Abstract
Objective
The increasing prevalence of type 2 diabetes mellitus (T2DM) calls for evolving a concomitant screening strategies for early disease detection and prediction of the complications. Progranulin (PRGN) was recently introduced as a biomarker of inflammation in T2DM. However, little data have been published as regarding progranulin in relation to diabetic micro angiopathy among Egyptian patients with T2DM. The aim of this study was therefore to investigate and evaluate serum progranulin as a biomarker for the presence and severity of micro vascular complications among Egyptian patients with T2DM.
Methods
A total of 90 age and sex matched participants were included in this cross sectional study. They were divided into group 1 included 30 non diabetic healthy controls and group 2 included 60 patients with type 2 diabetes mellitus. Furthermore, diabetic patients were categorized into two subgroups depending on the presence or absence of microvascular complications. Evaluation for diabetic nephropathy, neuropathy and retinopathy were determined. Furthermore, laboratory investigations were performed and serum progranulin levels were measured by a quantitative sandwich enzyme linked immune sorbent assay.
Results
The mean serum PRGN levels were significantly elevated in type 2 diabetic patients (20.90 ± 6.38 ng/ml) compared to control group (9.20 ± 1.41 ng/ml) (p < 0.001). Moreover,the serum PRGN levels were increased parallel to the severity of diabetic nephropathy (DN) and diabetic retinopathy (DR) with significantly highest detectable values were in macro albuminuric group of diabetic nephropathy as well as proliferative diabetic retinopathy (PDR) groups (P < 0.001). Besides, it worth mentioning that, the level of Serum progranulin started to increase significantly in stage 2 DN in spite of normal level of albuminuria. There were highly significant positive correlation between serum PRGN and disease duration, body mass index (BMI),fasting blood sugar (FBS), HbA1c, Total cholesterol (TC),triglyceride (TG), serum creatinine, ACR (r = 0.918, 0.623, 0.430, 0.539,0.910,0.842,0.759, 0.903, resp., P < 0.001) and a significant positive correlation with low density lipoprotein (LDL) (r = 0.344),but there was a highly significant negative correlation between serum PRGN and eGFR (r = −0.866, P < 0.001) in the studied diabetic patients.
Conclusion
Progranulin might be considered as a biomarker for diabetic micro angiopathy and its severity. In addition, there is a group of diabetic patients with decreased eGFR but without albuminuria in which serum PRGN level was indicated to be used as an early biomarker of diabetic nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a chronic progressive disease considering as a global epidemic. The condition is comorbid with presence of micro vascular complications [1].
Diabetic retinopathy (DR) is present with development of micro albuminuria in patients with type 1 diabetes mellitus (T1DM); while, it is present only with development of macro albuminuria in patients with T2DM. Previously, micro albuminuria has been approved to be a traditional marker for diagnosis of DN, monitoring its progression, and assessment of its associated conditions as cardiovascular complications. However, recent studies have shown that albuminuria is a less accurate predictor of overt nephropathy risk than thought previously as kidney damage can progress even when micro albuminuria has regressed. Furthermore, there is a lack of a strong association between albuminuria and glomerular filtration rate (GFR). As such, there is an increasing need to find and validate an earlier and reliable biomarker to diagnose DN as an alternative to albuminuria based staging method [2].
Recently activation of inflammatory processes may contribute to micro vascular damage with subsequent well recognized complications of diabetes. Furthermore, Lines of treatment of those complications in the future may include regulation of inflammatory processes by targeting factors that contribute to that vascular damage [3].
Progranulin is a 68–88 kDa cysteine-rich secreted glycoprotein with varied pleiotropic actions. The secreted full length form of progranulin has anti-inflammatory action, while the proteolytically cleaved subunits by elastase called granulin peptides (GRNs) have potent pro-inflammatory effects. It is encoded by PRGN gene and expressed by many cell types, including epithelial cells, macrophages, neurons and adipocytes [4]. Moreover, in rodents progranulin is expressed by Reno tubular epithelium but in humans the expression of PRGN in kidneys remains unknown [5].
