Abstract
In recent years, application of high-strength steel in automobile bodies has been expanded to improve collision safety and fuel efficiency. However, it has been reported that for high-strength steels with strengths of at least 780 MPa, cross-tension strength (CTS) in resistance spot-weld joints decreases as steel strength increases. Methods of improving joint strength include post-heating. Although many studies have been conducted on post-heating, the temperature and microstructural changes required by post-heating to improve joint strength, such as a decrease in hardness and relaxation of solidification segregation elements, remain unclear. The effect of post-heating on CTS was investigated through resistance spot-weld joints of 1.5-GPa-class hot-stamped steel. We analyzed the temperature at the nugget edge during post-heating by using SORPAS, and conducted a reproducible thermal-cycle test simulating post-heating to determine the temperature range and microstructural changes necessary to improve joint strength. CTS was improved in two regions by varying the current ratio. In the tempering region, CTS varied in a wide range, and the current ratio varied in a narrow range. In the solidification segregation relaxation region, CTS did not vary, and the current ratio varied in a wide range. To improve CTS by tempering with post-heating, all solute carbon in martensite must be segregated or precipitated; the temperature for tempering with post-heating should be between 630 and 720 °C. To improve CTS by solidification segregation relaxation with post-heating, the P-enriched area fraction must be reduced; the temperature range for solidification segregation relaxation with post-heating should be between 860 and 1450 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, application of high-strength steel in automobile bodies has been expanded to improve collision safety and fuel efficiency [1, 2]. High-strength steel is usually assembled into automobile bodies via resistance spot welding. However, it has been reported that for high-strength steels with strengths of at least 780 MPa, cross-tension strength (CTS) in resistance spot-weld joints decreases as steel strength increases [3, 4]. This may be due to hardening of the nugget [5, 6] or segregation of impurity elements such as P and S [6]. In addition to the impurity elements, there are other segregation elements such as Mn and Si, but their effect on CTS is small [7].If joint strength is decreased, the weld joint can peel off in a collision, resulting in automobile body performance that cannot be stabilized. Thus, resistance spot weld joint strength using high-strength steels must be improved.
Methods of improving joint strength include enlargement of the nugget and post-heating. Post-heating is a re-heating process after the main welding to form a nugget; tempering or diffusion of solidification segregation elements occurs in the nugget, improving its toughness. Many studies have been conducted on post-heating [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. For example, the study of tempering with post-heating revealed the decrease in hardness of nuggets or heat-effected zone (HAZ) and carbide precipitation in nuggets. In addition, the study of solidification segregation relaxation with post heating-revealed the decrease in segregation area of impurity elements. However, the temperature and microstructural changes required by post-heating to improve joint strength, such as a decrease in hardness and relaxation of solidification segregation elements, remain unclear.
In this study, the effect of post-heating on resistance spot-weld joints was evaluated for 1.5-GPa-class hot-stamped steel. We analyzed the temperature at the nugget edge during post-heating, and conducted a reproducible thermal-cycle test simulating post-heating to determine the temperature range and microstructural changes necessary to improve joint strength.
2 Experimental methods and analysis
2.1 Specimens
Hot-stamped 1.5-GPa-class steel was used to construct the specimens. Table 1 shows the chemical composition of the specimens. Specimens cut to a predetermined size were heated in a furnace at 900 °C for 5 min, placed between cooling dies, and quenched to obtain a tensile strength of 1.5 GPa.
2.2 Welding conditions
After quenching, two specimens cut to 30 mm × 50 mm were overlapped, and resistance spot welding was performed. The joint was used to observe the cross-section of the weld. Cross-tension joints were also fabricated using specimens cut to 50 mm × 150 mm, as specified in JIS Z 3137. Three cross-tension joints were fabricated for each current ratio.
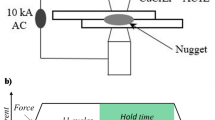
Figure 1 shows a diagram of the welding pattern; Table 2 presents the welding conditions. The main welding current was set to 6.5 kA to produce a nugget diameter of 4√t, where t is the sheet thickness. The sheet thickness for the cross-tension test was 2.0 mm; thus, the nugget diameter 4√t was 5.7 mm. After main welding, the specimens were cooled with electrodes. For post-heating, the current ratio was the post-heating current value (Ih2) divided by the main welding current value (Ih1), and was varied between 0.45 and 1.05. In this experiment, the cooling time is increased so that martensitic transformation occurs at the nugget. The post-heating time was also increased so that temperature was stable during post-heating at the nugget.
2.3 Cross-tension test
Tensile tests were performed on the cross-tension joints at room temperature at a tensile speed of 10 mm/min, as specified in JIS Z 3137.
2.4 Analysis conditions
To estimate the temperature increase during post-heating, “SORPAS 3D” resistance spot-welding simulation software (SWANTEC Software and Engineering ApS) was used for numerical analysis. The analytical model was quarter-symmetric, as shown in Fig. 2. The sheet thicknes was 2.0 mm, the same thickness as the experimental specimens. 22MnB5-USIBOR (hot-stamped) steel was selected as the material for the analytical model. The electrode shape was dome radius, diameter: φ 16 mm, tip diameter: φ 6 mm, tip radius: R40 mm same with the welding conditions in Section 2.2. The upper electrode was movable during the analysis; the lower electrode was not. The welding conditions were the same as those used in the experiments, as shown in Table 2.
