Abstract
Anderson-Fabry disease (AFD) is a lysosomal storage disease caused by the inappropriate accumulation of globotriaosylceramide in tissues due to a deficiency in the enzyme α-galactosidase A (α-Gal A). Anderson-Fabry cardiomyopathy is characterized by structural, valvular, vascular and conduction abnormalities, and is now the most common cause of mortality in patients with AFD. Large-scale metabolic and genetic screening studies have revealed AFD to be prevalent in populations of diverse ethnic origins, and the variant form of AFD represents an unrecognized health burden. Anderson-Fabry disease is an X-linked disorder, and genetic testing is critical for the diagnosis of AFD in women. Echocardiography with strain imaging and cardiac magnetic resonance imaging using late enhancement and T1 mapping are important imaging tools. The current therapy for AFD is enzyme replacement therapy (ERT), which can reverse or prevent AFD progression, while gene therapy and the use of molecular chaperones represent promising novel therapies for AFD. Anderson-Fabry cardiomyopathy is an important and potentially reversible cause of heart failure that involves LVH, increased susceptibility to arrhythmias and valvular regurgitation. Genetic testing and cardiac MRI are important diagnostic tools, and AFD cardiomyopathy is treatable if ERT is introduced early.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anderson-Fabry disease (AFD) is a lysosomal storage disorder caused by genetic deficiency of the enzyme encoded by the alpha-galactosidase A gene (α-Gal A) enzyme located on the X-chromosome (Xq22.1) [1]. This causes progressive, widespread intracellular accumulation of globotriaosylceramide (Gb3) and other sphingolipids throughout the body [1, 2]. Initial clinical manifestations of AFD are nonspecific and are often mistakenly attributed to alternative disorders, leading to missed or delayed diagnoses, by which time end-organ damage may be irreversible [1, 3]. Major manifestations of AFD are cardiac, renal and neurological; these can be partially attributed to vascular endothelial dysfunction [3, 4]. According to the organs involved, a number of different symptoms and signs can be found in patients with AFD, including pain and neuropathy in the distal limbs (acroparesthesias); gastrointestinal signs and symptoms, such as diarrhea, abdominal pain and early satiety; benign cutaneous lesions of small blood vessels (angiokeratomas); and corneal opacities (cornea verticillata) [5–8]. Neurological manifestations often present earliest, but these symptoms are not definitive due to their transient and variable nature [1, 9]. The most important manifestations of AFD from an outcomes perspective are renal, including proteinuria and renal failure; and cardiovascular, including left ventricular hypertrophy (LVH), diastolic dysfunction, valvular abnormalities and conduction defects [4, 10]. Anderson-Fabry disease is characterized by two major phenotypes: classical and variant, with considerable variation in clinical course [11, 12].
In this review, we provide an updated perspective on the prevalence, gender differences, diagnostic modalities and treatment of AFD with a focus on the cardiomyopathy associated with AFD.
Case report
A 37-year-old male with AFD was evaluated and treated over the past 10 years. Genetic testing showed a G43V mutation in the GLA gene, and plasma α-Gal A activity was markedly reduced at 0.31 nmol/hr/mL. His manifestations of AFD included acroparesthesias and marked renal and cardiac involvement without significant cerebrovascular disease. His medical therapy included enteric-coated ASA, ACE inhibition, statin therapy and enzyme replacement therapy (ERT), which was initiated in 2004. He progressed with biventricular hypertrophy, worsening aortic valvular regurgitation (Fig. 1a–c) and progressive renal dysfunction. He underwent an aortic valve replacement with a mechanical aortic valve prosthesis and subaortic septal myomectomy. Histopathological studies using PAS staining showed marked vacuolation in the myocardium, and electron microscopy revealed myocardial electron-dense lamellar deposits (Fig. 1d, e). Due to progression of his renal failure in the presence of proteinuria, a renal biopsy was performed which showed evidence of glomerulosclerosis. He was initiated on renal replacement therapy (hemodialysis) and is currently being evaluated for a kidney transplant.
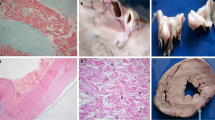
Clinical characteristics of a patient with advanced Anderson-Fabry disease. 12-lead ECG showing a normal sinus rhythm, short PR interval and left and right ventricular hypertrophy (a). Doppler echocardiography showing severe aortic valvular regurgitation (b) and two-dimensional echocardiography showing concentric left ventricular hypertrophy with mild ventricular dilation (c). Pathological findings obtained from a septal myomectomy specimen showing multiple vacuolated cells or perinuclear vacuoles on hematoxylin and eosin (H&E) staining (d), and ultrathin slide sections showing enlarged secondary lysosomes packed with storage material, which has a somewhat heterogeneous appearance using transmission electron microscopy (e)
Epidemiology of Anderson-Fabry disease
Classic Anderson-Fabry disease
Classically affected males have very low or absent α-Gal A activity, which results in severe systemic manifestations that typically begin in childhood or adolescence, and include acroparesthesias, angiokeratomas, cornea verticillata, hypohidrosis, mild proteinuria and gastrointestinal problems. Between 20 and 40 years of age, cardiac involvement typically becomes noticeable, and renal involvement usually progresses to the point of requiring dialysis or renal transplantation [13, 14]. Late manifestations usually include cardiac dysfunction, leading to heart failure, arrhythmias and myocardial infarction [15, 16]. Cerebrovascular manifestations include early stroke, thromboses and transient ischemic attacks, which lead to significant neurological deterioration and death [17].
Variant Anderson-Fabry disease
A variant phenotype is due to residual α-Gal A activity (>5 % of normal plasma α-Gal A) [18, 19]. Because of the low residual α-Gal A levels, these patients do not have all of the major clinical manifestations of classic AFD, but often present later in life with elements of AFD, including cardiomyopathy and renal disease [1, 20–22].
