Abstract
Background
Previous studies showed altered angiopoietin-like protein-8 (ANGPTL-8) circulating levels in type 2 diabetes mellitus (DM). Whether or not the alteration in ANGPTL-8 level can be a predictive maker for increased DM risk remains unclear.
Aim
Investigating possible role of ANGPTL-8 as a risk predictor of type2 DM, in addition to a set of factors likely to affect ANGPTL-8 level.
Methods
One hundred recently diagnosed persons with type 2 DM and 100 sex- and age-matched healthy controls were enrolled. Exclusion criteria included type 1 DM, acute infections, history of chronic kidney disease, malignancy, and blood loss or transfusion. Serum levels of ANGPTL-8, blood pressure, weight, height, glycosylated hemoglobin (HbA1c), fasting blood glucose, cystatin C, lipid profile, liver, and kidney function tests were assessed. The independent relationship between DM and ANGPTL-8 was tested in the unadjusted and multiple-adjusted regression models.
Results
Serum ANGPTL-8 levels showed significant elevation among persons with vs. without DM (p = 0.006), positive correlation with HbA1c (p < 0.001), and negative correlation with estimated GFR (eGFR) (p = 0.003) but no significant correlation to fasting glucose level. In the unadjusted model, patients in the third tertile of ANGPTL-8 had 4 times risk of DM (OR 4.03; 95% CI = 1.37–11.84). Data adjustment for cardiovascular diseases, smoking, body mass index, systolic blood pressure, alanine transaminase (ALT), and low-density lipoprotein (LDL) increased the direct relationship between ANGPTL-8 and DM (OR 6.26; 95% CI = 1.21–32.50). However, the risk significantly decreased after adjustment of Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR creatinine-cystatin (OR 2.17; 95% CI = 0.10–49.84).
Conclusion
This study highlights a possible predictive role of ANGPTL-8 in diabetic complications, particularly nephropathy. Larger prognostic studies are needed to validate the cause-effect relationship between ANGPTL-8 and deteriorated kidney functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a health problem with increasing deleterious effects on both population health and economy. The Middle East and North Africa (MENA) region is a region of a high prevalence of DM [1]. Egypt occupies the 8th rank on the list of the top 10 countries regarding the number of adults with DM (8.2 million). By 2045, Egypt will climb to number 6 in the list, with an expected average of 16.7 million people suffering from DM [2].
In the new era of predictive, preventive, and personalized medicine (PPPM), it is no longer enough to diagnose a disease and its accompanying complications and treat patients using the classical tools, but rather take the process to a higher level of developing platforms that can predict the onset of such events, prevent the disease or its complications, and administer treatment based on personalized variations between patients [3]. Applying this to the management of diabetes, three levels of desirable pre-diabetes and diabetes care were proposed by Golubnitschaja [4]. Those levels included prediction of susceptibility to diabetes and pre-stages of diabetes in addition to prediction of the undesirable complications.
Previous research has pointed out some parameters as predictive markers for the abovementioned levels, including age and socioeconomic status [5]; suboptimal health status [6]; indices of DNA damage [7]; some metabolic biomarkers such as serum sclerostin, irisin, and others [8, 9], markers of endothelial cell damage [10]; and glycosylated hemoglobin (HbA1c) [11, 12]. DM as a multifaceted disease still has room for research into more markers that can hold potential in PPPM [13]. Of such potential markers stands out angiopoietin-like protein 8 (ANGPTL-8).
ANGPTL-8 has been a marker of great controversy since its characterization a few years ago [14,15,16,17]. ANGPTL-8, also known as betatrophin, refeeding induced in fat and liver (RIFL), lipasin, and hepatocellular carcinoma-associated protein TD26, is a 198 amino acid adipokine/ hepatokine secreted mainly from the liver in humans and from the adipose tissue and liver in mice [18, 19]. It is an atypical member of the angiopoietin-like protein (ANGPTL) family, functionally considered to regulate blood lipid, in a nutrition-dependent manner [20]. Its levels were shown to be affected by fasting, and it seems to be a lipogenic factor as it is induced during adipogenesis. It promotes cleavage of ANGPTL-3 leading to inhibition of lipoprotein lipase, in addition to the elevation of serum triglyceride level [14, 21].