It was originally identified as a growth factor involved in regulation of wound healing or in diseases such as cancer progression [4]. Moreover, in acute condition of ischemia–reperfusion injury, lower levels of PRGN is detected in mice kidney; and treatment with recombinant PGRN in this condition could diminish inflammation suggesting its anti-inflammatory effect. While in obesity (a chronic condition), it was recognized as an adipokine related to insulin resistance and inflammation indicating its pro-inflammatory effects and metabolic function [5].
However, until now, study for evaluating the serum PRGN as a biomarker for diabetic micro angiopathy and its severity among patients with T2DM seems limited and has not been investigated in details. Therefore, we determined progranulin levels among Egyptian patients with T2DM and assessed its relation to micro vascular complications.
Methods
This cross-sectional study was carried in Al Zahraa University Hospital and Sayed Galal University Hospital from January 2018 to July 2018. A total of 90 age and sex matched individuals were enrolled; there were healthy controls (n = 30) and patients with T2DM (n = 60). Patients were defined according to American Diabetes Association (ADA) criteria [6] or if they were on anti-diabetic drugs.
Those with history of malignancy, diabetic macro vascular complications, other endocrine disease which affect glucose metabolism, liver disease, other causes of renal disease disease, inflammatory disease, infection, urinary tract infection, pregnancy, history of drug abuse and those with T1DM were excluded from that study.
Furthermore, diabetic patients were further subdivided into 40 diabetic patients with micro vascular complications who had either one or more of the following (diabetic retinopathy, nephropathy or neuropathy), in addition to 20 diabetic patients without micro vascular complications.
Diabetic neuropathy was defined by the presence of at least two of the following: symptoms, reduced vibration perception, insensitivity to monofilament at one or more of nine sites on either feet, and absent ankle tendon reflexes.
Diabetic retinopathy (DR) was assessed by ophthalmologist and classified based on severity into three subgroups normal, non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) using fundus examination. NPDR was diagnosed based on one or more of the following features: micro aneurysms,intra retinal hemorrhages, hard and soft exudates, venous beading and intra retinal micro vascular abnormalities while, those without these abnormalities in the retina were categorized as normal (NDR). On the other hand, PDR is considered if there was neovascularization, pre-retinal hemorrhages, vitreous hemorrhage, or pan retinal laser photo coagulation scars. The severity of DR in the worse affected eye was used for retinopathy grading. Some confusing cases were diagnosed through fundus fluorescein angiography (FFA) [7].
Patients with diabetic nephropathy were subdivided according to Urinary Albumin/Creatinine ratio (ACR) into: 20 diabetic patients with normo albuminuria (ACR <30 mg/g), 20 diabetic patients with micro albuminuria (ACR 30–300 mg/g), and 20 diabetic patients with macro albuminuria (ACR >300 mg/g). As well as according to eGFR into diabetic patients with increase in eGFR ≥90 and diabetics with eGFR <90. Estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2) was calculated using the modification of diet in renal disease (MDRD) equation: 186 x (Creat)-1.154 x (AGE)-0.203 x (0.742 if female) x (1.210 if African American) according to the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines for evaluation and stratification of chronic kidney disease.
The study was conducted according to Al-Azhar university Ethics Committee’s procedure and an informed consent was obtained from all participants included in the study.
Laboratory investigations included the following: complete blood count (CBC), fasting blood sugar (FBS) and 2 h post prandial blood sugar (PPG), HbA1c, fasting lipid profile: (Total Cholesterol(TC), Triglyceride(TG), high-density lipoprotein-cholesterol (HDL-c) and low-density lipo protein cholesterol (LDL-c), serum urea and serum creatinine, liver function test, complete urine analysis.
Abdominal ultrasound to detect the structural abnormality of both kidneys (grades of diabetic nephropathy). ECG to exclude macro vascular complication (Ischemic heart disease).
