Abstract
The hydrogen embrittlement (HE) fracture of advanced high-strength steels used in lightweight automobiles has received increasing public attention. The source, transmission, and movement of hydrogen, characterization parameters, and test methods of HE, as well as the characteristics and path of HE fractures, are introduced. The mechanisms and modes of crack propagation of HE and hydrogen-induced delayed fracture are reviewed. The recent progress surrounding micro and macro typical fracture characteristics and the influencing factors of HE are discussed. Finally, methods for improving HE resistance can be summarized as follows: (1) reducing crystalline grain and inclusion sizes (oxides, sulfides, and titanium nitride), (2) controlling nano-precipitates (niobium carbide, titanium carbide, and composite precipitation), and (3) increasing residual austenite content under the reasonable tension strength of steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As early as 1875, Johnson found that the toughness of steel was greatly reduced with hydrogen embrittlement (HE) [1]. The hydrogen was generated by the interaction between steel and corrosive acidic mediums entering the material, and then a brittle fracture occurred when stress was applied [2]. Since then, it has been gradually accepted that the brittle fracture of materials can be caused by the simultaneous action of stress and corrosion under the specific combination of medium, stress, and material, i.e., “stress corrosion cracking,” which is also known as “hydrogen-induced delayed fracture” [3,4,5,6,7,8]. In the early 1970s, the United States formulated automobile fuel consumption and safety regulations, which led to the application of steel with high strength and high formability in automobiles to cope with the international oil crisis and improve automobile safety. Professor Owen from Massachusetts Institute of Technology discussed and proposed the significance of critical heat treatment for the development of American automobiles [9], while Professor Ma also proposed the concept of lightweight automobiles [10]. In the early twentieth century, the industry deeply recognized the significance of lightweight automobiles, ultimately becoming an important direction of automotive development in China and the world [11]. Advanced high-strength steel is rapidly developing in China and is widely used in the automobile industry. However, due to the insufficient matching of strength and toughness, the HE sensitivity of high-strength steel increases, and the failure caused by HE (i.e., delayed fracture) often occurs during production and usage [12,13,14,15]. This kind of fracture has the characteristics of low stress and sudden onset, causing great harm to mechanical failure, which is the latent cause of sudden product accidents.
In steel smelting, modern metallurgical methods are used to improve the purity of steel, reduce the content of hydrogen or harmful gas, refine the inclusions, and reduce the number of inclusions, all of which are conducive to improving HE resistance. Therefore, in recent years, with the increase of high-strength steel consumption, the hydrogen embrittlement of high-strength steel and advanced high-strength steel has become an important research topic. Recent advances in hydrogen trapping and embrittlement in high-strength aluminum alloys and in atomic scale quantification of hydrogen embrittlement have been reported [16, 17], respectively, illustrating the interest in this issue. This paper reviews the research progress of HE and delayed fracture induced by hydrogen.
2 Hydrogen and Steel
2.1 Source, Transfer, Direction, and Fracture Process of Hydrogen in Steel
2.1.1 Source of Hydrogen
Hydrogen embrittlement mainly occurs in the smelting process of steel materials, the steel forming process, parts manufacturing, and use process [18]. The source, transfer, direction, and fracture process of hydrogen are shown in Fig. 1 [2].
Source, transport, direction, and fracture process of hydrogen in steel. In the figure, solid lines indicating strong correlations and dashed lines indicating weak correlations [2]
Hydrogen is carried into and remains in steel through the raw materials (especially wet ones) used in steel smelting. In component production, such as hot stamping sheet metal heating and the forming process of the inhalation of hydrogen, the amount of inhalation and the atmosphere in the furnace. When heated, the reaction process of hydrogen absorption includes the bare plate without coating and an aluminum–silicon coating plate. Hydrogen atoms from these reactions may enter the metal structure (Table 1) [18].
Hydrogen molecules attach to the steel surface and break down to form hydrated protons. The surface of the steel metal combines or reacts with the hydrogen, and these hydrogen atoms then enter the steel.
2.1.2 Hydrogen Transfer
The atomic radius of hydrogen is small. Therefore, hydrogen can rapidly diffuse into most metals, especially in body-centered cubic structure metals. The quenched martensite structure in hot stamping steel components has a high-density dislocation, and hydrogen atoms can move with dislocation similar to that found in a Cottrell atmosphere. This kind of transmission is orders of magnitude faster than lattice diffusion [19]. Therefore, the cracking rate induced by hydrogen is much higher than hydrogen’s standard lattice diffusion rate.
2.1.3 Direction of Hydrogen Movement
Hydrogen tends to aggregate in many microstructures (i.e., grain boundaries, inclusions, pores, dislocations, and solute atoms). Different structures have different degrees of hydrogen capture ability.
2.1.4 Process of Fracture Caused by Hydrogen
Hydrogen-induced cracking is usually a brittle fracture. However, ductile fractures or cleavage fractures can be observed depending on the degree of damage caused by the hydrogen. Brittle fracture cracks pass through the material matrix or brittle hydride. The typical fracture morphology includes micro-void coalescence, quasi-cleavage, intergranular, and cleavage fractures, as presented in Fig. 2 [20].
Fracture morphologies of hydrogen embrittlement of a micro-void coalescence dimple, b quasi-cleavage fracture, c intergranular fracture, d cleavage fracture [20]
2.2 Form and Action of Hydrogen in Steel
Hydrogen is always in one of the following microstructure states: interstitial atomic position, free surface, subsurface, grain boundary, dislocation, and atomic vacancy. The roles of hydrogen atoms in the various microstructures of steel are as follows:
-
(1)
In metal materials with few points, line, and plane defects, hydrogen can weaken the atomic bond force and the binding energy between atoms, thus reducing the resistance of dislocation movement and promoting the plastic deformation of the crack tip.
-
(2)
In micro-cracked materials, hydrogen increases dislocation activity at the crack tip. Hydrogen increases local plasticity at the crack tip. Under constant stress, if the crack has stopped, the hydrogen atoms will move and concentrate in the crack tip under the stress, therefore it makes the crack re-propagate.
-
(3)
Hydrogen atoms gather on dislocation lines and move with dislocation to the vicinity of grain boundaries, which may promote grain boundary fracture.
-
(4)
It is possible to form assemble atom groups or brittle hydrides on some slip planes due to the entry of hydrogen atoms, which results in strain localization and hardening. Final, the plastic deformation ability of materials will be reduced.
-
(5)
Hydrogen atoms enter and shield the dislocation line, which will reduce the dislocation’s elastic stress field and increase the dislocation’s activity.
-
(6)
Nano precipitates in metal matrix play as hydrogen traps. The dislocation can bring hydrogen atoms to the hydrogen trap and concentration, which leads to stress concentration and crack formation. However, the hydrogen traps also have the function of hydrogen fixation and hydrogen dispersion.
-
(7)
In lath martensite steels with high density distortion, phase transformation strengthening, lattice distortion strengthening, and dislocation density strengthening have occurred. When the hydrogen enters in the steel, it will reduce lattice distortion and facilitate dislocation movement. However, high density dislocation and dislocation entanglement make dislocation movement difficult, especially the combination of dislocation and hydrogen atoms. When the twin martensite present in the steel, the hydrogen will aggregation at the twin boundaries, resulting in the brittleness increasing of steel.