The breakthrough proposed by Yi et al. in 2013 [18], that ANGPTL-8 was a predictive marker of increased pancreatic beta cell proliferation and mass and improved insulin secretion in 1961 induced insulin resistance mouse models, failed to be reproduced [22]. However, it drove research about other potential roles of ANGPTL-8 in DM, especially in type 2. Most studies showed an association between type 2 DM and altered circulating ANGPTL-8 level, with most of them demonstrating increased levels of ANGPTL-8 [23, 24], while a few others showed ANGPTL-8 level to be decreased [25, 26] or unchanged [27]. Although the exact mechanism linking ANGPTL-8 to DM is not yet established, there is a general consensus that ANGPTL-8 is upregulated in cases of insulin resistance rather than insulin deficiency [28]. Whether or not the alteration in ANGPTL-8 level can serve as a predictive marker for the risk of developing DM or its complications remains unsettled.
Taking the association between DM and ANGPTL-8 into consideration, researchers attempted to uncover the possible underlying mechanisms causing variation in ANGPTL-8 concentration between persons with versus without DM. It is generally accepted that the concentration of any biomarker is affected by its synthesis, metabolism, degradation, and excretion. Thus, factors possibly affecting the ANGPTL-8 concentration in DM can fall into one of such categories. Most factors studied included those related to carbohydrate and lipid metabolism such as fasting blood glucose level, glycosylated hemoglobin (HbA1c), insulin [24, 26, 29,30,31], lipid profile [14, 25, 27, 29], and obesity [25, 32]. There was, however, a great variation in results of all studied parameters. For example, circulating ANGPTL-8 was either increased [29], decreased [25, 32], or unchanged [27, 33] in obesity, either positively [29, 31] or negatively correlated with insulin [25], and either positively correlated with atherogenic lipid profiles [27] or with HDL-C [25].
As mentioned above, another aspect that can regulate circulating ANGPTL-8 level is the kidney function, especially with the well-known unfavorable effect of DM on the kidneys that might result in diabetic nephropathy. Despite the relevance of this point, limited studies have been performed and again showed no consensus [20, 34, 35].
The current study is aimed at investigating the possible role of ANGPTL-8 as a risk predictor of type 2 DM and possible factors that might affect ANGPTL-8 circulating levels.
Subjects
Study protocol
The protocol was approved by the Ethics Committee of Alexandria Faculty of Medicine (IRB number 00007555) following the International Ethical Guidelines for Epidemiological studies [36].
Study design and participants
A matched case-control study was conducted in the outpatient clinics of the Alexandria Main University Hospital. The Alexandria Main University Hospital is a specialist’s referral center for the northern part of Egypt. It covers four governorates of Northern Egypt and serves approximately 14 million people. One hundred newly diagnosed persons with type 2 DM (diagnosed within 3 months) were randomly selected from adult patients admitted to the outpatient clinics. Persons with diabetes were included based on the American Diabetes Association (ADA) criteria for diagnosis of DM [37].
One hundred sex- and aged-matched controls were enrolled from healthy workers at the Alexandria Main University Hospital. All participants were aged ≥ 18 years. Exclusion criteria were the history of type 1 DM, pre-diabetes, acute infections, history of chronic kidney disease, malignancy, and blood loss or transfusion.
Material and methods
Information on the demographics, history of co-morbid conditions (hypertension, cardiovascular diseases, liver diseases, and neurological diseases), and medication history was collected. Previous conditions were defined based on the medical history obtained by the treating physician at the outpatient clinics. Renal function was assessed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-cystatin equation to calculate estimated GFR using both serum creatinine and cystatin C (eGFR cr-cys) [38].
