2011, vol. 140, Abstr. 34A, No. 4, 22 Oct 2011. Available from: http://doi.org/10.1378/chest.1116752.
Muthukrishnan P, Roukoz H, Grafton G, Jessurun J, Colvin-Adams M. Hydroxychloroquine-induced cardiomyopathy: a case report. Circ Heart Fail. 2011;4:e7–8.
Pieroni M, Smaldone C, Camporeale A, Ierardi C, Dell’Antonio G, Bellocci F, et al. Images in cardiology: chloroquine-induced transition from dilated to restrictive cardiomyopathy. J Am Coll Cardiol. 2011;57:515.
Gentille Lorente DI. Ocular and cardiac toxicity by chloroquine [in Spanish]. Rev Med Chile. 2011;139(10):1384–6.
Hartmann M, Meek IL, van Houwelingen GK, Lambregts HPCM, Toes GJ, van der Wal AC, et al. Acute left ventricular failure in a patient with hydroxychloroquine-induced cardiomyopathy. Neth Heart J. 2011;19:482–5.
Newton-Cheh C, Lin AE, Baggish AL, Wang H. Case records of the Massachusetts General Hospital. Case 11-2011: a 47-year-old man with systemic lupus erythematosus and heart failure. N Engl J Med. 2011;364:1450–60.
Hamilton A, Langerman F. Two for the price of one: simultaneous hepatic and cardiac toxicity from hydroxychloroquine. J Hosp Med. 2012;7:S193.
Bae SM, Jung HO, Ihm SM, Kim JJ, Chin JY, Kim T-S, et al. Hydroxychloroquine-induced cardiomyopathy that presented as pulmonary hypertension: a newly noted complication. Cardiology. 2012;123:197–200.
Azimian M, Gultekin SH, Hata JL, Atkinson JB, Ely KA, Fuchs HA, et al. Fatal antimalarial-induced cardiomyopathy: report of 2 cases. J Clin Rheumatol. 2012;18:363–6.
Tjeuw M, Joshua F, Katrib A, Bertouch J. Hydroxychloroquine induced myopathy and nephropathy. Intern Med J. 2011;41:13.
Tonnesmann E, Stroehmann I, Kandolf R, Wolburg H, Strach K, Musshoff F, et al. Cardiomyopathy caused by longterm treatment with chloroquine: a rare disease, or a rare diagnosis? J Rheumatol. 2012;39(5):1099–103.
Frustaci A, Morgante E, Antuzzi D, Russo MA, Chimenti C. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol. 2012;157(1):117–9.
Abbasi S, Tarter L, Farzaneh-Far R, Farzaneh-Far A. Hydroxychloroquine: a treatable cause of cardiomyopathy. J Am Coll Cardiol. 2012;60:786.
Morgan N, Patel S, Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:286–8.
Sultan A, Crass S, Bernstein M, Khalil S, Ahmed V, Rehman S. Hydroxychloroquine induced cardiomyopathy. J Gen Intern Med. 2013;28:S341.
Vereckei A, Fazakas A, Balo T, Fekete B, Molnar MJ, Karadi I. Chloroquine cardiotoxicity mimicking connective tissue disease heart involvement. Immunopharmacol Immunotoxicol. 2013;35:304–6.
Joyce E, Fabre A, Mahon N. Hydroxychloroquine cardiotoxicity presenting as a rapidly evolving biventricular cardiomyopathy: key diagnostic features and literature review. Eur Heart J Acute Cardiovasc Care. 2013;2:77–83.
Rojo-Ortega J, Iglesias-Cubero G, Rodríguez-Reguero J, Rojo-Manaute J, Argüelles-Collada J. Chloroquine-induced atrial lesions: histopathological studies. Rev Esp Patol. 2013;46:117–21.
Nandagudi A, Jury EC, Alonzi D, Butters TD, Hughes S, Isenberg DA. Heart failure in a woman with SLE, anti-phospholipid syndrome and Fabry’s disease. Lupus. 2013;22:1070–6.
Lopez-Ruiz N, Uribe CE. Chloroquine cardiomyopathy: beyond ocular adverse effects. BMJ Case Rep. 2014. https://doi.org/10.1136/bcr-2014-205751.
Yogasundaram H, Putko BN, Tien J, Paterson DI, Cujec B, Ringrose J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol. 2014;30:1706–15.
