Abstract
A recently developed advanced hot-formed (AHF) steel for automobile is introduced and three physical metallurgy concepts based on which the AHF steel was designed are reviewed, they are dynamic carbon partitioning (DCP), flash copper precipitation and bake toughening. AHF steel is an upgrade of the existing hot-formed steel especially suitable for making components with superior crashworthiness; it can be processed by regular hot stamping equipment and process. A kinetics model for DCP is expressed in detail, which can be used to calculate the volume fraction of retained austenite based on four materials and processing parameters. The flash copper precipitation used as an additional strengthening mechanism for AHF steel is also discussed and its ultrafast kinetics can be attributed to the enhancement of quenched-in vacancies on copper diffusion. Also, the bake toughening of AHF steel is addressed; the mechanism of which may be related to the elimination of the less stable block-like retained austenite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since 2008, China has continuously been the world’s largest auto production site and market. According to the Xinhua News [1], China produced 18.7 million light vehicles in 2013, or 22.7% of the total global production, which is an increase of 12% from 2012. Unfortunately, however, the fuel efficiency of vehicle, i.e. the fuel consumption per 100 km travel distance (L/100 km), in China remains comparatively higher than most of the developed countries, e.g. 50% higher than Japan and 14% higher than continental Europe [2]. It is believed that the rapid increased vehicle population plus the relatively lower standard of fuel efficiency has contributed to the heavy smog that frequently shrouds many cities in China in recent years. On the other hand, an internet survey published by Global Times on 20th April 2013 reported that after asking respondents to pick which of 7 issues most concerned them when purchasing their next new car in 3–5 years, the top two were safety (24.2%) and fuel efficiency (17.3%). This indicates that the automotive industry has to find a solution to improve both the fuel efficiency and the safety of vehicles at the same time to respond to the market demands. An apparent solution is to build automobile using stronger components because parts with greater strength can be thinner and lighter, thusly promoting both fuel efficiency and safety [3]. By now, it seems that the only established process for manufacturing components that have strength greater than 1,200 MPa is hot stamping [4].
Hot stamping is a thermo-mechanical forming process with intended martensite transformation. It was patented (GB1490535) by Plannja, a Swedish company, in 1977, for processing saw blades and lawn mower blades. In 1984, Sabb Automobile AB was the first to use this technique to manufacture hardened boron steel component for the Sabb 9000 model [5]. Since then, the number of hot-stamped components has increased from 3 million parts/year in 1987 to 107 million parts/year in 2007 [3], thanks to the successful commercialization of Al-coated steel sheet developed by Arcelor around 2001 (US Patent No. 6,296,805), which can effectively prevent oxidation from occurring during hot stamping. The first hot stamping line in China was, however, not established until 2009 by Baosteel. So far, hot-stamped components have found use in A-pillar, B-pillar, bumper, roof rail, rocker rail and tunnel, to name just a few applications.
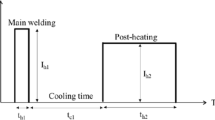
In hot stamping process, a blank that is usually made of boron steel is heated up in a furnace to be transformed into full austenite status, transferred to the press and subsequently formed and quenched in a closed die into full martensite. 22MnB5, typically containing (wt%) 0.23 C, 1.18 Mn, 0.22 Si, 0.16 Cr, 0.12 Ni and 0.002 B [6], is the most commonly used steel grade for hot stamping. The material initially exhibits a ferrite/pearlite microstructure with a tensile strength of about 600 MPa, but transforms into full martensite with an ultrahigh tensile strength up to 1,500 MPa after hot stamping process. The ultrahigh strength is important for vehicle weight reduction because it allows a reduction in sheet thickness, but it alone does not necessarily result in an increase of crash safety or absorbed crash energy, which is determined by the combination of strength and ductility or the production of strength and elongation (PSE) of the material. Unfortunately, the elongation of current hot-formed (HF) steel, e.g. 22MnB5, after hot stamping process is usually limited to the order of 6% [7], leading to a relatively low PSE level about 9 GPa%. Due to the low PSE level, the current HF steel is not sufficient to make structural components requiring good crashworthy