Prevalence in the general population
Historical estimates of AFD incidence were very low: 1 in 40,000 males, which recent data refutes as a gross underestimate, likely due to missing accounts of mild or variant disease phenotypes [23]. Neonatal dried bloodspot screening for α-Gal A activity deficiency in separate populations of Japanese, Austrian, Chinese–Taiwanese and Italian neonates revealed that the prevalence of AFD might be much higher than previously predicted [24–27]. Indeed of 21,170 Japanese neonates, 1 in 3,024 was test positive; of 34,736 Austrian neonates, 1 in 3,859 was test positive; of 110,027 Chinese–Taiwanese neonates, 1 in 1,642 was test positive; and of 37,104 Italian neonates, 1 in 3,100 were test positive (Fig. 2a). These results suggested a hitherto unrecognized pandemic of AFD. However, genetic testing subsequent to the dried blood spot screening programs revealed varying proportions of confirmed GLA mutations in newborns with α-Gal A deficiencies (Fig. 2b). In the aforementioned studies, the majority of the confirmed GLA mutations were associated with variant phenotypes. Neonates with low α-Gal A activity and no confirmed mutation might experience a rebound in enzyme activity with aging. Conversely, large genomic deletions over part of Xq22.1 can cause AFD and are typically not detected by GLA mutation analysis [28], which may have been the case for some newborns without a GLA mutation corresponding to low α-Gal A activity. The penetrance of variant AFD mutations is likely affected by the presence of systemic risk factors, which are likely to develop later in life, such as hypertension, obesity, diabetes and smoking habit. It is therefore difficult to ascertain the risk posed to neonates who are diagnosed with uncharacterized GLA variants or those associated with non-classical disease, so long-term follow-up studies are clearly required.
Prevalence in cohorts with cardiomyopathies
Screening studies have revealed that variant AFD might contribute significantly to the prevalence of unexplained or idiopathic cardiovascular disorders. In small screening studies of Japanese males, UK males and Italian females for late-onset hypertrophic cardiomyopathy, AFD, was detected in 3, 6.3 and 12 % of patients, respectively [20, 29, 30]. Furthermore, in a large consecutive cohort of 1,386 European patients with unexplained LVH, 0.5 % had GLA mutations associated with AFD [31]. The Chinese–Taiwanese neonatal screening study revealed a high prevalence of GLA mutations associated with cardiovascular variant AFD, which compounds the need for cardiologists to be aware that a small portion of patients they evaluate for hypertrophic cardiomyopathy might have variant or previously unrecognized classical AFD [4, 26]. Indeed, AFD cardiomyopathy can be clinically unrecognizable from other genetic hypertrophic cardiomyopathies, which suggests that systematic mutation screening is necessary to better define AFD patients [32]. Early recognition of those patients with variant AFD that would otherwise be classified as idiopathic cardiomyopathy could allow appropriate treatment and disease alleviation. All together, these data suggest that the prevalence of variant AFD is much higher than previously predicted [23]. However, some degree of caution must be exercised as the penetrance of mutations associated with variant AFD can vary considerably. A recent review by van der Tol et al. [33] suggests that new diagnostic algorithms are necessary to ensure that patients with AFD are appropriately recognized, including ruling out AFD in ambiguous cases of low α-Gal A activity.
Diagnostic and genetic testing: importance of gender
The challenge of elucidating whether uncharacterized genetic variants are responsible for clinical manifestations of illness identifies the need for more rigorous diagnostic criteria for AFD [33]. De novo mutations are rare, and thus, most affected males have mothers who are carriers, so family history is an important component of the differential diagnosis for AFD. In all cases, the differential diagnosis for AFD is initiated when an astute clinician recognizes the confluence of the characteristic signs and symptoms (Fig. 3), although, as previously mentioned, many of the earliest disease manifestations are nonspecific. Traditionally, AFD was diagnosed in suspected males by markedly deficient or absent α-Gal A activity in plasma or peripheral leukocytes. However, evidence of α-Gal A pseudodeficiencies, which are apparent deficiencies in α-Gal A enzyme activity in the setting of testing that do not manifest in clinically significant deficiencies [34], suggests that confirmation of AFD should be made using genetic testing when α-Gal A activity assay is non-diagnostic [35]. Non-diagnostic α-Gal A activity results are those that are 5–10 % of a reference value, because 5–10 % α-Gal A activity might be associated with the absence of clinically significant disease [1]. Nonetheless, the nonspecific signs, symptoms and complex multi-organ nature of AFD make it difficult to clinically suspect the disease without prior known family history [3]. Indeed, evidence of a relative with confirmed AFD can be diagnostic in the context of unclear test results [33]. Similarly, lysoGb3 has been proposed as a specific plasma biomarker for AFD, which could add clarity to the diagnostic algorithm by identifying clinically significant mutant varieties [36].
Diagnostic algorithm for Anderson-Fabry disease shows the separate paths taken after presentation for male and female patients that end in a positive diagnosis of AFD. GVUS genetic variant of unknown significance. This figure has been adapted from published recommendations by Laney et al. [39], van der Tol et al. [33] and Niemann et al. [36]
Clinical manifestations in heterozygous carrier females range from asymptomatic to severe disease, although symptoms may initially appear mild [37]. In an Anderson-Fabry disease registry of 1,077 enrolled females, 69.4 % had symptoms and signs of AFD [38], and the majority of female AFD patients develop clinically significant disease [39, 40]. Carrier detection by enzyme analysis is not reliable in females, since α-Gal A activities range from very low to normal in heterozygotes and any female patient being evaluated for AFD must undergo genetic testing [4, 32]. Similar to males, pedigree analysis [33] or plasma lysoGb3 analysis [36] could be used to rule in or out AFD in a female patient with a genetic variant of unknown significance (Fig. 3). Children can be evaluated for AFD provided the family will receive appropriate counseling and medical support, and while early initiation of ERT is supported [41], there is little evidence to suggest its use in children [42]. Prenatal screening can be accomplished using amniocentesis or chorionic villus sampling, provided the same conditions for testing asymptomatic children are met [43]. Genetic counseling is essential for all individuals diagnosed with AFD [39].
Cardiovascular complications in Anderson-Fabry disease
Patients with cardiomyopathy due to infiltrative myocardial diseases have a poor prognosis, and heart disease is now the leading cause of mortality in patients with AFD [9, 44]. As illustrated by our case report (Fig. 1), heart disease in patients with AFD is characterized by progressive left ventricular hypertrophy (LVH) leading to heart failure, valvular heart disease and arrhythmias [30, 45]. Cardiac-related death has overtaken renal disease as the most common cause of mortality in AFD [2, 9]. The primary gene defect in AFD is the alpha-galactosidase A gene, which is responsible for the cleavage between the two galactose residues in Gb3, with more than 400 different mutant alleles identified (Fig. 4a) [46]. Undigested Gb3 accumulates in the vascular endothelium and in the heart leading to the induction of hypertrophic, fibrotic and apoptotic pathways (Fig. 4b) [47]. A majority (60 %) of patients with AFD have a history of abnormal cardiovascular signs and symptoms, with hypertension and edema being the most prevalent, followed by murmur, dyspnea and angina [21, 48].