Laboratory investigations
All patients and controls were asked to fast overnight. The following day, a blood sample was withdrawn into two tubes: one EDTA-coated tube for analysis of glycosylated hemoglobin (HbA1c) and another RST™ tube with thrombin-based clot activator BD to obtain serum for analysis of all the remaining markers. Blood samples were transferred on dry ice to the lab within less than 2 h. Tubes designated for serum analysis were centrifuged; serum was withdrawn and aliquoted in Eppendorf tubes. On the day of sample collection, serum concentration of fasting blood glucose (FBG), cholesterol, triacylglycerol, high-density lipoproteins (HDL), very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), aspartate transaminase, alanine transaminase (ALT), albumin, urea, and creatinine was assessed using colorimetry. In addition, HbA1c level was measured in whole blood. Serum aliquots for ANGPTL-8 and cystatin C assays were stored at − 80 °C till the day of assay.
On the day of the assay, serum aliquots were brought to room temperature. Cystatin C was measured by N latex-enhanced nephelometry assay using Siemens Healthineers ProSpec system. Multiple dilutions of a human cystatin C calibrator obtained from human sera were used to generate a six-point calibration. The signal intensity was proportional to the concentration of cystatin C in the sample. Samples were autodiluted 1:100, and standards were autodiluted from 1:20 to 1:640. A volume of 30 μl of samples was used. One to 3 sera of known cystatin C concentration were included in each run to rule out the drift of the assay. Quality controls of low (1.06 mg/l) and high (1.93 mg/l) cystatin C concentrations were measured before and after each run. Runs with a change of more than 6% in the quality controls over the course of the assay were discarded. Initial measuring range was 0.27–9.4 mg/l, while the minimum measuring range was 0.06–1.9 mg/l.
ANGPTL-8 was assayed using human ANGPTL-8 ELISA kit, provided by Aviscera Bioscience, catalogue number SK00528-06. The kit showed a sensitivity of 160 pg/ml, intra-assay precision of 4–6%, and inter-assay precision of 8–10%. Specificity for human ANGPTL-8 was 100% with no cross-reactivity with any of the similar proteins. The procedure was performed according to the manufacturer’s protocol, and results were calculated using an automated ELISA reader.
Statistical analysis
Data was reported as mean ± standard deviation (SD), median and inter-quartile range, or percent frequency, as appropriate. Comparisons between groups were made by independent t test or the Mann-Whitney U test. The association between paired variables was analyzed by Pearson product moment correlation coefficient (r) or Spearman Rho correlation coefficient (Rho) and p value. Variables which showed positively skewed distributions were log transformed (log10) before the correlation study.
The relationship between circulating ANGPTL-8 (independent variable) and type 2 DM (dependent variable) was analyzed by multiple logistic regression models including all variables showing a significant correlation with DM at the bivariate analysis (Table 1, last column), as well as the bivariate correlates of ANGPTL-8. A series of risk factors serving as potential confounders of the link between DM and ANGPTL-8 were considered (all variables which were of statistical or clinical significance were included). Factors included demographic data as the history of smoking, body mass index (BMI), clinical history (cardiovascular disease), blood pressure, lipid profile (total cholesterol, triacylglycerol, LDL, HDL, VLDL), liver function tests (ALT, AST), serum albumin, and renal function. Glomerular filtration rate (eGFR cr-cys), and ANGPTL-8 were determined. Variables that significantly affected the link between plasma ANGPTL-8 and the study outcome (DM) in the bivariate analysis were then jointly included in a multiple logistic regression model. Three models were built: an unadjusted model between the tertiles of ANGPTL-8 and DM status, model adjusting for demographic and clinical data, and the third one combining the two sets of variables with eGFR cr-cys.
Assessment of the functional form of plasma ANGPTL-8 (untransformed, log-transformed, tertiles, or quartiles) into the logistic regression models was done using martingale residual analysis [39]. By this analysis, the “tertiles” were the most appropriate functional form of plasma ANGPTL-8 to be considered into the logistic regression models.
Odds ratios (OR) and their 95% confidence intervals (CI) were calculated. All calculations were done using a standard statistical package (SPSS for Windows Version 9.0.1, 11 Mar-1999, Chicago, Illinois, USA).