qualities. Thus, the market has a great demand right now for ultrahigh strength steel with combined mechanical properties of 1,500 MPa strength and 20 GPa% PSE value. A new steel grade, named quenching and partitioning (Q&P) steel, developed during the last decade [8–12], may fill into this category. The microstructure of Q&P steel after a Q&P process consists of a martensitic matrix and carbon-enriched retained austenite; the former ensures the steel an ultrahigh strength and the latter provides its superior elongation up to 15% [8–12]. To create such mixed microstructures of martensite and retained austenite, the Q&P process first involves austenitization, followed by quenching to a temperature (quenching temperature, T Q) between the martensite start (M s) and finish (M f) temperatures to obtain a specific fraction of martensite, kept at a constant temperature that is the same as T Q or slightly higher (partitioning temperature, T P) for a given period of time allowing carbon diffusion from the carbon-supersaturated martensite to the neighbouring austenite to occur, and finally, the steel is quenched to room temperature and the austenite that has been sufficiently carbon enriched remains metastable at room temperature, whereas the rest transforms into martensite. The temperature–time profile of the Q&P process is illustrated together with that of the hot stamping process in Fig. 1. It can be seen that the two profiles are totally incompatible. As indicated by the work of Liu et al. [13], normal Q&P steel can only reveal superior ductility if it is treated by Q&P process. In their work, typical Q&P steel was austenitized and consequently quenched to room temperature without interruption (to simulate the temperature–time profile of hot stamping process) and treated via typical Q&P processes. It was found that the elongation of the quenched (i.e. hot stamping simulated) sample is of a low value of 6.6%, while that of the Q&P processed is of a much higher value up to 14.8%. It can be concluded that neither the current HF steel nor the existing Q&P steel can provide a PSE level of 20 GPa% under the standard hot stamping process.
In this paper, an advanced hot-formed (AHF) steel for automobile that has a superior combination of strength in the order of 1,500 MPa and PSE level greater than 20 GPa% achieved via the standard or a slightly modified hot stamping process (see the next section for details) will be introduced and overviewed based on both the published and unpublished research including the recent work at the Northeastern University. A few newly developed physical metallurgy concepts based on which the AHF steel was developed, i.e. the dynamic carbon partitioning during martensite transformation, the flash precipitation of Cu in quenched martensite and the mechanisms of the bake toughening, will also be discussed.
2 Advanced Hot-Formed Steel—Definition and Achievement
The unique feature of hot stamping process is its capability of quenching a sheet blank into martensite while forming it into its final shape in a closed die, thusly the heat treatment and metal forming are completed at the same time in one step. In the process, the quenching medium is the water-cooled die, through the direct contact with which the heat preserved in the hot blank is extracted. Due to the structure of the die and the nature of the heat transfer between the die and the sheet, the only temperature–time profile obtainable during the hot stamping process is the simple continuous quenching. In other words, it is difficult, if not possible, to carry out a standard Q&P treatment by hot stamping process because it has no facility to heat up the sheet and to keep the sheet isothermally. Nevertheless, the quenching rate during the hot stamping process can be altered by altering the heat transfer coefficient between the die and the sheet using several possible means, such as to coat the surface of the die, to use different mold materials or to adjust the working temperature of the die [14]. Furthermore, a small modification may be made to the hot stamping process without affecting its equipment configuration and increasing its cycle time, i.e. to let the stamped part to be discharged from the closed die at an elevated temperature and subsequently to cool it to room temperature with a slower rate. In summary, two types of temperature–time profiles can be achieved using the water-cooled die of hot stamping, one is the regular continuous quenching (R) and another is continuous quenching followed by an elevated temperature discharge of the part from the die (E), as illustrated in Fig. 2. Accordingly, the hot stamping process can be performed in two slightly different ways, i.e. hot stamping with regular continuous quenching (HS-R) and hot stamping with elevated temperature discharge (HS-E).