Generally, the higher binding energy of hydrogen with hydrogen trap in various material defects, the better stability of hydrogen in hydrogen traps [21]. The binding energy of hydrogen in the material is demonstrated in Table 2.
2.3 Dissolution and Diffusion of Hydrogen in Metals
Hydrogen dissolution in metals, such as in Fe, Ni, Cu, Al, and Mg, is an endothermic reaction. The change of solubility of hydrogen in such metals with temperature can be expressed as following [22]:
where CH ([H]/[M]) is solubility of hydrogen in metals \(P_{{{\text{H}}_{2} }}\) is the hydrogen partial pressure, and T is the temperature.
Hydrogen atoms always exist in tetrahedral or octahedral spaces of the metal lattice. So the crystal structure type obviously effects on hydrogen solubility in metal. The results show that the solubility of hydrogen increases suddenly when body-centered cubic (BCC) α-Fe is transformed to face-centered cubic (FCC) γ-Fe, and then decreases when γ-Fe is transformed to δ-Fe [23]. Han et al. [24] used thermal desorption spectroscopy (TDS) to analyze the hydrogen content in the residual austenite (RA) and ferrite in transformation-induced plasticity (TRIP) steel. The study showed the hydrogen content in the RA and ferrite was 0.9 mass ppm and 0.3 mass ppm, respectively. This indicates that the FCC crystal structure has higher hydrogen solubility than the BCC crystal structure in steel.
There are two mode of diffusion of hydrogen in metals: normal and anomalous diffusion. When hydrogen atom jumps from one interstitial site to another, it is normal diffusion. When hydrogen atom diffuses along channels such as dislocations, grain boundaries and phase boundaries, it is called abnormal diffusion. The driving force of hydrogen diffusion is chemical potential difference or hydrogen concentration gradient, another one is stress induction. The microstructure of the material will have different effects on the diffusion of hydrogen, such as interface (original austenite grain boundary, martensite lath boundary, etc.), dislocation, RA, and hydrogen trapping phenomenon in the material will affect the diffusion ability and diffusion mode of hydrogen.
Hirta et al. [25] studied the migration path of a hydrogen atom in FCC, BCC, and hexagonal close packed (HCP) iron through first principles. Several conclusions show that the hydrogen atom would choose the path of the “octahedral gap—tetrahedral gap—octahedral gap” for migration in the FCC and HCP lattice gap. Through finite element analysis, when the hydrogen volume concentration in the structure is low, high angle grain boundaries play a significant role in controlling hydrogen diffusion. When the hydrogen volume concentration is high, relatively low energy traps such as dislocation are more prominent in controlling hydrogen diffusion and distribution [26]. Yzdipour et al. [27] studied the effect of grain boundaries on hydrogen diffusion by constructing microstructural models with considering different grain boundary surface ratios. The results showed that the grain size (grain boundary fraction) has a dual effect on the diffusion rate. The hydrogen diffusion rate is very low when the grain size is at the two extremes (10, 120 μm), and reaches the peak when the grain size is 46 μm. This phenomenon is mainly related to the combined effect of grain size on the surface area of grain boundaries per unit volume (which contributes to hydrogen diffusion) and the triple junctions of grain boundaries (which has the function of trapping hydrogen and hindering hydrogen diffusion). When the grain size is very small, it has a high density of grain boundary triple junctions, which has a strong trapping effect on hydrogen, and the hindrance effect on hydrogen diffusion is greater than the beneficial effect of grain boundary surface area per unit volume, thus reducing hydrogen diffusion. With the increase of grain size, the density of grain boundary triple junctions decreases gradually. In this case, the hindrance effect of hydrogen capture on reducing hydrogen diffusion is lower than the beneficial effect of unit grain boundary area on hydrogen diffusion, so the hydrogen diffusion rate increases. When the grain size increases to a certain extent, the grain boundary surface area per unit area drops sharply, and the beneficial effect of this factor on hydrogen diffusion rate drops sharply. At this time, the reduction of grain boundary triple junctions reduces the hindrance to hydrogen diffusion, which cannot compensate for the decrease of the beneficial effect of grain boundary surface area on hydrogen diffusion. Therefore, the diffusion rate of hydrogen with large grain size is slower than that of hydrogen with medium grain size. Momotani et al. [28] posited that the initial dislocation density and dislocation movement in martensitic steel have an important influence on hydrogen enrichment at the grain boundaries. Guedes et al. [29] suggested that hydrogen and dislocation interact. As the dislocations move, they drag the hydrogen Cottrell atmosphere along with them, promoting the diffusion of hydrogen. And hydrogen slows down dislocation movement by reducing the activation volume and energy. Both processes promote the accumulation of hydrogen atoms and lead to HE. Rudomilova et al. [30] found that the reversible low-energy hydrogen trap in steel greatly affected the diffusion of hydrogen in steel, while irreversible high-energy trap had no significant effect on hydrogen transport.
Due to the slow diffusion rate of hydrogen in austenite, it is different to use the thin film RA in quenched martensite as an effective hydrogen trap. However, the coherent and semi-coherent relationships formed at the interface between austenite and martensite can form a good hydrogen trap. Therefore, the effect of austenite morphology on hydrogen diffusion can be studied in more detail by using a more accurate two-dimensional diffusion model [31].
3 Test Method and Characterization Parameters of HE
Different HE test methods and characterization parameters should be selected for samples of different sizes and shapes (plate or rod), different strength levels and different service environments, as well as related generic technologies. For plate samples, constant strain bending test method [32] or U-shaped bending test method [33] can be selected. For rod specimens, constant load tensile test method [34,35,36] or slow strain rate tensile test [37] method can be preferred. For the key evaluation of the toughness property index under the tip notch of material, and the change of the related index when the hydrogen environment changes, the fracture toughness experiment method can be selected [38]. For evaluating the change of HE resistance of TWIP steel or TRIP steel before and after deformation, the cup-punching test method can be used [39]. In order to evaluate the HE resistance of ultra-high strength materials, the four-point bending test method was proposed in the literature [18], using 110 × 30 × t mm sample. The calculation equation of stress and the device is shown in formula (2) and Fig. 3.
where E is the elastic modulus, t is the thickness, and the H, A, y parameters are shown in Fig. 3. When the applied bending stress is 1260–1425 MPa and the loading sample does not crack for 150 h, it can be judged that there is no risk of delayed fracture caused by hydrogen.
The mechanical testing methods described above basically take the relative change value of mechanical properties without hydrogen damage and after hydrogen damage as the characterization parameters. Depended on the sample condition, test method and stress state beard by parts, and as well as the easy working process for test and characterization of the test specimens. Finally, the test methods and parameters are selected.
To explore the common technology and HE mechanism, the method of measuring diffusible hydrogen in steel can be adopted, which is thermal desorption spectroscopy (TDS). During the test, the hydrogen absorption rate and temperature variation curve were recorded by TDS. By analyzing the TDS curves, the stability of hydrogen in different hydrogen traps in steel can be studied. It is an effective tool to study the form of hydrogen present in steel. The diffusion coefficient of hydrogen in steel can also be measured [40], so that the hydrogen diffusion rate at different temperatures can be measured, which reflects the different stability of hydrogen adsorption of different hydrogen traps, and the binding energy of different hydrogen traps with hydrogen can be calculated, the different binding energy levels of hydrogen traps can be also determined.