Results
Study population characteristics
Table 1 summarizes the baseline characteristics of the study participants. More than one-third (38%) of persons with type 2DM and 29% of persons without diabetes were smokers. Patients who gave history of the cardiovascular diseases comprised 38% of the persons with diabetes and 34% of persons without DM. The mean ± SD of systolic blood pressure (mmHg) was significantly higher among persons with vs. without DM (137.7 ± 19.7 vs. 127.20 ± 12.9, respectively). ANGPTL-8 level (pg/ml) was significantly higher among persons with type 2 DM (33,000 (6050–148,500)) than without DM (1900 (1100–79,950)). The eGFR-cystatin was significantly lower among persons with type 2 DM (49.02, range 16.28–61.87 ml/min/1.73m2) than persons without diabetics (80.83, range 72.15–89.21 ml/min/1.73m2). Similar pattern was observed with eGFR-creatinine. Levels of triglycerides, LDL, VLDL, ALT, AST, urea, creatinine, glucose, and HbA1c were significantly higher in persons with DM, while levels of HDL and albumin were significantly lower in persons with vs. without DM (Table 1).
Functional correlates of DM: univariate and multivariate analyses
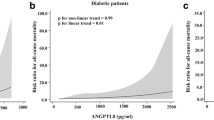
The associations of type 2 DM with other variables were substantially confirmed in correlation analyses. Type 2 DM associated inversely with HDL, albumin, and both eGFR cys and eGFR cr, while it was directly correlated to ANGPTL-8, systolic blood pressure, triglyceride, VLDL, FBG, HBA1c, urea, ALT, and AST (Table 1, last column). By the same token, plasma ANGPTL-8 correlated directly with HBA1c (Fig. 1a) and inversely with eGFR (Fig.1b). There was no correlation between plasma ANGPTL-8 level and FBG level (Fig.1c).
ANGPTL-8, DM, and renal function: multiple logistic regression analyses
The independent relationship between circulating levels of ANGPTL-8 with DM was tested in the unadjusted model and models adjusting for smoking status, BMI, clinical history of cardiovascular diseases, systolic blood pressure, ALT, and LDL data (model 1, Table 2) and for the two sets of variables combined together with CKD-EPI creatinine-cystatin-based e-GFR (model 2, Table 2). The unadjusted model showed that patients in the third tertile of ANGPTL-8 had four times risk of having DM (OR 4.03; 95% CI = 1.37–11.84). Data adjustment for smoking status, BMI, clinical history of cardiovascular diseases, systolic blood pressure, ALT, and LDL substantially increased the direct relationship between DM and ANGPTL-8 (OR 6.26; 95% CI = 1.21–32.50). However, the relationship between the third tertile of ANGPTL-8 and DM became largely insignificant after adjustment of eGFR cr-cys (OR 2.17; 95% CI = 0.10–49.84).
Discussion
Hepatic ANGPTL-8 is gaining more attention as a potential player in DM. Most studies have demonstrated altered circulating ANGPTL-8 levels in patients with type 2 DM, mainly increased levels [23, 24], yet the exact underlying mechanism explaining the association of ANGPTL-8 with type 2 DM is not fully understood. Moreover, available research hardly investigated the concept that ANGPTL-8 might be linked to diabetic complications such as retinopathy and diabetic kidney disease. Some studies proposed that ANGPTL-8 is a predictor of increased risk of DM [24, 40], but not to its complications.
Some factors that might regulate circulating ANGPTL-8 in DM were investigated in the literature. Most studied factors were related to the production and metabolism of ANGPTL-8; however, very limited research was done on the possible role of the kidney on affecting the ANGPTL-8 serum concentration.
Our study confirmed the hypothesis, that in persons with type 2 DM, circulating levels of ANGPTL-8 are significantly higher than in subjects without DM, which is similar to the conclusion reached by other previous studies [23, 24]. Higher levels of ANGPTL-8 may be related to the insulin resistance and the higher demand for insulin in type 2 DM subjects, in agreement with data reported in mice where ANGPTL-8 levels were upregulated by 3–4-folds in the db/db and ob/ob mice models compared with the wild-type [18]. On the other hand, Abu-Farha et al. showed strong positive associations between betatrophin and FBG in non-diabetic subjects. However, correlations with FBG were diminished in T2D subjects [41].