AHF steel is defined as a steel grade which has a PSE value greater than 20 GPa% and a tensile strength equivalent to that of regular HF steel (i.e. in the neighbourhood of 1,500 MPa) after a treatment of either HS-R or HS-E process. The excellent combination of strength and ductility can be attributed to the microstructure consisting of martensite and carbon-enriched film like retained austenite, which is similar to the microstructure in Q&P steel obtained by Q&P process, but is actually obtained using a regular hot stamping line. As will be detailed in the next section, the distinctive microstructure is a result of dynamic carbon partitioning (DCP) during martensite transformation.
The chemical compositions of a series of AHF steels developed recently at the Northeastern University are listed in Table 1. Also, the martensite transformation start temperatures (M s) either measured or calculated using the following equation are included in this table:
where M s is in Celsius degree and the alloying contents are given in wt% [15]. It can be seen that the AHF steel contains more than 1 wt% Si (similar to Q&P steel) and about 0.0025 wt% B (same to HF steel). The addition of the former is for preventing the formation of iron carbide during carbon partitioning and the latter is for retarding the pearlite transformation. In comparison with Q&P steel and HF steel, the AHF steel contains slightly higher alloy elements, such as Cr, Ni and Mo, for the purpose of increasing the hardenability of austenite allowing the martensite phase to be obtained under slower quenching rate to give enough time for DCP to occur. Mainly because of its lean alloy addition, the martensite transformation kinetics of regular HF steel, such as 22MnB5, is too fast to leave sufficient time for DCP to occur. The steel compositions listed in Table 1 can be classified into two groups according to their martensite transformation start temperatures (M s), i.e. the group with M s greater than 400 °C and the group with M s lower than 350 °C. DCP can take place faster in steels from the higher M s group because carbon diffusion rate increases strongly with temperature. This may enhance the carbon enrichment in untransformed austenite and stabilize the retained austenite phase. On the other hand, even though the DCP kinetics is relatively slower in steels from the lower M s group and thusly the carbon enrichment in untransformed austenite is restricted because of the lower temperature of martensite transformation, satisfactory fraction of retained austenite can still be obtained at room temperature with the help of the sufficient alloy addition. In comparison with both Q&P and the regular HF steels, the advantage of the AHF steel is that a controlled fraction of beneficial retained austenite phase can be obtained during martensite transformation under continuous quenching condition, avoiding the use of an isothermal stage that is crucial for Q&P process but incompatible with hot stamping process.
Typical mechanical properties of the AHF steels given in Table 1 treated by simulated HS-R and/or HS-E processes are listed in Table 2. Please note that the processing parameter \( \dot{T}_{\text{ms}} \) is the quenching rate of the sample around M s, T d is the temperature at which the sample was discharged from the closed die and \( \dot{T}_{\text{Td}} \) is the quenching rate of the sample at around T d. It can be seen that all steels produced a tensile strength in the neighbourhood of 1,500 MPa, an elongation in the range of 8.6–17.56% and extraordinary PSE levels from 20 to 25.3 GPa%.
The strength and elongation values shown in Table 2 are plotted against the well-known banana curve formed by most existing steels in Fig. 3. It can be seen clearly that the AHF steel introduced in this article overmatches all other steel grades in terms of its outstanding combination of strength and elongation.
The experimental data presented here have clearly verified the usefulness and the achievability of the AHF steel. However, neither the chemical composition nor the processing parameters of the newly developed steel has been fully optimized. Giving its great potential, interesting researchers are encouraged to take part in further research and development of the highly promising grade.
3 Dynamic Carbon Partitioning During Martensite Transformation
The occurrence of dynamic carbon partitioning (DCP) during martensite transformation is crucial for the success of AHF steel. Although martensite transformation is often referred to as a diffusionless transformation, the possibility of carbon diffusion from freshly formed martensite to its surrounding untransformed austenite during martensite transformation has been discussed both experimentally [20–22] and theoretically [23]. First of all, the driving force of carbon diffusion is its chemical potential difference in martensite and austenite and thusly is built up as soon as martensite transformation begins. Secondly, the carbon diffusion rate is quite fast in typical temperature range for martensite transformation—it may take from only 7.25 × 10−3 to 3.0 × 10−4 s to enrich the untransformed austenite [23]. Thus, it is possible that the diffusion of carbon occurs concomitantly with martensite transformation. Figure 4 shows the TEM images of retained austenite observed in steel F processed by a simulated hot stamping process with a quenching rate of 80 °C/s showing sufficient volume fraction of austenite can be retained after directly quenching, thus DCP may have occurred during martensite transformation.