4 Mechanism of HE in High-Strength Steel
4.1 Hydrogen Pressure Theory
The mechanism of HE is intuitive hydrogen pressure theory in early. That is the directional diffusion of hydrogen atoms under certain conditions to defect (point, line, plane defects, grain boundary or cracks). Its volume expansion is nearly 26 times during the hydrogen atom is gathered into hydrogen molecules, which lead to tensile stress, extend defects, holes and cracks formation, and expand the cause of material fracture. The theory is intuitive, easy to understand, which reasonably explains the formation of white spots in steel.
4.2 Brittle Hydride Formation (BHF) [41,42,43]
Brittle hydrides form after hydrogen enters transition metals such as Ti, Zr, and V. In particular, hydrogen tends to be concentrated in the stress concentration side, such as the crack tip, resulting in the formation of brittle hydride at the crack tip, which is easier for the promotion of crack propagation. However, most of these microalloying elements exist in the form of carbon and nitrogen compounds in hot-formed steels. Therefore, this mechanism makes it difficult to explain the HE in most high-strength steels.
4.3 Hydrogen-Enhanced Decohesion (HEDE) [44, 45]
In relatively complete crystals, the entry of hydrogen atoms will reduce the binding force between metal atoms, which is benefit to the dislocation movement to the grain boundary and other defects, resulting in strengthen the decohesion effect. If the hydrogen entry is uneven, it may cause deformation localization, resulting in uneven deformation and uneven distribution of damage and flow stress, finally, resulting in early failure of the material. For example, the movement of hydrogen atoms is blocked at the grain boundary and accumulates near the grain boundary, resulting in intergranular fracture.
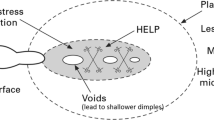
4.4 Hydrogen-Enhanced Local Plasticity (HELP) [46]
The hydrogen atom can form Cottrell atmosphere in dislocation line, which can reduce the motion activation energy of dislocation and promote dislocation movement. Hydrogen can reduce the elastic interaction between the dislocation line and the adjacent dislocation pinned by solute atom. Hydrogen can also reduce the equilibrium separation distance between the dislocations in the dislocation stacking arrangement. All of these are benefit of dislocation movement. Thus, the local plastic deformation is enhanced. A large number of dislocations carrying hydrogen atoms move to the stress concentration at the tip of the crack, resulting in the accumulation of hydrogen in these regions [45], increasing the formation of void and other defects, and finally resulting in micro-void Coalescence (MVC). The characteristics of transgranular fracture such as fish eye (or dimple) can be observed on the metal fracture surface after hydrogen charging, which is the proof of this theory.
4.5 Hydrogen-Enhanced Localized Plasticity and Hydrogen-Enhanced Decohesion (HELP + HEDE) [47,48,49]
In the quenched steel with a large amount of dislocated martensite and diffusible hydrogen, there will be microcracks and stress concentration at the tips of microcracks. Hydrogen will spread to the crack tip through the dislocation, expanding the stress concentration. The strengthening of stress concentration promotes dislocation movement to stress concentration point. The Cottrell atmosphere formed by hydrogen atoms gathered on the dislocation line can reduce the elastic stress field and dislocation movement resistance. When the dislocation carrying hydrogen atoms brings it to the crack tip, grain boundary and other stress concentration places, the hydrogen concentration increases, which reduces the polymerization energy between atoms such as grain boundary and makes the grain boundary prone to depolymerization, i.e., HELP. As a result, mixed fracture along crystal and micropit fracture in crystal appears in the hydrogen embrittlement fracture morphology of hot stamping steel.
The scanning electron microscope-electron channeling contrast imaging (SEM-ECCI) and high resolution electron backscatter diffraction (HR-EBSD) was used to observe the near-surface fracture characteristics and secondary cracks in hydrogen-induced martensitic steel cracking. The results show that the fracture characteristic possesses obvious local plastic deformation promoted by hydrogen [50]. In hot-formed martensitic steel, the fracture of hydrogen damage is the combination of intergranular fracture with quasi-cleavage fracture (IG + QC), and the HE mechanism is the combination between hydrogen-enhanced local depolymerization and hydrogen-induced local plastic deformation (HEDE and HELP). This theory has been fully verified by experiments. In 2020, Ma et al. also proposed a similar theory in their research on HE of hot stamping steel with niobium to explain the fracture characteristics of such steel after HE [8].
4.6 Adsorption-Induced Dislocation Emission (AIDE) [51, 52]
Lynch et al [52] observed pit morphology on the surface of low strength steel, and proposed the hydrogen AIDE theory. According to this theory, the entry of external hydrogen atoms would accelerate the dislocation movement on the surface of the crack tip, and the hydrogen atoms gathered at the crack tip would excite the dislocation emission and leave a hole there. The later holes’ connection would cause the crack tip propagation, in which the process just is similar to the HELP theory. Therefore, this theory is regarded as modified hydrogen enhances local plastic deformation theory.
4.7 Hydrogen-Enhanced Strain-Induced Vacancy (HESIV) [53]
Based on the strong interaction between hydrogen and vacancy, Nagumo et al. [53] proposed the theory of HESIV. It is believed that hydrogen can stabilize vacancies, and increase the number and size of voids. When the size and number of these voids grow some level, it is can be interconnected; at last the pits observed experimentally can be formed. However, the current experimental evidence is not enough to explain how vacancies grow into voids. As such, the theory remains controversial.
4.8 Formation of Low-Energy Dislocation Nanostructures Results in HE of Steel Strengthened by Nanoprecipitation [54]
Hydrogen traps in some high-strength steels were introduced in order to reduce or avoid the possibility of hydrogen accumulation and HE. When hydrogen enters in this steel, the hydrogen-enriched region shows higher dislocation mobility, promoting the formation of low-energy dislocation nanostructures. This structure acts as a hydrogen absorber, and then more hydrogen is collected, which will cause to severe misorientation cracks. A hydrogen charging test on low alloy ferritic steel with a nano-precipitated phase shows that hydrogen diffuses to the crack tip and accumulates at the tip, promoting the formation of dislocation cells. When hydrogen reaches the critical level, it leads to failure, so the low energy dislocation cell structure is considered to be the main reason for crack initiation.
In summary, there are various microscopic mechanisms of HE. The classical hydrogen pressure theory is simple and intuitive, and it is of application value to explain the occurrence of white spots in steels with high hydrogen content. By using of high-resolution 3D-APT, electron backscatter diffraction (EBSD) test technology and first principles, the location and movement locus of hydrogen atoms, as well as the hydrogen dislocation stress interaction field between the hydrogen atom and dislocation line is observed and calculated. The influence of hydrogen on dislocation movement was proposed, which accelerated the aggregation of the hydrogen atoms in the fine structure in steel or micro defect, resulting in the related mechanism of HE. Among them, the theory of HELP + HEDE can more strongly explain the occurrence of HE, and there is experimental evidence of fracture observation.