Pathophysiology of Anderson-Fabry disease. The schematic representation of a sphingolipid and the site of cleavage by α-galactosidase (a). The accumulation of sphingolipid in the heart leads to a progressive cardiomyopathy characterized by ventricular hypertrophy, valvular regurgitation, impaired coronary microvasculature and electrophysiological abnormalities (b)
Cardiac structural and functional abnormalities
Cardiac manifestation in patients with AFD includes LVH with impaired diastolic function; however, systolic function, as assessed by left ventricle ejection fraction, is preserved in the large majority of affected patients [15, 16, 49, 50]. Lysosomal Gb3 accumulation in the myocardium is responsible for only 1–3 % of mass in the hypertrophic heart. Interestingly, in a screen of AFD patients, left ventricular mass index inversely correlated with α-Gal A activity, which suggests that the extent of Gb3 accumulation determines the relative extent of pathogenesis in AFD [48]. Abnormal deposition of Gb3 induces pathologic processes such as inflammation and oxidative stress, which lead to hypertrophy and extracellular matrix (ECM) remodeling [47]. Unlike male patients, myocardial functional decline and fibrosis development do not always coincide with myocardial hypertrophy in female patients with AFD [30, 51, 52]. Initial staging and monitoring should therefore be based on LVH and replacement fibrosis in female patients with AFD [51]. There is a strong correlation between age and the severity of LVH, and evidence suggests that all female AFD carriers older than 45 years have LVH [53]. The development of heart failure secondary to AFD is a recognized burden for the variant AFD phenotypes, and hypertrophy, diastolic dysfunction and valvular dysfunction are all important pathogenic events driving the heart failure phenotype [21, 48, 54]. Reduction in LV cavity size and papillary muscle hypertrophy can also result in LV outflow tract obstruction and exercise intolerance in patients with AFD [55]. In addition, the high prevalence of obesity and hypertension as pan-ethnic health burdens coupled with presently long average life expectancy can further exacerbate variant forms of AFD, thereby contributing significantly to the incidence of heart failure [56–58].
Valvular abnormalities and vascular changes
Left heart valves are predominantly affected in AFD, even though the pathological accumulation of Gb3 is present in both sides of the heart. This could be explained by the increased pressure in the left heart leading to a more rapid deterioration of the mitral and aortic valves [16, 47]. Mitral leaflet thickening, with corresponding moderate or severe mitral regurgitation, is typically present in more than 50 % of patients, while minor aortic valve thickening is found in nearly 50 % of patients, which can progress to moderate or severe regurgitation (Figs. 1b and 4b) [15, 48]. Coronary microvascular dysfunction and reduced coronary perfusion reserve are characteristic of AFD, while gross abnormalities in blood flow are not apparent [59, 60]. Increased common carotid artery intima-media thickness (CCA-IMT) was present in males and females with AFD, and there was a strong positive correlation between LV mass and CCA-IMT [61]. Deacylated Gb3, globotriaosylsphingosine, is dramatically increased in the plasma of patients with AFD and stimulates proliferation of vascular smooth muscle cells, thereby providing a mechanism for the increased intima-media thickness seen in these patients [62]. Myocardial perfusion imaging using positron-emission tomography or nuclear medicine is necessary for assessment of coronary microvascular function.
Conduction defects and arrhythmias
Early manifestations of AFD on the ECG include a shortened PR interval, which is likely due to accelerated AV nodal conduction [63]. The PR interval may change with age and/or the grade of the disease: initial shortening in up to 40 % of affected men and later prolonged in the advanced phases of the disease which can progress to high-grade atrioventricular block. In adults, ECG signs of LV hypertrophy are present in up to 61 % of men and 18 % of women [15]. The QRS duration, meanwhile, has been reported up to 160–200 ms with the 12-lead amplitude-duration product having the best correlation with LV mass [54]. Later manifestations of AFD include supraventricular tachyarrhythmias, conduction defects and bradycardia, and ventricular arrhythmias and sudden cardiac death [64, 65]. A retrospective evaluation of 6 AFD patients with implantable devices revealed atrial and ventricular pacing 71 and 49 % of the time, respectively [66]. The high incidence of pacemaker implantation and utilization suggests that bradyarrhythmias have a significant impact on the natural history of AFD. Heart rate variability correlates with autonomic dysfunction and is lowered in men <18 years of age compared to age-matched normal subjects [67], which may represent a pro-arrhythmogenic electrophysiological substrate.
Imaging approaches to evaluate patients with Anderson-Fabry cardiomyopathy
Assessment of cardiac structural and functional abnormalities in AFD can be obtained by a number of imaging modalities, including echocardiography (LVH, wall thickness, diastolic filling, valvular abnormalities) and cardiac MRI. Echocardiography has been used as a standard noninvasive screening test for Anderson-Fabry cardiomyopathy for many years. However, only approximately 40 % of patients have LVH at the time of AFD diagnosis, which makes recognizing cardiac involvement difficult using conventional echocardiography [15]. In addition, changes in LV diastolic function, as assessed by conventional Doppler echocardiography, have also been variable [68, 69]. The challenge to conventionally diagnose AFD is the significant overlap with other cardiovascular pathologies in terms of structural and functional alterations. More specificity might be achieved by way of novel approaches that exploit unique elements of the AFD phenotype.
Echocardiography
Echocardiography can be used for assessing LV remodeling and hypertrophy, estimating LV mass and assessing valvular regurgitation. New methods of strain and strain rate (SR) echocardiography have had satisfactory results for the detection of subclinical stages of impaired myocardial contractility and diastolic dysfunction in AFD [70]. Tissue Doppler-derived strain and SR have been previously shown to be useful for detecting subclinical stages of impaired regional longitudinal and radial function in AFD patients with otherwise preserved ejection fraction [68]. In addition, peak systolic strain and SR improve with ERT, indicating that SR imaging may be a useful tool in monitoring the efficacy of treatment [45, 71]. Recent evidence shows that strain and SR analyses derived from two-dimensional speckle-tracking techniques can identify AFD, independent of concentric remodeling and hypertrophy, with more sensitivity and specificity than conventional tissue Doppler echocardiography [50]. After correcting for LVH, strain rate during isovolumic relaxation (SRIVR) and transmitral E-wave velocity to SRIVR ratio remained predictors of AFD by ROC analysis. Longitudinal systolic strain was also significantly lowered in AFD patients compared to healthy controls [50]. Speckle-tracking imaging can provide evidence of subclinical myocardial dysfunction associated with reduced contractile reserve and diastolic dysfunction in patients with AFD [72].