TEM images of retained austenite observed in steel F processed by a simulated HS-R with a quenching rate of 80 °C: a bright field image; b dark field image; c diffraction pattern of retained austenite [18]
Diffusion process is a function of temperature and time. In the case of athermal martensite transformation as the one that takes place during hot stamping process, the temperature range and time period, during which dynamic carbon partitioning occur, are determined by such processing parameters as martensite transformation start temperature, M s; part discharge temperature, T d quenching rate at M s, \( \dot{T}_{\text{ms}} \) and quenching rate at T d, \( \dot{T}_{\text{Td}}. \) Consequently, DCP and in turn the volume fraction of retained austenite should be a function of these parameters as well. Table 3 shows the dependence of volume fractions of retained austenite, f γ, measured using XRD on the above processing parameters. It can be seen that, as a general trend, the retained austenite volume fraction increases as \( \dot{T}_{\text{ms}} \) and \( \dot{T}_{\text{Td}} \) decrease and as M s and T d increase. This is reasonable because a slower \( \dot{T}_{\text{ms}} \)and/or \( \dot{T}_{\text{Td}} \) means a prolonged diffusion time and a higher M s and/or T d means higher diffusion temperatures, both may enhance the carbon partitioning.
An analytical expression that is capable of predicting the volume fraction of retained austenite in given steel after a given hot stamping process was developed recently. The derivation is briefly reviewed as follows.
According to the thermodynamics of martensite transformation [24, 25], the driving force for an athermal martensite transformation, ∆G, is determined by the sum of the Gibbs chemical free energy change, ∆G c, and the non-chemical free energy change, ∆G nc, companying the austenite-to-martensite transformation, i.e.
where the non-chemical free energy change, ∆G nc, is an energy barrier for the martensite transformation, has always a positive sign and consists of the elastic strain energy, the interfacial energy and energies with the formation of different types of defects (e.g. dislocations) during transformation. Raghavan and Antia [26] demonstrated that ∆G nc can statistically be expressed as a linear function of M s, i.e.
where A and B are constants. Substitution of Eq. (3) into (2) leads to
Following Magee [27], it can be assumed that the number of martensite plates formed in a unit volume of austenite, dN, increases linearly with the increment in the driving force ∆G due to undercooling below M s, i.e.
where ϕ is a constant. The martensite fraction increment df can be written as
where f is the martensite phase fraction in sample and V a is an average volume per martensite plate. Combination of Eqs. (5) and (6) gives
or
where d∆G c/dT is a constant [26] and dM s/dT = 0 if the transformation is truly diffusionless. However, if dynamic carbon partitioning occurs, the carbon concentration in untransformed austenite will be enriched from the original level C 0 to C 0 + ∆C. As a result, M s will be reduced from M s to M s − ξ△C, where ξ is a constant. Assuming that the diffusion of carbon from martensite to untransformed austenite is at steady state, the carbon enrichment, ∆C, can be written as
where K is a constant, D is the carbon diffusivity in austenite and t is the diffusion time. It should be noted that the steady-state assumption is not exact but reasonable because the carbon concentration gradient in austenite at the interface that controls the carbon diffusion rate from martensite to untransformed austenite will be refreshed every time once a new martensite plate forms since the nucleation of martensite is a complete diffusionless reaction and thusly remains more or less constant during the athermal transformation. Differentiating Eq. (9) and submitting it into Eq. (8) leads to
where \( \dot{T} = {\text{d}}T/{\text{d}}t \) is quenching rate. To simplify the integration of Eq. (10), the temperature-dependent \( \dot{T} \) and D can be approximated using their values at M s and notated as \( \dot{T}_{\text{ms}} \) and D ms. Thus, Eq. (10) can be integrated to be
where f γ is the volume fraction of retained austenite and \( \alpha = \phi V_{\text{a}} \frac{{{\text{d}}\varDelta G_{\text{c}} }}{{{\text{d}}T}} \)and \( \beta = \phi V_{\text{a}} BK\xi \) are both constant. Letting T be the room temperature, Eq. (11) can be used to calculate the volume fraction of retained austenite in the given steel treated by HS-R process under a given quenching rate. On the other hand, to calculate the volume fraction of retained austenite in a given steel treated by HS-E process, Eq. (11) can be rewritten as
where T d and T R are discharge temperature and room temperature, respectively; and D Td and \( \dot{T}_{\text{Td}} \) are the carbon diffusivity and quenching rate at temperature T d. It can be seen in Eqs. (11) and (12) that by altering quenching rate and discharge temperature during hot stamping process, the volume fraction of retained austenite and thusly the mechanical properties of the steel can be modified. For very large quenching rate, both Eqs. (11) and (12) become the classical Koistinen and Marburger equation [28], i.e.