5 Methods and Way to Improve HE Resistance
5.1 Grain Size Refinement
The austenite grain, martensite lath, and lath packet of hot stamping steel 22MnB5 are refined through niobium and vanadium composite microalloying (Fig. 4). Grain refinement can effectively increase the grain boundary area, including grain boundary, lath packet boundary, and lath boundary, and increase the number of low angle grain boundaries which can effectively absorb diffusible hydrogen, reduce the sensitivity of hydrogen-induced cracking, and increase the threshold of the hydrogen crack propagation, therefore, it can further inhibit hydrogen-induced cracking. After niobium and vanadium composite microalloying, niobium and vanadium carbide precipitation will occur. Niobium carbide precipitation can be used as a high energy hydrogen trap. Grain refinement can effectively increase the area of grain boundaries, including grain boundaries, lath bundle boundaries, and lath boundaries, which can effectively absorb diffusible hydrogen and improve HE resistance. The results of the constant bending experiment of U-shaped samples, the constant load tension experiment, the determination of hydrogen diffusion coefficient in the lattice, and the slow strain rate tension experiment all confirm that niobium-vanadium composite microalloying can effectively improve the hydrogen-induced delayed fracture resistance.
5.2 Strength and Microstructure Matching
The reasonable control of the materials’ strength, toughness and microstructure matching is critical for improving the resistance to HE. Usually, the delayed fracture resistance to hydrogen decreases as the material’s strength increases and toughness decreases, as shown in Fig. 5. Steel with tensile strength below 1000 MPa has low sensitivity to HE, that is, more hydrogen can be accommodated in steel without HE. When the tensile strength reaches 1500 MPa, the HE sensitivity increases sharply, and the critical hydrogen content is below 0.65 ppm, the critical hydrogen content is defined as the hydrogen content at which the tensile strength decreases by 30%.
The sensitivity of HE is also related to the matching of strength and toughness, microstructure characteristics. Figure 6 shows the microstructure comparison of hot-formed steel with different strength levels and compositions. It can be seen from the composition and microstructure comparison of different steels. The refinement of grain and martensite structure has an obvious effect on the HE resistance of microalloying, especially composite microalloying for hot-formed steels of different strength grades.
With the increase of steel strength, microstructure refinement becomes more important, especially when the steel strength is above 1800 MPa. When the strength of steel increases, the extreme sharp cold bending property decreases, which is not conducive to the improvement of the comprehensive strength and toughness properties. Especially when the carbon content is bigger than 0.35%, twin martensite will appear in the quenched structure. The twin martensite is hard and brittle, and hydrogen atoms will aggregation near twin martensite, which will increase the risk of HE. Microstructure refinement of ultra-high-strength steel can have both high strength and better toughness, so as to improve the hydrogen brittleness resistance of the material.
5.3 Hydrogen Trap Formation
The hydrogen trap is the position where the metal quickly adsorbs and captures hydrogen atoms. In 1948, Darken and Smith [55] proposed the concept. When the binding energy reaches 30 kJ/mol, the hydrogen trap is called a shallow trap. When the binding energy reaches 58 kJ/mol, it is called a deep hydrogen trap. When the binding energy is at 30–58 kJ/mol, it is usually referred to as a medium hydrogen trap. The higher the binding energy, the stronger the capture ability of hydrogen. The binding energy for hydrogen atoms of the complete crystal is usually 5 kJ/mol. There is an internal stress field around the crystal defect and the second phase. It can interact with hydrogen atoms and adsorb hydrogen around it, forming a trap to capture hydrogen.
The carbides of microalloy elements (Nb, V, and Ti) in steels are strong or quite strong hydrogen traps. These microalloy carbides not only have the effect of precipitation hardening but also have the function of refining the grain. Some carbide precipitations also inhibit the precipitation of harmful cementite in the tempering process of quenching steel [56]. However, it is proposed in the literature that the interface between cementite and matrix precipitated in quenched steel is also a strong hydrogen trap [57]. Several studies have examined the correlation condition produced by the hydrogen trap by VC, TiC, and NbC for the surface center cube carbide. The ability to capture hydrogen for the microalloy carbide reduces on NbC > TiC > VC at the same atomic concentration [58].
5.3.1 NbC
NbC is a strong hydrogen trap. Chen [59] used atom probe tomography (APT) technology with cold chain sample transmission to directly observe the deuterium atoms bound at the interface of NbC and matrix. A coherence interface between fine (< 10 nm) NbC and matrix and a non-coherence interface between a large (10–25 nm) NbC and matrix are high-energy hydrogen traps. The 3D-APT atom distribution image at the interface between NbC and α-Fe proves that hydrogen (deuterium atom) is adsorbed at the interface between niobium carbide precipitation and α-Fe.
Shi et al. [60] used the high-resolution electron microscope and density functional theory (DFT) test results to determine the atomic arrangement at the interface between NbC and α-Fe. Combined with the calculation result from the first principle, the NbC/α-Fe semi-coherence lattice interface was confirmed, and the position of the hydrogen depth trap in the core of the adaptive dislocation was determined, where its binding energy is 81 kJ/mol. The NbC size of 10 ± 3.3 nm shows the strongest ability to capture hydrogen. When the sample was subjected to external force, the NbC particles precipitated in the steel exhibited a pinning effect, and at the same time hindered the movement of grain boundaries. It can also hinder the dislocation move. Therefore, it also indirectly hinders the movement of hydrogen along with the dislocation and the accumulation of hydrogen [41].
Numerous studies have shown that the grains in the {111} // ND texture can rotate in the direction of shear stress in tension, extensive plastic deformation can be accumulated, and the driving force of the crack propagation can be reduced [61, 62]. This is conducive to improving the hydrogen-induced fracture resistance. This view is confirmed by the fracture observation and the orientation analysis of the crystal. However, the presence of niobium carbide prevents the austenite grains size from growing up, thus preventing the formation of the {111} // ND texture. It is a disadvantage of improving hydrogen-induced delayed fracture resistance [50].
In order to deeply understand the effect of microalloyed niobium on improving the hydrogen embrittlement resistance of steel, the dissolution generation and shape control of microalloyed carbides should be studied based on the solution product equation of Niobium microalloyed [63], so as to obtain the size, distribution and shape of niobium microalloyed carbides that have the most favorable influence on steel properties.
A schematic diagram of the influence and effect of NbC particles on the HE resistance of martensitic steel was proposed [50], this diagram showed that: NbC particles prevented the growth of austenite grains at the original austenite grain boundaries. When martensitic transformation occurred, lath martensitic grains were generated, and a large number of lath boundaries (or ∑3 boundaries) were generated. The nanosized NbC particles will evolve in two ways. The first is the evolution of hydrogen trap, the process is as follows: (1) generate NbC to induce irreversible hydrogen trap; (2) increase the hydrogen trap of primeval austenite grain boundaries and martensite lath boundaries. The distribution of hydrogen is homogenized to prevent hydrogen from the HEDE. The other path is through crystallography and dislocation evolution, the process is as follows. (1) The formation of hydrogen pinning dislocation air mass and hydrogen capture to prevent or reduce the harmful effects of HELP and dislocation proliferation. (2) Decrease the fraction of ∑3 boundary, increase crack growth resistance to prevent the HELP. (3) Weaken the beneficial effect of {111}//ND texture, that is, to promote fracture by strengthening the resistance of crack propagation. The first four points of the above two technical paths are beneficial. Nano-sized NbC is beneficial to the improvement of HE resistance, while (3) in the second path is harmful to the improvement of HE resistance because it weakens the formation of {111}//ND texture.