All biochemical parameters (Fasting blood sugar, liver function tests, kidney function tests, lipid profiles, serum calcium) were carried out by using the automated analyzer.
The determination of serum PRGN was carried by enzyme linked immunosorbent assays (ELISA) according to the manufacturers’ instructions (Human Progranulin ELISA Kit, KONO Biotech Co.,Ltd.,China). Five milliliter of blood samples were collected from each participant in the study after an overnight fast and then centrifuged at 3000 g for 10 min and the separated serum was stored at −80 °C until they were analyzed. Sensitivity was 7.8 ng/mL for PGRN and the specificity of the immunosorbent assay was estimated to be 100%. Whereas the inter and intra assay coefficient of variation was less than 10% for serum samples and was done under internal quality controls.
On the other hand, MedCalc® version 12.3.0.0 program was used for calculations of sample size, statistical calculator based on 95% confidence interval and power of the study 80% with α error 5%, According to a previous study [3] showed that the mean of PGRN (μg/L) with normal control 45.21 ± 4.75, simple diabetes mellitus 47.18 ± 4.51, early diabetic nephropathy (microalbuminuria) 53.98 ± 5.44 and clinical diabetic nephropathy,(macroalbuminuria) 86.84 ± 30.31, with p value <0.001 HS, so it can be relied upon in this study, based on this assumption, sample size was calculated according to these values produced a minimal samples size of 90 cases were enough to find such a difference., subdivided into group I: Control (N = 30), group II: diabetic patients without microvascular complications (N = 20), group III: diabetic patients with microvascular complications (N = 40).
Statistical methods
The recorded data were collected and analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). The data were presented as mean, standard deviations and ranges for the quantitative data with parametric distribution which was tested by Kolmogorov-Smirnov test of normality. Independent-samples t-tests were used to compare between 2 groups. The comparison between more than two groups with quantitative data and parametric distribution were done by using one way analysis of variance (ANOVA) followed by post Hoc test. Chi-square (x2) test of significance was used in order to compare proportions between two qualitative parameters. Inter relationships between variables were analyzed by Pearson correlation analysis. Binary logistic regression was used to predict the outcome of categorical variable based on one or more predictor variables. Multiple linear regression analyses were done, using serum PRGN as dependent variables. The independent variables were selected if they were correlated with PRGN using Pearson’s correlation coefficient. P value <0.05 was considered significant and P value >0.05 was considered insignificant while P values<0.001 was considered as highly significant.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Results
There was no significant difference between both groups as regards age, sex and BMI. Demographic and laboratory data of all studied groups are listed in (Table 1). Serum PRGN was significantly higher in diabetic group (20.90 ± 6.38 ng/ml) compared to control group (9.20 ± 1.41 ng/ml) (p < 0.001), (Table 1).
Furthermore, serum PRGN levels were significantly higher in type 2 diabetic patients with micro vascular complications (24.54 ± 4.36 ng/ml) and in those without complications (13.63 ± 1.91 ng/ml) than in healthy controls (9.20 ± 1.41 ng/ml) (p < 0.001), with the highest levels in diabetic patients with microvascular complications. (P < 0.001), (Table 2).
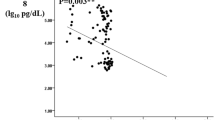
Regarding diabetic nephropathy (according to albuminuria), serum PRGN was significantly higher in subgroup 3 (diabetics with macroalbuminuria) (28.23 ± 2.18 ng/ml) compared with the other two subgroups (diabetics with microalbuminuria) and (diabetics without albuminuria) (P < 0.001), and in subgroup 2 (diabetics with microalbuminuria) (20.86 ± 2.40 ng/ml) compared with subgroup 1 (diabetics without albuminuria) (15.63 ± 1.91 ng/ml) (P < 0.001) (Table 3) (Fig. 1).