An interesting observation in this study was that, unlike other studies [24, 42], ANGPTL-8 correlated directly and significantly with HbA1c, but not with the FBG level, where there was no such significant correlation. This finding drew our attention that perhaps ANGPTL-8 is more likely associated with long-term glycemic control represented by HbA1c, rather than short-term glycemic control, represented by FBG levels. It is documented that HbA1c, is a reliable measure of the glycemic status and has a direct relation with the occurrence of diabetes-related complications as proved by many landmark studies [43]. A study by Penno et al. [44] has shown that HbA1c can predict diabetic kidney disease but not retinopathy. They reported that the prevalence of decreased eGFR and microalbuminuria was highest when HbA1c was above the median, and prevalence was lowest when HbA1c median was at lowest. An interesting finding was that HbA1c was a much stronger associate with development of diabetic kidney disease rather than FBG. Logistic regression models of univariate analysis showed that the risk of diabetic kidney disease was strongly increased at the HbA1c categories 8% (OR = 2.35; 95% CI 1.30–4.25), while it was much less with FBG (OR = 1.139; 95% CI 1.054–1.231). Gupta and Singh concluded in their study [11] that the level of glycemic control, as represented by HbA1c levels, was the strongest factor determining conversion from normoalbuminuria to microalbuminuria in patients with type 2 DM. Based on this observation and considering the association between ANGPTL-8 and both HBA1c and renal functions (discussed later), a new aspect of research studying the potential role of ANGPTL-8 in predicting diabetic complications, especially diabetic kidney disease, might be tackled.
Another worth-mentioning observation is that the ELISA kit used in our study (Aviscera bioscience, catalogue number SK00528-06) was reported in a study by Guo et al. [45] to show significantly higher concentrations of ANGPTL-8 in overweight persons with type 2 DM vs. overweight persons without DM, while it showed lower concentrations of ANGPTL-8 in obese persons with type 2 DM vs. obese persons without DM. This unusual observation by the authors was attributed to the ELISA kit itself, which was different from kits used in other studies including Wuhan EIAAB Science Co. ELISA kit (catalogue number E1164H) and C-terminal ELISA kit recognizing the region from 139 to 198 amino acids from Phoenix Pharmaceuticals (catalogue number EK-051-55) [29]. However, our study showed that ANGPTL-8 levels were significantly higher in persons with type 2 DM, regardless of their body weight, thus driving the attention that perhaps other associated factors in the diabetic persons enrolled in Guo et al. study might have led to lowering of ANGPTL-8 concentration in obese diabetic patients.
Moving on to the possible role of ANGPTL-8 as an associate of increased risk of DM, the current study showed that patients in the highest tertile of ANGPTL-8 had four times risk of having type 2 DM (OR 4.03) in the unadjusted models. Data adjustment for cardiovascular diseases, smoking, body mass index, systolic blood pressure, ALT, and LDL even increased the direct relationship between ANGPTL-8 and DM (OR 6.26).
This was concordant with the results obtained by Lee et al. [42], who also concluded that subjects in the highest baseline quartile of ANGPTL-8 levels had more than a threefold higher risk of incident diabetes than the subjects in the lowest quartile. Farha et al. [41] as well demonstrated that subjects in the highest tertile of ANGPTL-8 had higher odds of having type 2 DM (OR = 6.15), and Leiherer et al. [24] showed that in multivariate analysis, ANGPTL-8 proved to be an independent predictor of DM risk (OR 1.23). This potentiated the concept that ANGPTL-8 might be used to predict the incidence of DM. However, to our surprise, that was not true when we adjusted to kidney functions (which, to our knowledge, other authors have not done). Adjustment to eGFR cr-cys significantly decreased the risk (OR = 2.17).
This interesting result may set a base for a strong and independent relationship between circulating ANGPTL-8 and renal function among persons with type 2 DM. Such a relationship implies that impaired renal functions, in the form of low eGFR cr-cys, might be the most important associate to high ANGPTL-8 levels among patients with type 2 DM, rather than glycemic and lipid profiles.