Cardiac MRI
Cardiac magnetic resonance imaging (CMR) plays a critical role in evaluating the differential diagnosis of cardiomyopathies and to characterize the structure function of the heart in patients with AFD. Cardiac MRI can reliably detect hypertrophy, hypokinesia and areas of delayed enhancement in patients with AFD [73, 74] and is also a suitable tool for evaluating the beneficial effects of ERT on LV mass in AFD patients [75] and to delineate gender differences in LV remodeling [51, 52, 70]. Furthermore, CMR is sensitive to the way in which intracellular accumulation of Gb3 in AFD alters the biochemical environment of the heart. Non-contrast T1 mapping reveals differences in tissue pathophysiology and can elucidate biochemical differences that might not be structurally apparent, such as in idiopathic versus AFD cardiomyopathy [76]. While AFD-induced hypertrophy is structurally indistinguishable from other types of hypertrophic cardiomyopathy (Fig. 5a), our group [52] and Sado D et al. [77] have shown that non-contrast T1 mapping can distinguish AFD from other etiologies of concentric remodeling and hypertrophy, whereby septal T1 times are significantly lower in AFD patients than control or concentric remodeling patients (Fig. 5b, c). Structural analyses revealed that AFD and concentric hypertrophy did not differ in terms of cardiac mass, LV chamber volume, mass to LV chamber volume ratio or wall thickness. Likewise, LV hypertrophy was a common finding in diverse patient groups, including AFD, hypertension and cardiac amyloidosis [77]. In both studies, T1 relaxation time was significantly lower in AFD, which is likely to be a consequence of increased lipid content in the intracellular compartment of cardiomyocytes in AFD [52]. Furthermore, these studies established that T1 cutoff values could be derived that separated AFD from other conditions with LV hypertrophy with high sensitivity and specificity.
Cardiac magnetic resonance imaging in healthy controls, AFD and concentric remodeling/hypertrophy subjects in unsaturated SASHA acquisition (a) and pre-contrast septal myocardial T1 mapping (b). Histograms quantifying T1 pixel mapping show AFD patients have significantly reduced myocardial T1 as compared with control or concentric remodeling/hypertrophy patients (c). Figure reproduced with permission from Thompson et al. [52]
Renal disease in patients with Anderson-Fabry disease
End-stage renal disease (ESRD) was the most common cause of morbidity and premature mortality in AFD, particularly in classically affected males [22, 78]. Recent evidence indicates a reduction in morbidity and mortality attributable to ESRD, likely due to improvements in supportive care, use of ERT, dialysis and management of comorbidities such as hypertension [9]. Enzyme replacement therapy has produced clearance of renal Gb3 deposits with improvement in clinical outcomes [79, 80]. The decline of renal function in Anderson-Fabry nephropathy is adversely affected by male gender, advanced chronic kidney disease and severe proteinuria with urinary protein excretion being the most important predictor [81]. The diagnosis of AFD needs to be addressed in patients with progressive kidney disease; some AFD patients have been first identified in screening programs of dialysis patients [82]. The Fabrazyme and ARBs and ACE Inhibitor Treatment (FAACET) is a multicentre and open-label study of the safety and efficacy of renin-angiotensin system inhibition in the control of proteinuria and renal disease in patients with AFD which will provide important data to further guide therapy in these patients [83].
Patients with AFD have a worse 3-year dialysis survival rates compared with non-diabetic controls, which is likely driven by an underlying cardiomyopathy that often complicates volume control and dialysis regimen. Similarly, the 5-year survival rate of transplanted AFD patients is lower than that of controls. However, because Anderson-Fabry nephropathy does not recur in the allograft, renal transplantation might improve outcomes better than dialysis. With the advent of renal transplantation and dialysis, the mean lifespan of males with classical disease improved from an average of 41–50 years from 1967 to 2001 [84, 85]. However, transplantation is the best renal replacement therapy (RRT) option for AFD patients with ESRD.
Medical management of Anderson-Fabry disease
Patients with AFD should be followed regularly, regardless of disease status, by a multidisciplinary team that will be able to handle the heterogeneity and variability of AFD. Although signs and symptoms will dictate the frequency and extent of follow-up, comprehensive medical evaluation at least once a year is recommended in all males with AFD. Once a diagnosis of AFD is confirmed, all individuals should have a detailed medical and family history taken. All signs and symptoms should be carefully documented at baseline and then at least annually or more often, as the clinical situation dictates. Even in the absence of symptoms, all known heterozygous females should be considered at risk for developing disease manifestations and should have a complete baseline examination. Symptomatic heterozygotes should be followed annually with tests focused on their disease manifestations, while asymptomatic females can be re-evaluated every 2 years.
Risk factor management
Proactive modification of lifestyle, including smoking cessation, should also be recommended as a general measure for prevention and improvement of cardiovascular, cerebrovascular and renal disorders. An adapted version of the Dietary Approaches to Stop Hypertension (DASH) diet, tailored for the kidney issues prevalent in AFD, and combined with moderate exercise will help with weight management, blood pressure control, cholesterol reduction, as well as overall cardiopulmonary fitness [86, 87]. ACE inhibitor and statin therapy are important therapeutic agents to treat hypertension and dyslipidemia in patients with AFD, respectively, and are known to mediate vascular protective effects [87]. Stroke prophylaxis is important, and low enteric-coated ASA is the first therapy for AFD patients. Specialist care may be necessary to manage the development of advanced cardiovascular, renal or neurological manifestations [87, 88]. Care should be taken to manage AFD with the understanding that its systemic nature is potentially contraindicative for some conventional therapies used for more isolated organ diseases.