Regarding diabetic nephropathy (according to eGFR), serum PRGN was significantly higher in diabetic subgroup with eGFR <90 (22.23 ± 6.93 ng/ml) compared to diabetic subgroup with eGFR ≥90 (16.38 ± 3.75 ng/ml) (P < 0.001) (Table 4).
Regarding diabetic retinopathy, Serum PRGN was significantly elevated in diabetic patients with DR (25.68 ± 6.29 ng/ml) compared to those without DR (15.53 ± 4.29 ng/ml) (P < 0.001). When considering severity of DR, the serum PRGN level was significantly higher in the PDR group (29.20 ± 2.42 ng/ml), compared with NPDR groups (22.68 ± 4.29 ng/ml)(P < 0.001) (Table 5) (Fig. 2).
Also, Serum PRGN was significantly elevated in diabetic patients with Diabetic Neuropathy compared to those without (P < 0.001).
In diabetic patients, a highly significant positive correlation was detected between serum PRGN and disease duration, BMI, FBS, HbA1C,TC,TG,serum creatinine, ACR (r = 0.918, 0.623,0.430,0.539,0.910,0.842,0.759, 0.903, resp., p < 0.001), and a significant positive correlation with low density lipoprotein (LDL) (r = 0.344),(Table 4),further, there was a highly significant negative correlation between serum PRGN and eGFR (r = −0.866, P < 0.001) in the studied diabetic patients (Table 6).
Multiple linear regression analysis showed that age, HbA1c, TC, eGFR, ACR, urea, creatinine were independently related to serum PRGN levels (Table 7).
The logistic multivariate regression analysis was done to estimate the odd ratios and 95% CI for the relationships of serum PRGN levels with the development of DN, DR and T2DM. For each analysis, two groups were selected, and the group with more severe DN or sever DR coded as 1, was tested against the next less severe DN or sever DR group, which was coded as 0. Moreover, type 2 diabetic patients group coded as 1, and was tested against the control group. The group coded as 0 was used as reference for the analysis. We considered some potential confounders including Age (years),sex, BMI, fasting blood sugar; postprandial blood sugar; HbA1C, Duration of T2DM, total cholesterol; triglyceride,LDL and serum creatinine. We found that serum PRGN levels were a significant independent factor for DN (according to albuminuria or eGFR) and retinopathy among type 2 diabetic patients. Moreover, these associations were not affected by adjustment of the confounders (Table 8).
Comparing male and female in patients group in terms of PRGN levels, there was a non-significant difference in the mean ± SD of PRGN (20.96 ± 6.69) ng/ml in male compared with female of the same group (20.88 ± 6.31) ng/ml (P = 0.962) .
Discussion
Diabetes mellitus has now become a main worldwide health problem with projections of an increase in numbers from presently almost 200 million people to around 370 million by 2025 [8].
Micro vascular complications of T2DM are associated with severe morbidity, mortality and a huge economic burden. So, there is an important need to identify new biomarker able to identify disease onset and progression and can be used as a therapeutic target for management of these complications.
Progranulin is an evolving molecule which has both pro and anti-inflammatory properties. It plays varied functions in different tissues, cells and metabolic conditions. Firstly, it was identified as a growth factor and was considered as a potential biomarker in cancer. Also, it was considered as an adipokine associated with obesity, glucose intolerance, insulin resistance [4].
Furthermore,serum PGRN level is usually low, being up regulated in the inflammatory state suggesting its involvement in chronic subclinical inflammation associated with the pathogenesis of diabetic micro angiopathy, and also, it correlated with changes in disease metrics over time which point to its potential use as a biomarker for disease occurrence and progression in several pathologies [9].
In the present study, we found statistically significant increase in the mean serum progranulin levels in diabetic patients compared to healthy controls. Moreover, serum PRGN levels were significantly elevated in type 2 diabetic patients with micro vascular complications and in those without complications than in control subjects, with the highest levels among diabetic patients with microvascular complications. This is in agreement with Ezz and Abd El A zeem [4] study which reported that serum PRGN concentrations were elevated in type 2 diabetic patients compared to those of healthy control group and that increase was augmented in patients with nephropathy. Similar to other study [9], serum progranulin levels showed no statistically significant difference between different genders among diabetic group which indicate that, there was no impact of sex on serum PRGN levels.