Very scant research has focused on the interaction between renal functions and circulating ANGPTL-8 levels and again reaching controversial results. In consensus with our study, Maurer et al. showed a negative correlation between circulating ANGPTL-8 and CKD-EPI eGFR [20]. Some other studies identified weak positive correlations between circulating ANGPTL-8 and serum creatinine levels, yet this was not statistically significant [28, 46, 47].
A suggested mechanism for increased circulating ANGPTL-8 levels in association with impaired kidney functions would be impaired excretion of ANGPTL-8. However, this was argued by the following studies: Chen et al. [35] showed increased serum ANGPTL-8 levels in persons with diabetes with normo-, micro- and macroalbuminuria as compared with healthy control and positively correlated with albumin/creatinine ratio. An important observation in Chen’s study is that in type 2 DM patients, there was an increased urinary ANGPTL-8 level as compared with healthy subjects, but there were no differences among normoalbuminuria, microalbuminuria, and macroalbuminuria groups. This ruled out that reduced clearance of ANGPTL-8 results from the decreased glomerular filtration in the different groups. Another study that also opposed the possible role of renal excretion in determining circulating ANGPTL-8 levels was that of Ebert et al. [34]. In their study, they compared circulating ANGPTL-8 levels in diabetic persons with sustained renal function and diabetic persons undergoing hemodialysis. ANGPTL-8 was significantly positively correlated with the eGFR. Subjects with eGFR > 50 ml/min/1.73m2 had a significantly higher ANGPTL-8 levels than patients on hemodialysis.
ANGPTL-8 might be increased in association with impaired renal functions in the same manner that other several hepatokines increased. Studies have detected an increase in ANGPTL3 (whose cleavage is promoted by ANGPTL-8) [48], chemerin [49], FGF21 [50], and leptin [51] in association with impaired renal functions. The mechanism of such an increase is not fully understood though.
Conclusion
Our study highlights two findings that perhaps were not clearly addressed in the literature. First is the possible link between ANGPTL-8 and the long-term rather than short-term glycemic control, as represented by a strong association between ANGPTL-8 and HbA1c rather than fasting blood glucose. The second is the strong association between ANGPTL-8 and the impaired renal functions. Taking into consideration that HbA1c has been implicated as a predictor of early diabetic kidney disease, our findings might set a path for scientific research investigating the possible predictive role of ANGPTL-8 in detecting early diabetic kidney disease. There are several questions to be answered: Is the increased ANGPTL-8 level associated with the impaired renal functions, a cause or effect of diabetic kidney disease, or is it a compensatory mechanism trying to restore the renal functions? Large-scale prognostic studies are required to uncover the mechanism of this association.
Limitations
Our study was not free of limitations. One limitation was the unavailability of the urine samples to study albumin and ANGPTL-8 excretion, which would have helped determine whether the increased circulating ANGPTL-8 level associated with deteriorating renal functions was due to impaired excretion. In addition, the authors could not reach a solid conclusion about the potential role of ANGPTL-8 in predicting diabetic complications because follow-up of patients was not possible. This study can be considered a preliminary one, aiming at understanding the factors behind increased circulating ANGPTL-8 in type 2 DM. The results, however, require validation on a larger scale follow-up studies, with tools to study the underlying mechanisms linking kidney functions to circulating ANGPTL-8 levels.
Expert recommendations
Diabetes mellitus is a multifaceted, widely spread disease affecting millions of people all around the globe and has a considerable impact on the economy. It is no longer sufficient to rely on the classical methods of diagnosis and treatment. In the era of predictive, preventive, and personalized medicine, search for factors that can fulfill such an approach has become mandatory as regards DM [1, 3, 4, 10, 52]. A novel biomarker that might be of potential use in becoming of relevance in predictive and preventive measures in type 2 DM and/or its complications is ANGPTL-8. Since our study showed ANGPTL-8 to be strongly associated with both HbA1c and the deteriorated kidney functions, we recommend further large-scale prognostic research conducted to determine the potential cause-effect relationship between kidney functions and ANGPTL-8 circulating levels and the possible predictive role that ANGPTL-8 circulating levels can have in determining future diabetic complications, particularly early screening of diabetic kidney disease in type 2 DM.