Enzyme replacement therapy
Prior to 2001, there was no treatment for patients with AFD. However, in 2001, enzyme replacement therapy (ERT) was developed as a treatment for this rare condition [79]. Successful development of two ERT alternatives, agalsidase α (Replagal) and agalsidase β (Fabrazyme), has resulted in a considerable progression in the treatment of patients with AFD and can modify renal and cardiac function. Clinical trials of enzyme replacement therapy (ERT) for AFD have proven the safety and efficacy of recombinant human forms of α-Gal A in the short term [1, 2, 79, 80]. Indeed, ERT has now been demonstrated to slow progression of a composite endpoint of renal, cardiac, central nervous system events and death in the AGAL-008-00 trial [80]. Early ERT contributes to resolving existing intracellular Gb3 deposits in the coronary capillary endothelial cells without affecting the intracellular deposits within cardiomyocytes [2, 79, 89]. The early recognition of heart disease is critical since ERT has proved to be effective in mediating cardiac clearance of Gb3 thereby improving cardiac function [2, 9, 45, 71]. Enzyme replacement therapy, if initiated early, may reverse some end-organ damage and can at least halt progression of the disease including cardiomyopathy [2, 45, 79, 90]. Based on registry data, in patients with baseline cardiac hypertrophy, ERT resulted in a sustained reduction in LV mass index after 5 years (from 71.4 to 64.1 g/m2) and a significant increase in midwall fractional shortening from 14.3 to 16 % after 3 years [91]. Importantly, in patients without baseline hypertrophy, LV mass index and midwall fractional shortening remained stable [91]. Furthermore, ERT improves pain scores and quality of life assessments in AFD patients [92].
The following criteria based on multi-modality assessment may be used to initiate ERT with fulfilling two or more criteria being an indication for ERT (Level of evidence II; Grade B) [88]:
-
1.
LV wall thickness greater than 12 mm in males and 11 mm in females.
-
2.
LVH by ECG or increased LV mass index (echo or cardiac MRI).
-
3.
Diastolic filling abnormalities (E/A ratio >2 and the deceleration time (DT) should be ≤140 ms)
-
4.
Rate of LV mass increase of 5 g/m2/year as determined by at least 3 measurements in 12 months.
-
5.
Abnormal longitudinal or radial strain rate of LV wall.
-
6.
Increased left atrial size on 2D echocardiography
-
7.
Conduction abnormalities and/or arrhythmias: Type II/III AV block, atrial arrhythmias or LBBB.
-
8.
Moderate-to-severe mitral or aortic valvular regurgitation.
-
9.
Late enhancement of left ventricular wall, especially in females, using cardiac MRI.
The current algorithm for deciding ERT use does not entirely avoid inappropriate use or missed opportunities for treatment, so improvement might make use of new research findings. The aforementioned reports from our group [52] and the UK group [77] that support the use of non-contrast T1 mapping in AFD, potentially for detection of subclinical cardiomyopathy, could be useful for ERT decision-making. Presently, however, there is no published evidence for the use of non-contrast T1 mapping in the latter capacity. Indeed, published guidelines, including the Canadian guidelines mentioned above, only support the use of more standard cardiac metrics for determining ERT candidacy. A randomized-control trial is therefore needed to evaluate whether non-contrast T1 mapping could improve ERT use to reduce inappropriate prescriptions or initiate ERT as early as possible in individuals who can benefit.
It is also worth noting that ERT may not be effective at reducing adverse renal outcomes [93]. Furthermore, ERT may lose its effectiveness after the appearance of end-organ damage, such as the occurrence of cardiac fibrosis [94, 95]. The potential inadequacy of ERT in some AFD patients, whereby disease progression is not appreciably arrested coupled with the high annual cost of ERT (approximately $200,000), creates a need for novel alternatives to ERT or possible adjuvant therapies for the existing ERT regimen [94, 96]. Moreover, inadequate distribution of administered enzyme to various organs and tissues due to inequities in flow dynamics and receptor distribution in various tissues may limit ERT effectiveness [97]. Beyond technical considerations that may limit the applicability of ERT in AFD, there is conflicted evidence with respect to the long-term clinical benefit of ERT [42, 98]. Indeed, little is known about how ERT affects patient mortality or adverse outcomes in a multisystem perspective [98], although there is demonstrated benefit for adverse cardiovascular outcomes [99]. Furthermore, the positive effect of ERT on LVH [91], imaging assessment of myocardial fibrosis and strain [45, 71], and myocardial deposition of Gb3 [2] only represents improvement in surrogate rather than hard measures of adverse cardiovascular outcomes. Registry data, surveillance programs, industry-led follow-up studies or new randomized-control trials with dedicated longer follow-up periods may provide information with regard to hard cardiovascular outcomes [98]. Given the aforementioned high cost of ERT for AFD, clarification of the long-term risks and benefits will be paramount.
New directions: chaperone therapy and gene therapy
Competitive α-Gal A inhibitors, or molecular chaperones, represent a paradoxical target for new therapies, whereby inhibitor molecules such as galactose and 1-deoxygalactonojirimycin interact with and allow proper folding of otherwise unstable α-Gal A variants [100, 101]. These chaperones could be used in tandem with ERT to induce a confluence of recombinant human and chaperone-assisted-native α-Gal A to achieve a suitable level of enzymatic activity to maintain healthy function [46, 102, 103]. Only some mutant forms of α-Gal A will be responsive to chaperones; computational modeling and pharmacogenomic screens will expedite the process of finding candidate mutant varieties [104, 105]. Gene therapy is currently in development to replace the mutated α-Gal A gene, but it is difficult to develop a single viral vector to efficiently deliver the gene to all target organs [106]. The successful delivery of a plasmid encoding α-Gal A into Hep G2 cells with a solid-lipid nanoparticle based non-viral vector represents an exciting, and possibly more widely applicable therapeutic direction [107].
Conclusions
Anderson-Fabry disease is an important metabolic disorder that can cause severe, variable disease manifestations in affected individuals. Large-scale metabolic and genetic screening studies have revealed AFD to be more prevalent in populations of diverse ethnic origins. Anderson-Fabry cardiomyopathy, which is characterized by structural, valvular, vascular and conduction abnormalities, is now the most common cause of mortality in patients with AFD. Since AFD is an X-linked condition, women have a milder but significant burden of AFD cardiomyopathy. Genetic testing is widely available and plays a critical role in the evaluation of patients with AFD. With the advent of effective enzyme replacement therapy and the continued advancement in diagnostic and evaluative methods, we should increasingly be able to provide a diagnosis early in the disease course before organ damage becomes irreversible. Novel echocardiographic and cardiac MRI techniques can aid the continued pursuit of new effective therapies, such that options are available for all patients suffering this debilitating condition.