Malchair P, Labori M, Rubio-Rivas M, Salazar-Mendiguchia J, Baixeras N, Corbella X. Hydroxychloroquine myocardial toxicity in a patient with systemic lupus erythematosus. Eur J Case Rep Intern. 2015;2(3):1–3. https://doi.org/10.12890/2015_000204.
Shaw D, Rohekar G. Hydroxychloroquine-induced cardiomyopathy presenting as heart failure: a case report. J Rheumatol. 2015;42:1284.
Zerbib Y, Guillaumont MP, Touati G, Duhaut P, Schmidt J. Early cardiotoxicity of hydroxychloroquine [in French]. Rev Med Interne. 2016;37:209–11.
O’laughlin J, Mehta P, Wong B. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016;2016:4626279.
Pavsic N, Mraz J, Strazar ZD, Gabrijelcic J, Toplisek J, Kozelj M, et al. Shunt reversal due to deterioration of chloroquine-induced cardiomyopathy in a patient with primary Sjogren syndrome. Int J Cardiol. 2016;215:145–6.
Tselios K, Gladman DD, Harvey P, Mak S, Chantal M, Butany J, et al. Hydroxychloroquine-induced cardiomyopathy in systemic lupus erythematosus. J Clin Rheumatol. 2016;22(5):287–8.
Chatre C, Filippi N, Roubille F, Pers Y-M. Heart involvement in a woman treated with hydroxychloroquine for systemic lupus erythematosus revealing fabry disease. J Rheumatol. 2016;43(5):997–8.
Sabato LA, Mendes LA, Cox ZL. Restrictive cardiomyopathy associated with long-term use of hydroxychloroquine for systemic lupus erythematosus. J Pharm Pract. 2017;30(5):571–5.
Shaikh S, Grewal V, Turk A, Montfort J, Slawsky M, Catanzaro J. A rare cause of myocardial injury in a patient with systemic lupus erythematosus: hydroxychloroquine induced cardiomyopathy. J Am Coll Cardiol. 2017;69:2376.
Abdin A, Poss J, Kandolf R, Thiele H. Hydroxychloroquine-induced cardiomyopathy in a patient with limited cutaneous systemic sclerosis. Clin Res Cardiol. 2017;106(3):234–6.
Di Girolamo F, Claver E, Olive M, Salazar-Mendiguchia J, Manito N, Cequier A. Dilated cardiomyopathy and hydroxychloroquine-induced phospholipidosis: from curvilinear bodies to clinical suspicion. Rev Esp Cardiol (Engl Ed). 2018;71(6):491–3.
Putko BN, Wen K, Thompson RB, Mullen J, Shanks M, Yogasundaram H, et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev. 2015;20:179–91.
Rosenmann E, Kobrin I, Cohen T. Kidney involvement in systemic lupus erythematosus and Fabry’s disease. Nephron. 1983;34:180–4.
Rahman P, Gladman DD, Wither J, Silver MD. Coexistence of Fabry’s disease and systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:475–8.
Martinez P, Aggio M, Rozenfeld P. High incidence of autoantibodies in Fabry disease patients. J Inherit Metab Dis. 2007;30:365–9.
Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest. 2004;34:236–42.
Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, et al. Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet. 2009;374:1986–96.
White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–58.
Kreger BE, Anderson KM, Kannel WB. Prevalence of intraventricular block in the general population: the Framingham Study. Am Heart J. 1989;117:903–10.
Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–7.
Thompson RB, Chow K, Khan A, Chan A, Shanks M, Paterson I, et al. T(1) mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ Cardiovasc Imaging. 2013;6:637–45.
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–8.
Tett SE. Clinical pharmacokinetics of slow-acting antirheumatic drugs. Clin Pharmacokinet. 1993;25:392–407.
Kim K-A, Park J-Y, Lee J-S, Lim S. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res. 2003;26:631–7.
Author information
Authors and Affiliations
Contributions
CC and YMP collected the data and analyzed and interpreted the patient data regarding the cardiac complications of chloroquine/hydroxychloroquine. CC and YMP wrote a draft of the manuscript. FR, HV, and CJ provided expertise in heart disease and imaging modalities and participated in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Clotilde Chatre, François Roubille, Hélène Vernhet, Christian Jorgensen, and Yves-Marie Pers have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatre, C., Roubille, F., Vernhet, H. et al. Cardiac Complications Attributed to Chloroquine and Hydroxychloroquine: A Systematic Review of the Literature. Drug Saf 41, 919–931 (2018). https://doi.org/10.1007/s40264-018-0689-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-018-0689-4