References
Duarte AA, Mohsin S, Golubnitschaja O. Diabetes care in figures: current pitfalls and future scenario. EPMA J. 2018;9(2):125–31.
International diabetes federation. Diabetes atlas, 8th edn. 2017. Available at : www.diabetesatlas.org. Accessed 15 June 2019.
Golubnitschaja O, Baban B, Boniolo G, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23.
Golubnitschaja O. Advanced diabetes care: three levels of prediction, prevention & personalized treatment. Curr Diabetes Rev. 2010;6(1):42–51.
Suwannaphant K, Laohasiriwong W, Puttanapong N, et al. Association between socioeconomic status and diabetes mellitus: the National Socioeconomics Survey, 2010 and 2012. J Clin Diagn Res. 2017;11(7):LC18–22.
Ge S, Xu X, Zhang J, et al. Suboptimal health status as an independent risk factor for type 2 diabetes mellitus in a community-based cohort: the China suboptimal health cohort study. EPMA J. 2019;10(1):65–72.
Soliman N, El-Shabrawi M, Omar S. DNA fragmentation damage as a predictive marker for diabetic nephropathy in type II diabetes mellitus. J Endocrinol Metab Diabetes S Afr. 2018;23(2):32–5.
Saadeldin MK, Elshaer SS, Emara IA, et al. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: a case control study. PLoS One. 2018;13(11):e0206761.
Lee H, Park T, Kim B. Metabolic markers predictive of prediabetes in the Korean population. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-201-LB.
Golubnitschaja O, Costigliola V. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14.
Gupta M, Singh JP. Correlation of microalbuminuria with glycosylated haemoglobin in patients of diabetes having nephropathy. Int J Adv Med. 2017;4(3):805–8.
Sena CM, Bento CF, Pereira P, et al. Diabetes mellitus: new challenges and innovative therapies. EPMA J. 2010;1(1):138–63.
Golubnitschaja O, Costigliola V. EPMA summit 2014 under the auspices of the presidency of Italy in the EU: professional statements. EPMA J. 2015;6(1):4.
Ren G, Kim JY, Smas CM. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab. 2012;303(3):E334–51.
Quagliarini F, Wang Y, Kozlitina J, et al. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci U S A. 2012;109(48):19751–6.
Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun. 2012;424(4):786–92.
Fu Z, Yao F, Abou-Samra AB, et al. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun. 2013;430(3):1126–31.
Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2013;153(4):747–58.
Luo M, Peng D. ANGPTL8: an important regulator in metabolic disorders. Front Endocrinol (Lausanne). 2018;9:169.
Maurer L, Schwarz F, Fischer-Rosinsky A, et al. Renal function is independently associated with circulating betatrophin. PLoS One. 2017;12(3):e0173197.
Zhang R, Abou-Samra AB. Emerging roles of lipasin as a critical lipid regulator. Biochem Biophys Res Commun. 2013;432(3):401–5.
Yi P, Park JS, Melton DA. Retraction notice to: betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2017;168(1–2):326.
Li S, Liu D, Li L, et al. Circulating betatrophin in patients with type 2 diabetes: a meta-analysis. J Diabetes Res. 2016;2016:6194750.
Leiherer A, Muendlein A, Geiger K, et al. Betatrophin is associated with type 2 diabetes and markers of insulin resistance. Diabetes. 2018;67(Supplement 1). https://doi.org/10.2337/db18-2445-PUB.
Gomez-Ambrosi J, Pascual E, Catalan V, et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99(10):E2004–9.
Gokulakrishnan K, Manokaran K, Pandey GK, et al. Relationship of betatrophin with youth onset type 2 diabetes among Asian Indians. Diabetes Res Clin Pract. 2015;109(1):71–6.
Fenzl A, Itariu BK, Kosi L, et al. Circulating betatrophin correlates with atherogenic lipid profiles but not with glucose and insulin levels in insulin-resistant individuals. Diabetologia. 2014;57(6):1204–8.
Espes D, Lau J, Carlsson PO. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia. 2014;57(1):50–3.