References
Clarke JT (2007) Narrative review: Fabry disease. Ann Intern Med 146:425–433
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S et al (2001) Safety and efficacy of recombinant human alpha-galactosidase A–replacement therapy in Fabry’s disease. N Engl J Med 345:9–16
Rao DA, Lakdawala NK, Miller AL, Loscalzo J (2013) Clinical problem-solving. In the thick of it. N Engl J Med 368:1732–1738
Arbustini E, Narula N, Dec GW, Reddy KS, Greenberg B, Kushwaha S et al (2013) The MOGE(S) classification for a phenotype-genotype nomenclature of cardiomyopathy: endorsed by the world heart federation. J Am Coll Cardiol 62:2046–2072
Hoffmann B, Beck M, Sunder-Plassmann G, Borsini W, Ricci R, Mehta A (2007) Nature and prevalence of pain in Fabry disease and its response to enzyme replacement therapy—a retrospective analysis from the Fabry outcome survey. Clin J Pain 23:535–542
Keshav S (2006) Gastrointestinal manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G (eds) Fabry disease: perspectives from 5 years of FOS, chap 28. Oxford PharmaGenesis, Oxford
Orteu CH, Jansen T, Lidove O, Jaussaud R, Hughes DA, Pintos-Morell G et al (2007) Fabry disease and the skin: data from FOS, the Fabry outcome survey. Br J Dermatol 157:331–337
Samiy N (2008) Ocular features of Fabry disease: diagnosis of a treatable life-threatening disorder. Surv Ophthalmol 53:416–423
Mehta A, Clarke JT, Giugliani R, Elliott P, Linhart A, Beck M et al (2009) Natural course of Fabry disease: changing pattern of causes of death in FOS—Fabry outcome survey. J Med Genet 46:548–552
Oqvist B, Brenner BM, Oliveira JP, Ortiz A, Schaefer R, Svarstad E et al (2009) Nephropathy in Fabry disease: the importance of early diagnosis and testing in high-risk populations. Nephrol Dial Transpl 24:1736–1743
Ashton-Prolla P, Tong B, Shabbeer J, Astrin KH, Eng CM, Desnick RJ (2000) Fabry disease: twenty-two novel mutations in the alpha-galactosidase A gene and genotype/phenotype correlations in severely and mildly affected hemizygotes and heterozygotes. J Investig Med 48:227–235
Germain DP (2001) A new phenotype of Fabry disease with intermediate severity between the classical form and the cardiac variant. Contrib Nephrol (136): 234–240
Grunfeld JP, Lidove O, Joly D, Barbey F (2001) Renal disease in Fabry patients. J Inherit Metab Dis 24(Suppl 2):71–74
Branton M, Schiffmann R, Kopp JB (2002) Natural history and treatment of renal involvement in Fabry disease. J Am Soc Nephrol 13(Suppl 2):S139–S143
Linhart A, Palecek T, Bultas J, Ferguson JJ, Hrudova J, Karetova D et al (2000) New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J 139:1101–1108
Linhart A, Lubanda JC, Palecek T, Bultas J, Karetova D, Ledvinova J et al (2001) Cardiac manifestations in Fabry disease. J Inherit Metab Dis 24(Suppl 2):75–83
Tuttolomondo A, Pecoraro R, Simonetta I, Miceli S, Arnao V, Licata G et al (2013) Neurological complications of Anderson-Fabry disease. Curr Pharm Des 19:6014–6030
Elleder M, Dorazilova V, Bradova V, Belohlavek M, Kral V, Choura M et al (1990) Fabry’s disease with isolated disease of the cardiac muscle, manifesting as hypertrophic cardiomyopathy. Cas Lek Cesk 129:369–372
von Scheidt W, Eng CM, Fitzmaurice TF, Erdmann E, Hubner G, Olsen EG et al (1991) An atypical variant of Fabry’s disease with manifestations confined to the myocardium. N Engl J Med 324:395–399
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M et al (1995) An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med 333:288–293
Linhart A, Kampmann C, Zamorano JL, Sunder-Plassmann G, Beck M, Mehta A et al (2007) Cardiac manifestations of Anderson-Fabry disease: results from the international Fabry outcome survey. Eur Heart J 28:1228–1235
Mignani R, Feriozzi S, Schaefer RM, Breunig F, Oliveira JP, Ruggenenti P et al (2010) Dialysis and transplantation in Fabry disease: indications for enzyme replacement therapy. Clin J Am Soc Nephrol 5:379–385
Scriver CR (1995) The metabolic and molecular bases of inherited disease, vol II. McGraw-Hill, New York, p 2742
Inoue T, Hattori K, Ihara K, Ishii A, Nakamura K, Hirose S (2013) Newborn screening for Fabry disease in Japan: prevalence and genotypes of Fabry disease in a pilot study. J Hum Genet 58:548–552
Mechtler TP, Stary S, Metz TF, De Jesus VR, Greber-Platzer S, Pollak A et al (2012) Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet 379:335–341
Lin HY, Chong KW, Hsu JH, Yu HC, Shih CC, Huang CH et al (2009) High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet 2:450–456
Spada M, Pagliardini S, Yasuda M, Tukel T, Thiagarajan G, Sakuraba H et al (2006) High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet 79:31–40
Feldt-Rasmussen U, Dobrovolny R, Nazarenko I, Ballegaard M, Hasholt L, Rasmussen AK et al (2011) Diagnostic dilemma: a young woman with Fabry disease symptoms, no family history, and a “sequencing cryptic” alpha-galactosidase a large deletion. Mol Genet Metab 104:314–318
Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ et al (2002) Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 105:1407–1411
Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA et al (2004) Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 110:1047–1053
Elliott P, Baker R, Pasquale F, Quarta G, Ebrahim H, Mehta AB et al (2011) Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry disease survey. Heart 97:1957–1960
Havndrup O, Christiansen M, Stoevring B, Jensen M, Hoffman-Bang J, Andersen PS et al (2010) Fabry disease mimicking hypertrophic cardiomyopathy: genetic screening needed for establishing the diagnosis in women. Eur J Heart Fail 12:535–540
van der Tol L, Smid BE, Poorthuis BJ, Biegstraaten M, Deprez RH, Linthorst GE et al (2014) A systematic review on screening for Fabry disease: prevalence of individuals with genetic variants of unknown significance. J Med Genet 51:1–9
Bach G, Rosenmann E, Karni A, Cohen T (1982) Pseudodeficiency of alpha-galactosidase A. Clin Genet 21:59–64
Yasuda M, Shabbeer J, Benson SD, Maire I, Burnett RM, Desnick RJ (2003) Fabry disease: characterization of alpha-galactosidase A double mutations and the D313Y plasma enzyme pseudodeficiency allele. Hum Mutat 22:486–492
Niemann M, Rolfs A, Stork S, Bijnens B, Breunig F, Beer M et al (2014) Gene mutations versus clinically relevant phenotypes-Lyso-Gb3 defines fabry disease. Circ Cardiovasc Genet 7:8–16