In this study, we tried to find out the possibility of using serum progranulin as a biomarker for diagnosis of diabetic microvascular complications (DN and DR) as early as possible. Therefore, we tested PGRN levels in type 2 diabetic patients with different stages of DN (according to eGFR and ACR system) as well as diabetic patients with DR.
Regarding staging of diabetic retinopathy, Serum PRGN was significantly elevated in diabetic patients with DR compared with those without. Moreover, serum progranulin level was increased parallel to the severity of DN with the highest levels among diabetic patients with PDR.
Regarding staging of diabetic nephropathy (according to ACR classification), serum PRGN was significantly higher in diabetic patients with macro albuminuria compared with the other two subgroups (diabetics with micro albuminuria) and (diabetics without albuminuria), and in diabetic patients with micro albuminuria compared with diabetic patients without albuminuria. Moreover, the mean serum PRGN levels increased parallel to the severity of DN with the highest levels among type 2 diabetic patients with advanced nephropathy. Xu et al. [3] study which was conducted on eighty-four type 2 diabetic patients in the different stages of DN (according to ACR) clarified that by comparing with the control group, serum PRGN levels was markedly elevated in sera of type 2 diabetic patients with micro angiopathy including (clinical diabetic nephropathy) (CDN) and PDR but did not differ distinctly between (simple diabetic nephropathy) (SDM) and micro albuminuric patients or background retinopathy group. This discrepancy may be due to that in our study the elevation of PRGN levels in normoalbuminuric group whose their mean eGFR were (95.59 ± 28.90 ml/min/1.73 m2) by the presence of 10 diabetic patients in this group had stage 2 diabetic nephropathy with mild decrease in their eGFR (79.89 ± 7.58 ml/min/1.73 m2). Thus we can conclude that serum PRGN levels increased significantly early with renal affection even before the appearance of albuminuria (stage 2 DN) which may make it more sensitive marker for DN than ACR system. Regarding diabetic nephropathy (according to GFR), serum PRGN was significantly higher in diabetic subgroup with eGFR <90 compared to diabetic subgroup with eGFR ≥90. This is in agreement with the study of Richter et al., [10] who reported that PRGN serum levels increased along with the stages of chronic kidney disease.
In the present study, we found that there was a highly significant positive correlation between serum PGRN and disease duration, BMI, FBG, HbA1c and lipid profile. This is in consistency with Ezz and Abd El A zeem [4] study that found similar results and suggested that association may be explained by its role in adiposity and insulin resistance. Additionally, some authors were found that administration of progranulin caused glucose intolerance and insulin insensitivity through triggering autophagy via TNFR1 in the mice adipose tissue, and ablation of PGRN prevented diet-induced insulin resistance. Exactly, PGRN might mediate adipose insulin resistance, by inducing autophagy via activated oxidative stress and endoplasmic reticulum stress [11]. On the other hand, the correlation between PGRN and T2DM, was reported in Korean populations by Youn et al., [12] and was also, established in Chinese populations [13], indicating that the relationship is not population specific.
In this study, correlations between serum progranulin and other renal parameters (sCr, ACR, eGFR) were done in attempt to explain the presence of higher levels of serum PRGN with the more severe stages of DN.
We found that there was a highly significant positive correlation between serum PRGN and sCr in the diabetic groups. This indicates that serum PRGN levels increase proportionally with the elevation in sCr. This is in consistency with Xu et al. [3] study that showed that there was a significant positive correlation between serum PRGN and sCr. Furthermore, there was a highly significant positive correlation between serum PRGN and the severity of albuminuria (ACR) in the diabetic groups. On the other hand, there was a highly significant negative correlation between serum PRGN and eGFR in the diabetic groups and this correlation became more significantly higher while the decrease in eGFR became more advanced. This is compatible with Xu et al. [3] study that showed a significant positive correlation between serum PRGN and eGFR. So, we concluded that the serum PRGN significantly increased with renal affection and decline of eGFR.