5.3.2Titanium Carbide (TiC), Titanium Nitride (TiN) and Dual Precipitates (TiC and Cu).
TiC is a NaCl crystal structure, and it is a typical, people interested in precipitation particle of a hydrogen trap in steel. Wei et al. [63] observed the microstructural variation of TiC at different tempering temperatures by using of high-resolution TEM. When the tempering temperature is ranging from 550 to 800 °C the morphology of titanium carbide is a slat and structurally a semi-coherent state with the matrix. When the tempering temperature is ≥ 800 °C the shape of titanium carbide is disk-shaped, which is an incoherent state with the matrix. The thickness of the disk is gradually increased. A flat shape gradually becomes spherical. From the initial precipitated strip of a semi-coherent lattice state with a size of 16 nm, then gradually grow up to a hemispheric having a certain thickness and diameter 40–2 μm [64]. The binding energy of fine coherent TiC particle in medium carbon steel with hydrogen is 46–59 kJ/mol. The binding energy of the titanium carbide particles having semi-coherent or incoherent in medium steel with hydrogen is 80–95 kJ/mol. However, the ability and quantity of hydrogen atoms trapped by titanium carbon increases with the tempering temperature, that is, the coarsening of titanium carbide particles is reduced.
In 2010, the direct observation for titanium carbide as hydrogen trap to capture hydrogen atoms was carried on by using of 3D APT [65]. Chen was carried on the direct observation for vanadium, molybdenum carbide as hydrogen trap to capture hydrogen atoms by using 3D APT [66].
Adding Cu and Ti element to the steel, the resulting in nano-sized composite precipitate has a better capture capacity of hydrogen [67]. Experimental steel is austenitized at 1200 ℃ to make the Ti precipitation particles completely solution. Then, the quenching and tempering process is performed. The Cu particles and titanium carbide composite precipitation are distributed in the matrix. The binding energy for hydrogen atom captured by composite precipitated particles was determined by using of TDS is 37.7–58 kJ/mol. With variation of the tempering temperature, the cause produced binding energy scope is resulting from interaction of the Ti, Cu composite precipitation phase which increases the position of the hydrogen trap, but also limits the size of the hydrogen trap. Ti, Cu composite precipitation which improved high-strength steel HE resistance has achieved good results reported in the recently published literature [68].
In addition, in ultra-high-strength steel, using Cu as a microalloy element is a new alloying idea. At the same time, Cu is cheaper than general microalloy elements, and Cu has a better anti-environmental corrosion ability. When combining of titanium with nitrogen, TiC will be formed which possess cubic structure. It is possible that the titanium nitride will become a source of cleavage, which is unfavorable to the improvement of steel strength and toughness. But in hot forming boron steel smelting, Ti will be usually used to deoxygenation and nitrogen fixation and play the role of boron in improving the hardenability of steel. However, the amount and size of TiN precipitation must be controlled. Therefore, the reasonable control of the addition amount of Ti and the solution and precipitation process of titanium carbide can play the role of titanium carbide in improving the HE resistance of hot-stamping steel.
5.3.2 VC
VC particles of appropriate sizes can act as hydrogen traps and effectively improve the delay fracture resistance of steel. The VC particles act as hydrogen traps, and the binding energy with hydrogen is 33–35 kJ/mol [69]. It is a moderate hydrogen trap. Hydrogen atoms can be in the VC crystal vacancy or at the VC particles’ interface and matrix [65]. According to the solution product equation of VC [70], the dissolution temperature of VC is relatively low. In the presence of manganese, the dissolution temperature of VC is lower. When the heating temperature is > 750 °C VC in general steel can start to dissolve during the general quenching with a heating temperature of 880–930 °C (e.g., 22MnB5 hot-stamping steel), and a majority of the VC particles will be dissolved. The dissolution of VC particles can effectively enhance the hardenability of steel [71]. During the tempering process after quenching, the precipitated particles of VC can improve the delayed fracture resistance of the steel. During the austenitic heating, insoluble VC particles can also refine the grains. Therefore, the microalloy element V has multiple beneficial effects in steel.
5.3.3 Microalloy Carbon and Nitride Composite Precipitation Phase
Nb, V, Ti are the most commonly used microalloying elements in steel, tungsten and molybdenum are commonly used carbide forming elements in steel, which are elements effectively improving hardenability of steel. The compound applications of these elements can effective avoid the shortcoming of single addition, and fully play the compound influence of these elements on the property of steel. In recent years, the niobium vanadium composite microalloy hot stamping forming steel, titanium molybdenum, niobium molybdenum, high hardenability, high delay fracture resistance of hot-stamping steel, and tungsten molybdenum niobium vanadium composite microalloy, high fatigue hot-stamping wheel steel at all are developed [12]. The niobium and niobium vanadium composite microalloyed 22MnB5 steel was charged with hydrogen, and the tensile test was performed after saturation.
The strength and ductility of 22MnB5 can be improved by microalloying, especially by tensile after hydrogen charging. The niobium-vanadium composite microalloying has higher strength and higher elongation. When only niobium microalloyed, the strength decreased to a certain extent after hydrogen charging, while the strength and elongation of 22MnB5 without microalloying decreased greatly after hydrogen charging. These contrasts indicate that, Microalloying and composite microalloying significantly improve the hydrogen brittle resistance. Under the hydrogen-charged current density of 3.0A/m2, the samples were saturated with hydrogen filling. The tensile test was then performed. Measure results of elongation loss are: The elongation rate loss of 22MnB5 is 58%; the elongation rate loss of 22MnB5 with niobium microalloying is 46%. However, the niobium-molybdenum composite microalloying, the elongation rate loss of 22MnB5 with niobium-molybdenum composite microalloying is 20%. These data suggest that the significant effect of composite microalloying on increasing the delay fracture resistance of 22MnB5 martensite grade steel.
0.015% or 0.03% titanium added to niobium-molybdenum hot-stamping steel to investigate effect on the HE resistance of the steel [72]. The results show that titanium carbide generated by the addition of titanium, can serve as the nucleation and growing position of niobium carbide and molybdenum carbide. The formation of the fine compound niobium-titanium carbide, and niobium, titanium and molybdenum carbide possess the pinning effect, therefor it can prevent the grains from growing up. When the titanium content increases, the precipitation of TiC will increase, and then the effect of preventing grain growth will increase. Measurements of the binding energy of the composite carbide to hydrogen by using of TDS indicate that the binding energy of TiC particles to hydrogen was increased from 83 kJ/mol (0.015% Ti) to 106 kJ/mol (0.030% Ti). Refinement of the precipitated phase particles also increases the position of the stable hydrogen trap.
The increase of Ti content extend temperature range of Ti (C, N) precipitation, promote the growth in its volume fraction, but also promote precipitation of (Ti, Nb, Mo) C fine particles. Complex small (Nb, Mo, Ti) C precipitation phase, can greatly increase the activation energy of hydrogen traps and the number of high-energy hydrogen traps. After filling hydrogen, the elongation loss is very small, which showed good resistance to HE [72].