MacDermot KD, Holmes A, Miners AH (2001) Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 38:769–775
Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U et al (2008) Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab 93:112–128
Laney DA, Bennett RL, Clarke V, Fox A, Hopkin RJ, Johnson J et al (2013) Fabry disease practice guidelines: recommendations of the national society of genetic counselors. J Genet Couns 22:555–564
Wang RY, Lelis A, Mirocha J, Wilcox WR (2007) Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med 9:34–45
Juan P, Hernan A, Beatriz SA, Gustavo C, Antonio M, Eduardo T et al (2014) Fabry Disease: multidisciplinary evaluation after 10 years of treatment with agalsidase beta. JIMD Rep. doi:10.1007/8904_2014_310
Anderson LJ, Wyatt KM, Henley W, Nikolaou V, Waldek S, Hughes DA et al (2014) Long-term effectiveness of enzyme replacement therapy in Fabry disease: results from the NCS-LSD cohort study. J Inherit Metab Dis. doi:10.1007/s10545-014-9717-4
Desnick RJ (2007) Prenatal diagnosis of Fabry disease. Prenat Diagn 27:693–694
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL et al (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342:1077–1084
Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S et al (2009) Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation 119:524–529
Shin SH, Kluepfel-Stahl S, Cooney AM, Kaneski CR, Quirk JM, Schiffmann R et al (2008) Prediction of response of mutated alpha-galactosidase A to a pharmacological chaperone. Pharmacogenet Genomics 18:773–780
Linhart A (2006) The heart in Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G (eds) Fabry disease: perspectives from 5 years of FOS, chap 20. Oxford PharmaGenesis, Oxford
Wu JC, Ho CY, Skali H, Abichandani R, Wilcox WR, Banikazemi M et al (2010) Cardiovascular manifestations of Fabry disease: relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase A activity. Eur Heart J 31:1088–1097
Sadick N, Thomas L (2007) Cardiovascular manifestations in Fabry disease: a clinical and echocardiographic study. Heart Lung Circ 16:200–206
Shanks M, Thompson RB, Paterson ID, Putko B, Khan A, Chan A et al (2013) Systolic and diastolic function assessment in fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J Am Soc Echocardiogr 26:1407–1414
Niemann M, Herrmann S, Hu K, Breunig F, Strotmann J, Beer M et al (2011) Differences in Fabry cardiomyopathy between female and male patients: consequences for diagnostic assessment. JACC Cardiovasc Imaging 4:592–601
Thompson RB, Chow K, Khan A, Chan A, Shanks M, Paterson I et al (2013) T1 mapping with cardiovascular MRI is highly sensitive for fabry disease independent of hypertrophy and sex. Circ Cardiovasc Imaging 6:637–645
Kampmann C, Baehner F, Whybra C, Martin C, Wiethoff CM, Ries M et al (2002) Cardiac manifestations of Anderson-Fabry disease in heterozygous females. J Am Coll Cardiol 40:1668–1674
Gambarin FI, Disabella E, Narula J, Diegoli M, Grasso M, Serio A et al (2010) When should cardiologists suspect Anderson-Fabry disease? Am J Cardiol 106:1492–1499
Calcagnino M, O’Mahony C, Coats C, Cardona M, Garcia A, Janagarajan K et al (2011) Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J Am Coll Cardiol 58:88–89
Flegal KM, Carroll MD, Kit BK, Ogden CL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307:483–490
Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A et al (2013) Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 310:959–968
Kalliokoski RJ, Kalliokoski KK, Sundell J, Engblom E, Penttinen M, Kantola I et al (2005) Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J Inherit Metab Dis 28:563–573
Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R et al (2006) Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart 92:357–360
Barbey F, Brakch N, Linhart A, Rosenblatt-Velin N, Jeanrenaud X, Qanadli S et al (2006) Cardiac and vascular hypertrophy in Fabry disease: evidence for a new mechanism independent of blood pressure and glycosphingolipid deposition. Arterioscler Thromb Vasc Biol 26:839–844
Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R et al (2008) Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc Natl Acad Sci USA 105:2812–2817
Pochis WT, Litzow JT, King BG, Kenny D (1994) Electrophysiologic findings in Fabry’s disease with a short PR interval. Am J Cardiol 74:203–204
Shah JS, Hughes DA, Sachdev B, Tome M, Ward D, Lee P et al (2005) Prevalence and clinical significance of cardiac arrhythmia in Anderson-Fabry disease. Am J Cardiol 96:842–846
Eckart RE, Kinney KG, Belnap CM, Le TD (2000) Ventricular fibrillation refractory to automatic internal cardiac defibrillator in Fabry’s disease. Review of cardiovascular manifestations. Cardiology 94:208–212
Acharya D, Robertson P, Kay GN, Jackson L, Warnock DG, Plumb VJ et al (2012) Arrhythmias in Fabry cardiomyopathy. Clin Cardiol 35:738–740
Kampmann C, Wiethoff CM, Whybra C, Baehner FA, Mengel E, Beck M (2008) Cardiac manifestations of Anderson-Fabry disease in children and adolescents. Acta Paediatr 97:463–469
Pieroni M, Chimenti C, Ricci R, Sale P, Russo MA, Frustaci A (2003) Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 107:1978–1984
Toro R, Perez-Isla L, Doxastaquis G, Barba MA, Gallego AR, Pintos G et al (2009) Clinical usefulness of tissue Doppler imaging in predicting preclinical Fabry cardiomyopathy. Int J Cardiol 132:38–44
Weidemann F, Breunig F, Beer M, Sandstede J, Stork S, Voelker W et al (2005) The variation of morphological and functional cardiac manifestation in Fabry disease: potential implications for the time course of the disease. Eur Heart J 26:1221–1227
Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W et al (2003) Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation 108:1299–1301
Soullier C, Obert P, Doucende G, Nottin S, Cade S, Perez-Martin A et al (2012) Exercise response in hypertrophic cardiomyopathy: blunted left ventricular deformational and twisting reserve with altered systolic-diastolic coupling. Circ Cardiovasc Imaging 5:324–332
Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ et al (2003) Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 24:2151–2155