Furthermore, serum PRGN was found to be a significant risk factor for DN (according to albuminuria or e GFR) and DR through multivariate logistic regression analysis even after adjustment for Age (years),sex, BMI,fasting blood sugar; postprandial blood sugar;HbA1C, Duration of T2DM, total cholesterol; triglyceride,LDL and serum creatinine (confounders). This data suggests that PGRN is associated with the presence of diabetic nephropathy, retinopathy and neuropathy, and may be involved in its pathogenesis which was in agreement with the study of Richter et al., [10] who reported that chronic kidney disease (CKD) stage (eGFR) was the strongest independent predictor of PRGN level in five hundred thirty two type 2 diabetic patients with CKD stages 1–5 as well as observed that serum PGRN levels remained significantly different between the five subgroups of CKD, being higher in stage 5 even after adjustment for age, sex and BMI. In addition to, a more recent study revealed that both ACR and serum Cr were associated with serum PGRN level after adjustment for clinical covariates and the inflammatory markers interleukin-6 and TNFα in patients with type 2 diabetes [14].
The biological mechanisms by which PRGN play a role in mediating increased albumin excretion and retinal injury is not fully known. Accumulating evidences have indicated that PRGN is thought to contribute to the pathogenesis of diabetic microangipathy through inflammatory mechanisms as it considered as an adipocytokine associated with obesity promoting IL-6 expression in adipocytes, glucose intolerance and insulin resistance impairing insulin signaling. Additionally, it is a chemo attractant protein that recruits monocytes into adipose tissue, promoting chronic inflammatory response with subsequent increase in the pro inflammatory cytokines levels [12]. Xu et al. [3] showed that circulating progranulin levels and other inflammatory markers (TNF-훼 and IL-6) was markedly increased in type 2 diabetic patients with microangiopathy with significant positive correlation between serum PGRN levels and those inflammatory markers. Decrease in circulating PGRN levels can be obtained with long term diet intervention and exercise training program [15].
However, the exact mechanisms underlying the increase of progranulin in patients with diabetic micro angiopathy needs further studies. Since both the cellular source of serum progranulin and its mechanisms of secretion are multiple, it is unclear whether the elevation of serum progranulin levels in patients with diabetic micro angiopathy reveals a higher production or a reduced clearance.
Reduced renal elimination may be one of the explanations for that elevation in the serum progranulin level especially with progression of micro vacular complication of T2DM. However, Richter et al., [10] as well as Nicoletto et et al., [16] did not find any correlation between serum and urinary progranulin in patients with type 2 diabetes, which limits their conclusions. Therefore, the exact mechanisms underlying the increase of progranulin in patients with diabetic micro angiopathy needs further studies. While, in type 1 diabetic patients, urinary PGRN levels were predictive of early kidney function decline and albuminuria. Schlatzer et al. [17] investigated PGRN in urine of seventy-four patients with type 1 diabetes mellitus and concluded that a panel of three proteins (urinary levels of Tamms-Horsfall glycoprotein, clusterin and human α-1 acid glycoprotein) plus progranulin could be used to predict early signs of DKD. However, Progranulin level in urine of patients with T2DM and diabetic kidney disease (DKD) remain unknown. This indicates that there were potential differences in the mechanisms of kidney damage in each type of diabetes.