Factor α in Eqs. (11) and (12) can be taken as a value about −0.011 for most plain-carbon steels [28] and β can be determined by the best fitting experimental data into Eqs. (11) and/or (12).
4 Flash Precipitation of Cu in Quenched Martensite
Steel martensite is strengthened mainly by the interstitial solid solution of carbon. It has been shown that the strength of martensite is proportional to the square root of carbon concentration [29]. As a result, there is a side effect of carbon partitioning that decreases the strength of martensite because it may cause carbon to dilute in martensite. It can be estimated that the strength of martensite steel may be reduced to σ q/21/2, where σ q is the strength of water-quenched martensite, by fully carbon partitioning. It is clear that to maintain the strength of AHF steel at the level of 1,500 MPa, one or more additional strengthening mechanisms must be employed.
In recent works performed at the Northeastern University [16], Cu precipitation was explored as a possible strengthening mechanism for martensite. Actually, Cu precipitation is a well-known effective way to harden ferrite/pearlite-based high-strength steels [30]. In that case, a prolonged incubation time, usually as long as tens of minute or even hours, is required for the precipitation to take place. By contrast, however, Cu precipitation may take place in freshly formed martensite during quenching under a rate as fast as 80 °C/s as demonstrated in Fig. 5, where Cu-rich particles of a few nanometres can be seen clearly. In a recent study, the hardness values measured for two sets of samples from steels C (Cu free) and D (containing 0.64 wt%Cu) quenched using different media were compared and it was found that as quenching medium was changed from water (mean cooling rate is about 730 °C/s) or oil (mean cooling rate is about 100 °C/s) to air (mean cooling rate is about 15 °C/s), the hardness difference between steels C and D is increased from almost nothing to 65 and 86 HV, respectively. Assuming that the effect of dynamic carbon partitioning is the same in steels C and D, the observed hardness difference can be attributed to the contribution of Cu precipitation strengthening. The maximum contribution of Cu precipitation to the strength of steel D was estimated to be about 134 MPa [16].
It is evident that Cu precipitation in martensite may take place very fast even dynamically during quenching. To distinguish it from the laggard precipitation of Cu in ferrite/pearlite, Cu precipitation in martensite during quenching may be called flash precipitation for its ultrafast kinetics. It is believed that Cu precipitation is a classical nucleation growth process controlled by Cu diffusion. As a substitutional element, the diffusion of Cu in bcc ferrite is generally slow. However, the diffusivity of substitutional element can be strongly affected by the concentration of excess vacancies, an increase by the ratio c q/c 0, where c q is the quenched-in vacancy concentration and c 0 is the equilibrium concentration. Noting that the equilibrium concentration of vacancies increases exponentially with increasing temperature [31] and that the typical austenitizing temperature is 950 °C and the possible temperature range for Cu precipitation is below M s, the ratio c q/c 0 can be estimated to be as large as 102–103. Thus, the occurrence of flash precipitation of Cu in martensite can be attributed to the enhanced diffusion of Cu by the high concentration of vacancies retained during quenching.