The comparison of hydrogen trapping capacity between undissolved TiC and fine (Ti, Mo)C precipitated by tempering shows that: Mo atoms replaced part of the Ti atoms in TiC, resulting in small (Ti, Mo)C, which binding energy with hydrogen is less than that of TiC, therefore, small (Ti, Mo)C particle is a low energy hydrogen trap [73]. In a 40CrMo quenched tempered steel, composite carbide precipitation has a significant impact on the HE resistance of steel. After quenching at 880 °C and 450 °C tempering, there are insoluble globular titanium-molybdenum carbide precipitations in steel. The binding hydrogen energy is 142.6 kJ/mol, but such particles cannot absorb hydrogen by an electrochemical hydrogen charge at room temperature, and it has no effect on HE in this process. Fine titanium molybdenum carbide precipitation induced by tempering, which binding energy to hydrogen is only 17.0–21.1 kJ/mol. And it can serve as a provisional hydrogen trap for electrochemical hydrogen charge at room temperature. The hydrogen trapped by the fine tempering-induced precipitation of (Ti, Mo)C will slowly diffuse out of the precipitation phase, and the increase of diffused hydrogen in the material will induce HE. The (Ti, Mo)C precipitation under this structure is not a beneficial but a harmful hydrogen trap.
To sum up, hydrogen trap is varied, the size of the specific to observe through the experiment of hydrogen trap, binding energy of trap with hydrogen, precipitation particle structure, the structure of interface between precipitation particle and matrix, and other factors to determine the nature of the hydrogen trap. Boning et al. showed that by DFT calculation, the hydrogen binding energy of MC/BCC-Fe PBs (phase boundary) was strongly dependent on the type of MC, occupation site, and foreign alloying elements [74]. The segregation of exogeneous alloyed atoms in PBs and the relationship between the local atomic environment (atoms at tetrahedral or octahedral apex) and the hydrogen binding energy are discussed. It is considered that exogeneous alloyed atoms are more likely to be partitioned in PBs than not in grains, which results in local relaxation of interface atoms and changes the local chemical environment of hydrogen atoms. It is generally believed that the binding energy of hydrogen is high in regions with low local electron density, and exogenously alloyed atoms tend to reduce the local electron density. However, the stability of hydrogen trapping is related to the ability of Fe atoms to provide electrons. Thus, although the local charge density can be reduced by exogenously alloyed atoms, an unfavorable trap will still occur due to the restriction of binding conditions. Therefore, not all carbide precipitation is effective for hydrogen capture. The Bader volume of hydrogen reflects by its size and shape, during the change of charge characteristics in the local atomic environment. Therefore, it is considered to be an effective descriptor for the quantifying thermodynamics of hydrogen trapping energetics at MC/BCC-Fe PBs.
5.4 Residual Austenitic Control
The RA is a toughness microstructure in advanced high-strength steel, such as in dual phase (DP) steel, complex phase steel, TRIP steel, manganese TRIP steel, martensite steel, and quenching and partitioning (Q&P) steel. Austenite belongs to the face center cubic structure; it has strong toughness and hydrogen storage capacity. However, the diffuse rate of hydrogen in austenite at room temperature is far less than that in martensite or ferrite. When undergoing electrochemical hydrogen charging, the rate at which hydrogen enters the austenite is very slow. Based on these characteristics, a series of studies, experiments, and explorations about the impact of austenite on HE has been carried out [15, 75]. The major results and progress are as follows:
-
(1)
The RA has a high ability to dissolve hydrogen, but the hydrogen diffuses very slowly in RA. Austenite has strong toughness, so it is a stronger hydrogen trap than ferrite. At room temperature, the diffusion coefficient of hydrogen in low alloy martensite steel is DH = 3.7 × 10–11 m2/s [76], and that in austenite stainless steel is DH = (2–7) × 10–16 m2/s [77]. Therefore, it is difficult for hydrogen atoms to diffuse into austenite when undergoing electrochemical hydrogen charging. Still, the interface is the best position to catch hydrogen as a strong hydrogen trap.
-
(2)
The morphology of RA in steel consists of strip, slice, and block. Its stability is related to the chemical composition of RA. For the mechanical stability of RA with higher carbon and manganese, the mechanical stability of granular or small volume is higher. Namely, the mechanical stability of RA has chemical stability, size stability, and shape stability. Improving the stability of RA in steel is an effective means to improve the HE resistance of steel containing RA. Firstly, the chemical stability of RA can be improved by increasing carbon, manganese, molybdenum and other solid dissolved gold elements in RA. Reducing the martensite transition point (Ms) and increasing the strain martensite transition point (Md), which is, improving the mechanical stability of austenite. The morphology and size of austenite can also be changed by different heat treatment processes to improve its mechanical stability.
-
(3)
If martensitic transformation occurs in RA, the delayed fracture resistance of steel will be reduced. This transformation is a deformation-induced martensite transformation, such as tensile deformation. The martensite-induced by the force deformation during the use of components is hard and brittle, which leads to the decrease of steel strength and toughness [75] and the increase of hydrogen embrittlement sensitivity. This again shows that the properties of the material and the function of the components and parts are two concepts [78]. Another reason is that the hydrogen enriched at the interface between RA and matrix martensite is inherited into the deformable martensite, which increases the sensitivity of hydrogen embrittlement [79]. Since the strain-induced martensite transformation and the volume expansion generated by the transformation will deform the surrounding matrix and form a large number of dislocations, the high phase transition stress and hydrogen-enriched strain-induced martensite will have a high brittle ability, which may provide a large number of nucleation sites for microcracks and be more sensitive to HE. The size of RA increases the content of carbon decreases, the content of manganese decreases, and the stability of RA decreases. Both the TRIP effect and TWIP effect in TRIP steel and TWIP steel lead to the increase of hydrogen embrittlement sensitivity. In these two types of steels, increasing the stability and amount of RA has an important impact on the improvement of hydrogen embrittlement resistance of these steels, which are important considerations for the improvement of component function in automobile material selection and service conditions.
-
(4)
The addition of 1% or 3% Cu in some high manganese steels, such as Fe-0.8C-15Mn-7Al, can improve the stability of austenite and increase the volume fraction of austenite. At the same time, a large number of Cu-rich B2 particles precipitated at the interface of austenite show a semi-coherent relationship, which increases a large number of mismatch dislocations at the interface [80]. The hydrogen embrittlement resistance is effectively improved.
There are various forms of RA in steel, and its stability is affected by morphology, size, chemical composition and heat treatment process. The presence of RA not only enhances the strength and toughness of steel, but also effectively improves the hydrogen embrittlement resistance. The stability of RA is affected by many factors. The beneficial effect of RA on the strength, toughness and hydrogen embrittlement resistance of steel can be maintained only when the mechanical stability of RA is improved and no strain-induced martensite transformation occurs during use or in the manufacturing process of parts.
5.5 Elimination of Residual Stress
Residual stress is ubiquitous in metallic materials. When advanced high-strength steel is formed with stamping, the residual stress is generated due to its deformation heterogeneity in the micro and macro scale [81]. Residual stress can be divided into microscopic residual stress and macroscopic residual stress. Micro-residual stress exists in different physical phase structures and grains inside or at the grain boundary. Residual stress is vital for the crack origin of hydrogen-induced delayed fracture. Macroscopic residual stress acts on the workpiece scale and controls the crack propagation process of delayed fracture. The concentration point of microscopic residual stress is often in the second phase, inclusions, or other microscopic defects, including cracks. Residual stress diffuses the hydrogen atoms or makes them concentrate toward the tensile stress or the abovementioned defects, which is a harmful factor in reducing the HE resistance of the material. In addition, the different mechanical response characteristics of the material itself, such as Bauschinger effect, will also cause hydrogen atoms to concentrate at the tensile stress of the interphase stress or microstructure defects, resulting in the composite effect of hydrogen damage and external force, and accelerate the destruction process of the material. However, further research is needed in this area. It is more difficult to study because of more and more complex factors, but the relevant research results have important reference value for the using safety of components.