Another explanation for increased serum PGRN level in diabetic patients with DN may be as a compensatory mechanism to reduce renal deterioration, since it was demonstrated that PGRN could attenuate inflammation in an acute condition [5]. This hypothesis is supported in a mice model of renal ischemia-reperfusion injury, Zhou et al. [5] observed that PGRN deficiency in the mice kidney was associated with higher elevation of blood urea nitrogen and serum creatinine, severe morphological injury and higher inflammatory response and its administration in vitro diminished inflammation after renal ischemia–reperfusion injury through a nucleotide-binding oligomerization domain containing 2 (NOD2)-mediated immune response [5]. Therefore, PGRN plays a protective role in acute kidney injury and has an anti-inflammatory effect in the kidney after renal ischemia–reperfusion injury. Similarly, progranulin deficient mice exposed to lipopolysaccharide (LPS) injection as an endotoxin-induced acute kidney injury in mice model, presented increases of inflammatory markers, blood urea nitrogen and serum creatinine [18]. Furthermore, administration of recombinant progranulin before LPS treatment in wild-type mice was associated with reduced renal injury [18]. Finally, in a mice model of hyper homocysteinemia (a risk factor for kidney disease), progranulin deficient mice also existing exacerbated kidney injury, that could be improved by pretreatment with recombinant human progranulin [19]. Also, some authors have reported that binding PGRN to TNFR-1 could impair tumor necrosis factor alfa (TNF-α) binding to its receptor, resulting in an anti-inflammatory effect [20]. Lastly, there is little evidence regarding the association of PGRN and DKD in T2DM patients and further studies are needed to recognize whether increased serum PGRN in diabetic patients with DKD could play an anti-inflammatory role or whether its accumulation in serum is only due to the decreased eGFR.
Conclusion
Serum PRGN level was significantly increased in patients with T2DM compared to control subjects and in type 2 diabetic patients with micro vascular complications than those without. Moreover, the mean serum PRGN levels increased parallel to the severity of diabetic nephropathy and diabetic retinopathy with the highest levels among patients with advanced complications suggesting that serum PRGN level can be used as a biomarker for the presence and severity of microvascular complications among patients with T2DM. Moreover, serum PRGN can be used as an early marker for diabetic nephropathy in diabetic patients with stage 2 DN even before the appearance of albuminuria.
We recommend that PRGN level could be used in the follow-up of type 2 diabetic patients and for anticipating its micro vascular complications and its severity and could be considered as a potential therapeutic target for the management of these complications. A further study is recommended using larger number of patients and longer duration to validate the present results. Furthermore, Prospective or longitudinal studies are needed to assess whether increased PRGN levels in type 2 diabetic patients would indicate micro vascular damage or not.
Abbreviations
- PRGN:
-
Progranulin
- T2DM:
-
Type 2 Diabetes Mellitus
- DR:
-
Diabetic Retinopathy
- DN:
-
Diabetic Nephropathy
- PDR:
-
Proliferative Diabetic Retinopathy
- NPDR:
-
NonProliferative Diabetic Retinopathy
- SDM:
-
Simple Diabetes Mellitus (normoalbuminuria)
- EDN:
-
Early Diabetic Nephropathy (microalbuminuria)
- CDN:
-
Clinical Diabetic Nephropathy (macroalbuminuria)
References
Huang L ,Xie Y ,Dai S and Zheng H .Neutrophil-to-lymphocyte ratio in diabetic microangiopathy. Int J Clin Exp Pathol 2017;10(2):1223–1232. www.ijcep.com /ISSN:1936-2625/IJCEP0037314.
Karlberg C, Falk C, Green A, Sjølie AK, Grauslund J. Proliferative retinopathy predicts nephropathy:a 25-year follow-up study of type 1 diabetic patients. Acta Diabetol. 2012;49:263–8. https://doi.org/10.1007/s00592-011-0304-y.
Lin X, Zhou B, Huixia L, Jiali L, Junhui D, Weijin Z, et al. Serum levels of progranulin are closely associated with microvascular complication in type 2 diabetes. Dis Markers. 2015;2015, Article ID 357279:9. https://doi.org/10.1155/2015/357279.
Ezz MK, Abd El Azeem EM. Assessment of progranulin in Egyptian type 2 diabetic patients as a novel biomarker for diabetic nephropathy. Int J Biosci. 2016;9(6):350–9.