Fu Z, Berhane F, Fite A, et al. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep. 2014;4:5013.
Zhang R. Abou-Samra AB. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc Diabetol. 2014;13:133.
Hu H, Sun W, Yu S, et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. 2014;37(10):2718–22.
Tuhan H, Abaci A, Anik A, et al. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Res Clin Pract. 2016;114:37–42.
Battal F, Turkon H, Aylanc N, et al. Investigation of blood Betatrophin levels in obese children with non-alcoholic fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2018;21(2):111–7.
Ebert T, Kralisch S, Hoffmann A, et al. Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2014;99(12):E2510–7.
Chen CC, Susanto H, Chuang WH, et al. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc Diabetol. 2016;15:3.
Cioms W. International ethical guidelines for epidemiological studies. Geneva. 2009.
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2017;39(Suppl 1):S13–22.
Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2013;367(1):20–9.
Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51(4):1469–82.
Pan R, Zhang H, Yu S, et al. Betatrophin for diagnosis and prognosis of mothers with gestational diabetes mellitus. J Int Med Res. 2019;47(2):710–7.
Abu-Farha M, Abubaker J, Al-Khairi I, et al. Higher plasma betatrophin/ANGPTL8 level in type 2 diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep. 2015;5:10949.
Lee SH, Rhee M, Kwon HS, et al. Serum betatrophin concentrations and the risk of incident diabetes: a nested case-control study from Chungju metabolic disease cohort. Diabetes Metab J. 2018;42(1):53–62.
Fortwaengler K, Parkin CG, Neeser K, et al. Description of a new predictive modeling approach that correlates the risk and associated cost of well-defined diabetes-related complications with changes in glycated hemoglobin (HbA1c). J Diabetes Sci Technol. 2017;11(2):315–23.
Penno G, Solini A, Bonora E, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care. 2013;36(8):2301–10.
Guo K, Lu J, Yu H, et al. Serum betatrophin concentrations are significantly increased in overweight but not in obese or type 2 diabetic individuals. Obesity (Silver Spring). 2015;23(4):793–7.
Yamada H, Saito T, Aoki A, et al. Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr J. 2015;62(5):417–21.
Ebert T, Kralisch S, Wurst U, et al. Betatrophin levels are increased in women with gestational diabetes mellitus compared to healthy pregnant controls. Eur J Endocrinol. 2015;173(1):1–7.
Mahmood D, Makoveichuk E, Nilsson S, et al. Response of angiopoietin-like proteins 3 and 4 to hemodialysis. Int J Artif Organs. 2014;37(1):13–20.
Pfau D, Bachmann A, Lossner U, et al. Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care. 2010;33(1):171–3.
Stein S, Bachmann A, Lossner U, et al. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care. 2009;32(1):126–8.
Merabet E, Dagogo-Jack S, Coyne DW, et al. Increased plasma leptin concentration in end-stage renal disease. J Clin Endocrinol Metab. 1997;82(3):847–50.
Golubnitschaja O. Time for new guidelines in advanced diabetes care: paradigm change from delayed interventional approach to predictive, preventive & personalized medicine. EPMA J. 2010;1(1):3–12.
Acknowledgments
The authors would like to acknowledge the efforts of the medical postgraduate student Mohamed Abd Allah ElKelany, in data collection and entry.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of idea, study design, laboratory investigations, interpretation of results, writing and revising the manuscript, providing intellectual content of critical importance to the work described, and final approval of the version to be published. In addition, Dr. Yasmine Amr Issa carried out all laboratory investigations, Dr. Samar Samy Abd ElHafeez was responsible for statistical analysis, and Dr. Noha Gaber Amin executed the recruitment, examination, and data collection of patients. All authors are also accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval for human studies
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Alexandria Faculty of Medicine Ethics of the research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Issa, Y.A., Abd ElHafeez, S.S. & Amin, N.G. The potential role of angiopoietin-like protein-8 in type 2 diabetes mellitus: a possibility for predictive diagnosis and targeted preventive measures?. EPMA Journal 10, 239–248 (2019). https://doi.org/10.1007/s13167-019-00180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-019-00180-3