Koeppe S, Neubauer H, Breunig F, Weidemann F, Wanner C, Sandstede J et al (2012) MR-based analysis of regional cardiac function in relation to cellular integrity in Fabry disease. Int J Cardiol 160:53–58
Messalli G, Imbriaco M, Avitabile G, Russo R, Iodice D, Spinelli L et al (2012) Role of cardiac MRI in evaluating patients with Anderson-Fabry disease: assessing cardiac effects of long-term enzyme replacement therapy. Radiol Med 117:19–28
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57:891–903
Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G et al (2013) Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging 6:392–398
Desnick RJ, Wasserstein MP, Banikazemi M (2001) Fabry disease (alpha-galactosidase A deficiency): renal involvement and enzyme replacement therapy. Contrib Nephrol (136):174–192
Eng CM, Banikazemi M, Gordon RE, Goldman M, Phelps R, Kim L et al (2001) A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 68:711–722
Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M et al (2007) Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med 146:77–86
Wanner C, Oliveira JP, Ortiz A, Mauer M, Germain DP, Linthorst GE et al (2010) Prognostic indicators of renal disease progression in adults with Fabry disease: natural history data from the Fabry Registry. Clin J Am Soc Nephrol 5:2220–2228
Kotanko P, Kramar R, Devrnja D, Paschke E, Voigtlander T, Auinger M et al (2004) Results of a nationwide screening for Anderson-Fabry disease among dialysis patients. J Am Soc Nephrol 15:1323–1329
University of Alabama at Birmingham. The Fabrazyme and ARBs and ACE Inhibitor Treatment (FAACET) Study. http://www.clinicaltrials.gov/ct2/show/NCT00446862. Accessed July 2 2014
Colombi A, Kostyal A, Bracher R, Gloor F, Mazzi R, Tholen H (1967) Angiokeratoma corporis diffusum–Fabry’s disease. Helv Med Acta 34:67–83
MacDermot KD, Holmes A, Miners AH (2001) Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet 38:750–760
Tyson CC, Nwankwo C, Lin PH, Svetkey LP (2012) The dietary approaches to stop hypertension (DASH) eating pattern in special populations. Curr Hypertens Rep 14:388–396
Eng CM, Germain DP, Banikazemi M, Warnock DG, Wanner C, Hopkin RJ et al (2006) Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med 8:539–548
West ML, Casey R, Clarke JT, Iwanochko M, Moore DF, Sirrs S (2012) Canadian Fabry disease treatment guidelines 2012. http://www.garrod.ca/wp-content/uploads/Canadian-FD-Treatment-Guidelines-2012.pdf. Accessed July 2 2014
Thurberg BL, Fallon JT, Mitchell R, Aretz T, Gordon RE, O’Callaghan MW (2009) Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation 119:2561–2567
Schiffmann R, Murray GJ, Treco D, Daniel P, Sellos-Moura M, Myers M et al (2000) Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci USA 97:365–370
Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G et al (2009) Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: an analysis of registry data. Lancet 374:1986–1996
Alfadhel M, Sirrs S (2011) Enzyme replacement therapy for Fabry disease: some answers but more questions. Ther Clin Risk Manag 7:69–82
Rombach SM, Smid BE, Linthorst GE, Dijkgraaf MG, Hollak CE (2014) Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: a systematic review and meta-analysis: effectiveness of ERT in different disease stages. J Inherit Metab Dis 37:341–352
Pieroni M, Camporeale A, Della Bona R, Sabini A, Cosmi D, Magnolfi A et al (2013) Progression of Fabry cardiomyopathy despite enzyme replacement therapy. Circulation 128:1687–1688
Weidemann F, Niemann M, Stork S, Breunig F, Beer M, Sommer C et al (2013) Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 274:331–341
Hersher R (2012) Small biotechs raring to cash in on the orphan disease market. Nat Med 18:330–331
Murray GJ, Anver MR, Kennedy MA, Quirk JM, Schiffmann R (2007) Cellular and tissue distribution of intravenously administered agalsidase alfa. Mol Genet Metab 90:307–312
El Dib RP, Nascimento P, Pastores GM (2013) Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev 2:CD006663
Sirrs SM, Bichet DG, Casey R, Clarke JT, Lemoine K, Doucette S et al (2014) Outcomes of patients treated through the Canadian Fabry disease initiative. Mol Genet Metab 111:499–506
Guce AI, Clark NE, Rogich JJ, Garman SC (2011) The molecular basis of pharmacological chaperoning in human alpha-galactosidase. Chem Biol 18:1521–1526
Suzuki Y (2013) Chaperone therapy update: fabry disease, GM1-gangliosidosis and Gaucher disease. Brain Dev 35:515–523
Benjamin ER, Khanna R, Schilling A, Flanagan JJ, Pellegrino LJ, Brignol N et al (2012) Co-administration with the pharmacological chaperone AT1001 increases recombinant human alpha-galactosidase A tissue uptake and improves substrate reduction in Fabry mice. Mol Ther 20:717–726
Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M et al (2001) Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med 345:25–32
Wu X, Katz E, Della Valle MC, Mascioli K, Flanagan JJ, Castelli JP et al (2011) A pharmacogenetic approach to identify mutant forms of alpha-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum Mutat 32:965–977
Siekierska A, De Baets G, Reumers J, Gallardo R, Rudyak S, Broersen K et al (2012) Alpha-Galactosidase aggregation is a determinant of pharmacological chaperone efficacy on Fabry disease mutants. J Biol Chem 287:28386–28397
Lee CJ, Fan X, Guo X, Medin JA (2011) Promoter-specific lentivectors for long-term, cardiac-directed therapy of Fabry disease. J Cardiol 57:115–122
Ruiz de Garibay AP, Delgado D, Del Pozo-Rodriguez A, Solinis MA, Gascon AR (2012) Multicomponent nanoparticles as nonviral vectors for the treatment of Fabry disease by gene therapy. Drug Des Devel Ther 6:303–310
Acknowledgments
Brendan Putko is partially supported by a studentship award from the Alberta HEART STEADI-HF training program, and Gavin Y. Oudit is supported by operating grants from the Canadian Institute of Health Research and the Heart and Stroke Foundation of Canada.
Conflict of interest
Gavin Y. Oudit has received research funding from Pfizer and Glaxo-Smith Kline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Putko, B.N., Wen, K., Thompson, R.B. et al. Anderson-Fabry cardiomyopathy: prevalence, pathophysiology, diagnosis and treatment. Heart Fail Rev 20, 179–191 (2015). https://doi.org/10.1007/s10741-014-9452-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-014-9452-9