5 Mechanisms of Bake Toughening
Bake toughening (BT) is a new processing concept analogous to the well-known bake hardening process, but it is aimed at improving the ductility and toughness rather than the hardness of the hot-stamped parts. Table 4 [32] lists the volume fractions of retained austenite (f γ), Charpy impact energies (A k), tensile strengths (UTS) and total elongations (El) measured for a series of samples of steel B that were processed by hot stamping simulation under different situations. It can be seen that the toughness and ductility of the hot-stamped samples in terms of Ak and El can be significantly improved by a simulated bake process at 200 or 280 °C for 5–60 min, while their strengths (UTS) are only slightly reduced.
The significance of bake toughening can be seen more clearly in Fig. 6, where the measured impact energies of the simulated hot-stamped samples before and after a bake toughening treatment are plotted against the volume fraction of retained austenite in each sample. It can be seen that the impact energy of samples before bake toughening treatment (diamond points linked by dashed lines) tends to increase with volume fraction of retained austenite. This trend is expected because it is widely believed that the so-called transformation-induced plasticity (TRIP) effect originated from retained austenite may be beneficial to the toughness of material [33]. However, the arrows shown in Fig. 6 indicate that the impact energy of a given sample jumps from its original level (diamond point) to a significant higher value (squire point) as a result of bake toughening treatment. It is more important to note that the volume fraction of retained austenite of each given sample was more or less reduced after the bake treatment despite the increase of the measured impact energy. This observation suggests that there may be two types of retained austenite in terms of their morphology and stability; one is film-like and more stable (see Fig. 4) and the other is block-like and less stable (see Fig. 7). Less stable retained austenite may be transformed into martensite before or during the early stage of plastic deformation and, therefore, may have little contribution to ductility. Moreover, the relatively large block of retained austenite may result in large and brittle martensite plates, which may become microcracks leading to the fracture of the material. Thus, the mechanisms of the bake toughening may be as follows: (1) removing the harmful block-like and less stable retained austenite by decomposing it into lower bainite and (2) further stabilizing the undecomposed austenite by carbon partitioning allowing it to be transformed into martensite at later stage of plastic deformation. In addition, other tempering mechanisms may also contribute to the toughness improvement. Nevertheless, it can be concluded from the above discussion that the automobile parts made by AHF steel is not only suitable for the bake process, but its combined mechanical properties can also be improved by the process significantly.
Impact energies measured for simulated hot-stamped steel B before and after bake toughening treatment [32]
SEM image of block-like retained austenite observed in Steel F processed by HS_R [18]
6 Conclusions
-
(1)
Advanced hot-formed (AHF) steel, which has a PSE value greater than 20 GPa% and a tensile strength equivalent to that of existing HF steel, is an upgrade of HF steel especially suitable for making automotive components with super crashworthiness. It was designed based on three new physical metallurgy concepts: dynamic carbon partitioning, flash copper precipitation and bake toughening.
-
(2)
Dynamic carbon partitioning (DCP), which takes place during quenching, makes AHF steel to form a microstructure same as that of Q&P steel via a regular hot stamping process. Based on the newly developed DCP kinetic model, the volume fraction of austenite retained during quenching can be calculated using only four parameters: M s, T d, \( \dot{T}_{\text{ms}} \) and \( \dot{T}_{\text{Td}} \).
-
(3)
AHF steel can be strengthened by flash copper precipitation; the ultrafast kinetics of which is caused by the enhancement of quenched-in vacancies on copper diffusion.
-
(4)
Bake toughening (BT) is a new processing concept analogous to the well-known bake hardening, but it is aimed at improving the ductility and toughness of the hot-stamped parts. AHF steel is fully compatible with bake process and can be bake toughened due to the elimination of the less stable block-like retained austenite.