Zhou M, Tang W, Fu Y, Xu X, Wang Z, Lu Y, et al. Progranulin protects against renal ischemia/reperfu- Sion injury in mice. Kidney Int. 2015;87:918–29.
American Diabetes Association. Diagnosis and classification of diaberes mellitus. Diabetes Care. 2014;37(Suppl. 1):S81–90.
Wilkinson CP, Ferris FL, Klein RE, et al. Global diabetic retinopathy project group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82.
International-Diabetes-Federation. (2015). IDF diabetes atlas (7th ed.). Brussels, Belgium: International Diabetes Federation.
Shafaei A, Marjani A, Khoshnia M. Serum Progranulin levels in type 2 diabetic patients with metabolic syndrome. Rom J Intern Med. 2016;54(4):211–6.
Richter J, Focke D, Ebert T, Kovacs P, Lössner U, et al. Serum levels of the adipokine progranulin depend on renal function. Diabetes Care. 2013;36(2):410–4.
Guo Q, Xu L, Li H, Sun H, Liu J, Wu S, et al. Progranulin causes adipose insulin resistance via increased autophagy resulting from activated oxidative stress and endoplasmic reticulum stress. Lipids Health Dis. 2017;16:25. https://doi.org/10.1186/s12944-017-0425-6.
Youn BS, Bang SI, Klöting N, Park JW, Lee N, et al. Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes. 2009;58(3):627–36.
Qu H, Deng H, Hu Z. Plasma progranulin concentrations are increased in patients with type 2 diabetes and obesity and correlated with insulin resistance. Mediat Inflamm. 2013;2013, Article ID 360190:6. https://doi.org/10.1155/2013/360190.
Kamei N, Yamashita M, Nishizaki Y, Yanagisawa N, Nojiri S, Tanaka K, et al. Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Sci Rep. 2018;8:15302. https://doi.org/10.1038/s41598-018-33590-w.
Nicoletto BB, Canani LH. The role of progranulin in diabetes and kidney disease. Diabetol Metab Syndr. 2015;7:117. Published 2015 Dec 21. https://doi.org/10.1186/s13098-015-0112-6.
Nicoletto BB, Krolikowski TC, Crispim D, Canani LH. Serum and urinary progranulin in diabetic kidney disease. PLoS One. 2016;11:e0165177. https://doi.org/10.1371/journal.pone.0165177.
Schlatzer D, Maahs DM, Chance MR, Dazard JE, Li X, Hazlett F, et al. Novel urinary protein biomarkers predicting the development of microalbuminuria and renal function decline in type 1 diabetes. Dia- betes Care. 2012;35:549–55. https://doi.org/10.2337/dc11-1491.
Xu X, Gou L, Zhou M, Yang F, Zhao Y, Feng T, et al. Progranulin protects against endotoxin-induced acute kidney injury by downregulating renal cell death and inflammatory responses in mice. Int Immunopharmacol. 2016;38:409–19. https://doi.org/10.1016/j.intimp.2016.06.022.
Fu Y, Sun Y, Zhou M, Wang X, Wang Z, Wei X, et al. Therapeutic potential of progranulin in hyperhomo- cysteinemia-induced cardiorenal dysfunction. Hypertension. 2017;69:259–66. https://doi.org/10.1161/HYPERTENSIONAHA.116.08154 PMID: 27872232).
Chen X, Chang J, Deng Q, Xu J, Nguyen TA, Martens LH, et al. Progranulin does not bind tumor necrosis factor (TNF) receptors and is not a direct regulator of TNF-dependent signaling or bioactivity in immune or neuronal cells. J Neurosci. 2013;33(21):9202–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albeltagy, E.S., Hammour, A.E. & Albeltagy, S.A. Potential value of serum Progranulin as a biomarker for the presence and severity of micro vascular complications among Egyptian patients with type 2 diabetes mellitus. J Diabetes Metab Disord 18, 217–228 (2019). https://doi.org/10.1007/s40200-019-00406-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-019-00406-1