In steel smelting, the use of modern metallurgical methods to improve the purity of steel, reduce hydrogen or harmful gas content, refine inclusions, reduce the number of inclusions, which are conducive to improve hydrogen embrittlement resistance.
6 Summary
The demand for lightweight automobiles and safety improvement leads to a variety and amount increasing of advanced high-strength steel applications. HE is an essential factor affecting the performance and reliability of the component of high-strength steel. This paper reviews the research progress of HE concerning advanced high-strength steel for automobiles. The present paper introduces the source and transmission of hydrogen in high-strength steel or automobile parts, interaction between hydrogen atoms and microscopic defects in steel, and the characterization parameters and measurement methods of HE. The proposed hydrogen embrittlement mechanism is reviewed based on the fracture characteristics of hydrogen-induced delayed fracture and the interaction between hydrogen atom and dislocation. Based on the interaction theory of hydrogen atom and dislocation, the factors affecting HE are discussed in detail. The methods for improving HE resistance are proposed as follows: refining grain size and hydrogen trap control fully playing, the beneficial effect of composite microalloying the RA and reasonably controlling residual stress, this can effectively improve the hydrogen embrittlement resistance of high-strength steel and advanced ultra-high-strength steel, and meet the reliability requirements of high-strength components.
References
W.H. Johnson, Nature 11, 393 (1875)
W.T. Anthoy, I.M. Bernstein, Stress Corrosion Cracking and hydrogen embrittlement, Review of advances in physical Metallurgy (Metallurgical Industry Press, Beijing, 1985), pp.482–505
P. Hirthj, Metall. Trans. A 11, 861 (1980)
M. Wang, E. Akiyamae, K. Tsuzaki, Corros. Sci. 49, 4081 (2007)
Q. Liu, A. Atrens, Corros. Rev. 31, 85 (2013)
M. Dadfarnia, A. Nagao, S. Wang, M.L. Martin, B.P. Somerday, P. Sofronis, Int. J. Fract. 196, 223 (2015)
H. Bhadeshia, ISIJ Int. 56, 24 (2016)
M.T. Ma, H.Z. Lu, Y.S. Chen, B.Y. Liu, Automobile Technol. Mater. 4, 1 (2021)
S.W. Owen, Met. Technol. 7, 1 (1980)
M.T. Ma, B.R. Wu, Duplex Steel-Physical and Mechanical Metallurgy (Metallurgical Industry Press, Beijing, 1988), pp.1–10
M.T. Ma, H.L. Yi, H.Z. Lu, Eng. Sci. 9, 71 (2012)
M.T. Ma, S.W. Jiang, G.Y. Li, Y. Feng, J. Zhou, H.Z. Lu, F.H. Li, Mater. Mech. Eng. 44, 1 (2020)
Y.J. Zhang, W.J. Hui, H. Dong, Acta Metall. Sin. Engl. Lett. 49, 1153 (2013)
J.Y. Li, H.B. Zhang, W.Z. Tan, G.P. Zhou, X.H. Wang, D.G. Ma, C.L. Liu, Analysis of Delayed Cracking Of Hot Stamping Steel, Study on Hydrogen-Induced Delayed Fracture of Chinese Automobile EVI and High Strength Steel (Beijing Institute of Technology Press, Beijing, 2019), pp.318–324
R.G. Davies, Metall. Trans. A 12, 1667 (1981)
H. Zhao, P. Chakraborty, D. Ponge, T. Hickel, B. Sun, C.H. Wu, B. Gault, D. Raabe, Nature 602, 437 (2022)
S. Hu, Y. Yin, H. Liang, Y.Z. Zhang, Y. Yan, Mater. Des. 218, 110702 (2022)
C.B. Sebastian, S. Thierry, A. Anis, Hydrogen Embrittlement resistance of Al-Si coated 1.8GPa press hardened steel solutions for body-in-white(BIW) application//7 international conference for hot sheet metal forming of high-performance steel CHS2, 2019, June 2–5th, Lulea Sweden, edited by Mats Oldenburg, Jens Hardell, Daniel Casellas. 2019: 179–189.
T. John, W.T. Anthony, I.M. Bernstein, J.R. Rebecca, Metall. Trans. A 7, 821 (1976)
T. Shinko, G. Hénaff, D. Halm, G. Benoit, G. Bilotta, M. Arzaghi, Int. J. Fatigue 121, 197 (2019)
Q.H. Liu, H.W. Tang, T.Z. Si, Mater. Prod. 51, 134 (2018)
R.A. Oriani, Acta Metall. 18, 147 (1970)
E. Fricke, H. Stüwe, G. Vibrans, Metall. Mater. Trans. A 2, 2697 (1971)
J. Han, J.H. Nam, Y.K. Lee, Acta Metall. 113, 1 (2016)
K. Hirata, S. Iikubo, M. Koyama, K. Tsuzaki, H. Ohtani, Metall. Mater. Trans. A 49, 5015 (2018)
T. Das, R. Chakrabarty, J. Song, S. Yue, Int. J. Hydrog. Energy 47, 1343 (2022)
N. Yazdipour, A.J. Haq, K. Muzaka, E.V. Pereloma, Comput. Mater. Sci. 56, 49 (2012)
Y. Momotani, A. Shibata, T. Yonemura, B. Yu, N. Tsuji, Scr. Mater. 178, 318 (2020)
D. Guedes, L. Cupertino Malheriros, A. Oudriss, S. Cohendoz, J. Bouhattate, J. Creus, F. Thebault, M. Piette, X. Feaugas, Acta Metall. 186, 133 (2020)
D. Rudomilova, T. Proek, P. Salvetr, A. Knaislová, G. Luckeneder, Mater. Corros. 71, 909 (2019)
A. Turk, G.R. Joshi, M. Gintalas, M. Callisti, E.I. Galindo-Nava, Acta Metall. 194, 118 (2020)
T/CSAE 155–2020 U-shaped constant bending load test method for hydrogen-induced delayed fracture sensitivity of ultra-high strength automotive steel plates.
M.T. Ma, G.D. Wang, D.F. Wang, Introduction to Automotive Lightweight (Chemical Industry Press, Beijing, 2020), pp.158–178
J.S. Kim, Y.H. Lee, D.L. Lee, K.T. Park, C.S. Lee, Mater Sci. Eng. A 505, 105 (2009)
M. Wang, E. Akiyama, K. Tsuzaki, Corros. Sci. 48, 2189 (2006)
S. Hiroshi, T. Kenichi, Y. Hagihara, Strain-Aged High-Strength Steel with High-Resistance to Delayed Fracture and Its Mechanism. Paper presented at Material Mechanics Conference, The Japan society of Mechanical Engineers, Tokyo, 24–26 October 2007.