References
China world’s largest auto production site in 2013: report, (English News, Xinhua News, 2014), http://news.xinhuanet.com/english/china/2014-01/14/c_133044814.htm. Accessed 14 Jan 2014
Z. Wang, Y. Jin, Energy Sav. Environ. Prot. (in Chinese) 7, 17 (2008)
J. Aspacher, in 1st International Conference on Hot Sheet Metal Forming of High-Performance Steel (Kassel, Germany, 2008), p. 77
K.I. Mori, Trans. Nonferrous Met. Soc. China 22, s496 (2012)
G. Berglund, in 1st International Conference on Hot Sheet Metal Forming of High-Performance Steel (Kassel, Germany, 2008), p. 175
M. Naderi, Ph.D. Thesis, RWTH Aachen, 2007
A. Mittal, Catalogue—European Edition, 2014
J.G. Speer, D.K. Matlock, B.C. DeCooman, J.G. Schroth, Acta Mater. 51, 2611 (2003)
J.G. Speer, D.V. Edmonds, F.C. Rizzo, D.K. Matlock, Curr. Opin. Solid State Mater. 8, 219 (2004)
E. de Moor, S. Lacroix, A.J. Clarke, J. Penning, J.G. Speer, Metall. Mater. Trans. A 39, 2586 (2008)
E. De Moor, C. Föjer, A.J. Clarke, J. Penning, J.G. Speer, in Proceedings of the Conference on New Developments on Metallurgy and Applications of High-Strength Steels, ed. by T. Perez (TMS, Warrendale, 2009), p. 721
A.M. Streicher, J.G. Speer, D.K. Matlock, B.C. DeCooman, in International Conference on Advanced High-Strength Sheet Steels for Automotive Applications Proceedings, ed. by J.G. Speer (AIST, Warrendale, 2004), p. 51
H. Liu, X. Lu, X. Jin, H. Dongb, J. Shi, Scr. Mater. 64, 749 (2011)
H. Karbasian, A.E. Tekkaya, J. Mater. Process. Technol. 210, 2103 (2010)
B.C. de Cooman, J.G. Speer, I.Y. Pyshmintsev, N. Yoshinaga, Materials Design: The Key to Modern Steel Products, 1st edn. (Media Grips, Bad Harzburg, 2007), p. 176
S. Yan, X.H. Liu, W.J. Liu, H.F. Lan, H.Y. Wu, Acta Metall. Sin. (in Chinese) 49, 917 (2013)
T. Lin, Ph.D. thesis, University of Chinese Academy of Sciences, 2014 (in Chinese)
Y.N. Duan, Master Theses, Northeastern University, 2012 (in Chinese)
Y. Wang, Master Theses, Northeastern University, 2013 (in Chinese)
B.V.N. Rao, G. Thomas, in International Conference on Martensitic Transformations (HIT Press, Cambridge, l979) p. 12
M. Sarikaya, G. Thomas, J.W. Steeds, in International Conference on Solid-solid Phase Transformations, ed. by H.I. Aaronson, D.E. Laughlin, R.F. Sekerka, C.M. Wayman, (TMS-AIME, Pittsburgh, 1982) p. 1421
T.Y. Hsu, X. Li, Acta Metall. Sin. (in Chinese) 19, 83 (1983)
T.Y. Hsu, J. Phys. IV 5, 351 (1995)
L. Kaufman, M. Cohen, Prog. Metall. Phys. 7, 165 (1958)
T.Y. Hsu, H.B. Chang, Acta Metall. 32, 343 (1984)
V. Raghavan, D. Antia, Metall. Mater. Trans. A 27, 1127 (1996)
C.L. Magee, in Phase Transformations, (Arner. Soc. Metals, Metals Park, Ohio, 1970), p. l15
D.P. Koistinen, R.E. Marburger, Acta Metall. 7, 59 (1959)
J.M. Chilton, P.U. Kelly, Acta Metall. 16, 637 (1968)
Y. Okamura, M. Okushima, H. Tamehiro, T. Kasuya, M. Tanaka, R. Yamaba, H. Inoue, A. Seto, Nippon Steel Technol. Rep., No. 66 (1995)
A.C. Damask, G.J. Dienes, Point Defects in Metals, 1st edn. (Gordon and Breach, New York/London, 1963)
S.T. Wu, Master Theses, Northeastern University, 2014 (in Chinese)
A. Kokosza, J. Pacyna, Arch. Mater. Sci. Eng. 31, 87 (2008)
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51034009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
Liu, W.J. An Introduction to Advanced Hot-Formed Steel for Automobile. Acta Metall. Sin. (Engl. Lett.) 27, 373–382 (2014). https://doi.org/10.1007/s40195-014-0076-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-014-0076-9