S. Takagi, Y. Toji, M. Yoshino, K. Hasegawa, ISIJ Int. 52, 316 (2012)
G.L. Pioszak, R.P. Gangloff, Corrosion 73, 1132 (2017)
B. Sun, J.P. Lin, X.L. Gao, Hot Work. Technol. 44, 183 (2015)
K. Bergers, E. Camisão de Souza, I. Thomas, N. Mabho, J. Flock, Steel Res. Int. 81, 499 (2010)
Kirchheimr, Acta Metall. 55 5139 (2007)
Kirchheimr, Acta Metall. 55 5129 (2007)
G. Westlaked, Argonne Natl Lab. 3, 1 (1969)
A. Orianir, Ber Bunst für Phys. Chem. 76, 848 (1972)
D. Beachemc, Metall. Mater. Trans. B 3, 441 (1972)
K. Rnbaumh, Sofronisp, Mater. Sci. Eng. A 176 191 (1994)
Y.A. Du, L. Ismer, J. Rogal, T. Hickel, J. Neugebauer, R. Drautz, Phys. Rev. B 84, 144121 (2011)
T. Yoshimasa, K. Hikaru, A. Ryo, A. Shigeo, H. Kimitaka, Y. Yamamoto, M. Shunsuke, T. Nobuo, Mater. Sci. Eng. A 661, 211 (2016)
C.S. Marchic, B. Somerdayb, Technical reference on hydrogen compatibility of materials. Geology (2005). https://doi.org/10.2172/1055634
S.Q. Zhang, J.F. Wan, Q.Y. Zhao, J. Liu, F. Huang, Y.H. Huang, X.G. Li, Corros. Sci. 164, 108345 (2020)
A. Pundta, R. Kirchheimr, Annu. Rev. Mater. Res. 36, 555 (2006)
S. Lynchs, Corros. Rev. 30, 105 (2012)
M. Nagumom, Fundamentals of Hydrogen Embrittlement. Springer, 2016
P. Gong, J. Nutter, P.E.J. Rivera-Diaz-Del-Castillo, W.M. Rainforth, Sci. Adv. 6, 6152 (2020)
L.S. Darken, R.P. Smith, Corrosion 5, 1 (1949)
H. Wu, B. Ju, D. Tang, R. Hu, A. Guo, Q. Kang, D. Wang, Mater Sci. Eng. A 622, 61 (2015)
A. Nagao, K. Hayashi, K. Oi, S. Mitao, ISIJ Int. 52, 213 (2012)
F.G. Wei, T.K. Hara, Adv. Mater. (2011). https://doi.org/10.1007/978-3-642-17665-4_11
Y.S. Chen, H.Z. Lu, J.T. Laing, Science 367, 171 (2020)
R.J. Shi, Y. Ma, Z.D. Wang, Acta Metall. 200, 686 (2020)
M. Masoumi, L.P.M. Santos, I.N. Bastos, S.S.M. Tavares, M.J.G. da Silva, H.F.G. de Abreu, Mater. Des. 91, 90 (2016)
V. Venegas, F. Caleyo, T. Baudin, J.H. Espina-hernández, J.M. Hallen, Corros. Sci. 53, 4204 (2011)
M.T. Ma, Advanced Automotive Steel (Chemical Industry Press, Beijing, 2008), pp.375–399
S.M. Lee, J.Y. Lee, Acta Metall. 35, 2695 (1987)
J. Takahashi, K. Kawakami, Y. Kobayashia, T. Taruib, Scr. Mater. 63, 261 (2010)
F.G. Wei, K. Tsuzaki, Metall. Mater. Trans. A 37, 331 (2006)
Y.C. Lin, I.E. McCarroll, Y.T. Lin, W.C. Chung, J.M. Cairney, H.W. Yen, Acta Mater. 196, 516 (2020)
Y. Si, Y.S. Tang, X. Zhou, K.J. Li, Y.L. Ma, M.T. Ma, Automob. Technol. Mater. 6, 16 (2022)
J. Lee, T. Lee, Y.J. Kwon, D.J. Mun, J.Y. Yoo, C.S. Lee, Met. Mater. Int. 22, 364 (2016)
Q.L. Yong, The Second Phase in Iron and Steel (Metallurgical Industry Press, Beijing, 2006), pp.146–147
M.T. Ma, Z.G. Li, Spec. Steel 10, 11 (2001)
J. Yoo, M.C. Jo, M.C. Jo, S. Kim, J. Oh, J. Bian, S.S. Sohn, S. Lee, Mater. Sci. Eng. A 791, 139763 (2020)
X. Jin, L. Xu, W. Yu, K. Yao, J. Shi, M. Wang, Corros. Sci. 166, 108421 (2020)
B. Zhang, J. Su, M. Wang, Z. Liu, Z. Yang, M. Militzer, H. Chen, Acta Mater. 208, 116744 (2021)
Y. Zhang, W. Hui, X. Zhao, C. Wang, W. Cao, H. Dong, Eng. Fail. Anal. 97, 605 (2019)
J. Han, J.H. Nam, Y.K. Lee, Acta Mater. 113, 1 (2016)
M.R. Louthan Jr., R.G. Derrick, Corros. Sci. 15, 565 (1975)
M.T. Ma, Heat Treat. 29, 1 (2014)
X. Zhu, W. Li, H.S. Zhao, L. Wang, X.J. Jin, Int. J. Hydrog. Energy 39, 13031 (2014)
J. Yoo, M.C. Jo, D.W. Kim, H. Song, M. Koo, S.S. Sohn, S. Lee, Acta Mater. 196, 370 (2020)
G.E. Totten. Handbook of Residual Stress and Deformation of Steel (ASM international, 2002)
V. Renzo, M.T. Michele, B. Linda, S. Corsinovi, D.C Daniele, Hydrogen Induced Delayed Fracture in hot Stamped Al-Si Coated Boron Steels, in 7th International Conference Hot Sheet Metal Forming of High-performance Steel June 2–5, (Lulea, Sweden, 2019), p. 191–200
M.T. Ma, Y.S. Zhang, Research progress in Hot Stamping of Ultra-High Strength Steel, Automotive Advanced Manufacturing Technology Tracking Research 2016 (Beijing Institute of Technology Press, Beijing, 2016), pp.15–75
S.M. Myers, S.T. Picraux, J. Appl. Phys. 50, 5710 (1979)
A.I. Shirley, C.K. Hall, Scr. Mater. 17, 1003 (1983)
W.Y. Choo, J.Y. Lee, Metall. Trans. A 13, 135 (1982)
I. Maroef, D.L. Olson, M. Eberhart, G.R. Edwards, Metall. Rev. 47, 191 (2002)
F.G. Wei, T. Hara, K. Tsuzaki, Metall. Mater. Trans. B 35, 587 (2004)
S.M. Lee, J.Y. Lee, Metall. Trans. A 17, 181 (1986)
Y.D. Park, I.S. Maroef, D.L. Olson, Weld. J. 81, 7 (2002)
Acknowledgements
This work was financially supported by the State Key Laboratory of Vehicle NVH and Safety Technology (NVHSKL-202104), and the innovation research group of universities in Chongqing (CXQT21030, CXQT19031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that there are no conflicts of interest to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, MT., Li, KJ., Si, Y. et al. Hydrogen Embrittlement of Advanced High-Strength Steel for Automobile Application: A Review. Acta Metall. Sin. (Engl. Lett.) 36, 1144–1158 (2023). https://doi.org/10.1007/s40195-022-01517-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-022-01517-0