2.5 Reproducible thermal-cycle test simulating post-heating
In resistance spot-weld joints, temperature increase during post-heating varies with the distance from the center of the nugget, resulting in a temperature distribution in the nugget. It is difficult to determine the microstructure at the nugget edge. To accurately identify microstructural changes at the nugget edge during post-heating, we conducted a reproducible thermal-cycle test in which the maximum temperature could be controlled to produce a uniform microstructure in the nugget.
Figure 3 shows the specimen preparation process. Resistance spot welding was performed on specimens with dimensions of 4 mm × 30 mm × 200 mm. The nugget diameter was 5.7 mm; the same as that of the cross-tension joint. Table 3 presents the welding conditions. The top and bottom surfaces of specimens with a nugget were ground 1 mm to remove indentations and produce uniform temperature during current heating in reproducible thermal-cycle tests. The reproducible thermal-cycle test specimens had a thickness of 2 mm.
To investigate the temperature range required for tempering and solidification segregation relaxation by post-heating, reproducible thermal-cycle test specimens were subjected to a thermal history to simulate post-heating. The heating rate was 500 °C/s, the same as that in post-heating. Air cooling was performed immediately after the target temperature (400–1400 °C) was reached.
2.6 Vickers hardness test
A Vickers hardness test was performed on nuggets from reproducible thermal-cycle test specimens using a 300 gf load at 0.25-mm intervals. Figure 4 shows the Vickers hardness test measurement position. The indentations on the upper-right and lower-right fusion lines were used as reference positions for the measurements. The average Vickers hardness was calculated from 25 measurements.
2.7 Observation and EPMA measurement
To observe the nugget microstructure, the weld cross-section was embedded and polished, corroded with picric acid solution, and observed using a light microscope through the center of the nugget. SEM observations and EPMA measurements of P, Si, and Mn were performed at the nugget edge.
3 Results
3.1 Results of cross-tension test and cross-sectional observation
Figure 5 shows the cross-tension test results and the cross-sectional macrostructure for resistance spot-weld joints. The plots in Fig. 5 show average CTS values; the error bars indicate maximum and minimum values. CTS did not change with a current ratio from 0 (main welding only) to 0.55. Although a peak in CTS was observed at a current ratio of 0.60, there was variation, as indicated by the error bars. The cause of variation is discussed in Section 3.4. When the current ratio was increased, stable strength enhancement was achieved with a current ratio of 0.70–0.80. However, with a current ratio of 0.90 or more, the CTS was equivalent to that with main welding only. It was considered that as the nugget may have been almost entirely re-melted by post-heating and be generated solidification segregation again, the segregation state with a current ratio of 0.90 or more was similar to that of main welding only.
The cross-sectional macrostructure observed by light microscopy indicated no differences with a current ratio from 0 to 0.65; traces of re-melting in the center of the nugget were observed with a current ratio of 0.80. In addition, the corrosion state around the re-melting area changed, indicating solidification segregation relaxation occurred.
3.2 SORPAS analysis results
Numerical analysis was performed using SORPAS to compare experimental and numerical nugget diameters or relate the current ratio to the maximum temperature at the nugget edge during post-heating. We believe that the CTS is dependent on the microstructure, such as hardness and solidification segregation, at the nugget edge. Figure 6 shows the nugget diameter analyzed by SORPAS. In SORPAS, as in the experiment, the nugget diameter was equivalent to 4√t(≒5.7 mm). This suggests that SORPAS can describe the experiment. Figure 7 shows the relationship between the maximum temperature at the nugget edge during post-heating and the CTS. The first CTS peak, at a current ratio of 0.60, was estimated to reach approximately 700 °C at the nugget edge; the second CTS peak, at a current ratio of 0.70–0.80, reached 1000–1400 °C. The results suggest that tempering occurred at a current ratio of 0.60 with post-heating, and solidification segregation relaxation occurred at a current ratio of 0.70–0.80.
3.3 Results of Vickers hardness test for reproducible thermal-cycle test specimens
To clarify the effect of tempering on CTS improvement, the relationship between maximum temperature and hardness was investigated through reproducible thermal-cycle tests. Based on the results, we estimated the effective temperature range for tempering with post-heating.
Figure 8 shows SEM photographs of reproducible thermal-cycle test specimens. Figure 9 shows the Vickers hardness test results. The red line in Fig. 9 indicates the CTS results in Fig. 7, with a maximum temperature at the nugget edge during post-heating between 500 and 800 °C. At a maximum temperature of 460 °C in reproducible thermal-cycle tests, there was almost no decrease in hardness and little carbide precipitation. The tempering time was short and the temperature was low. At a maximum temperature between 630 and 720 °C in reproducible thermal-cycle tests, the hardness decreased significantly and produced a minimum value. As the maximum temperature increased, hardness increased to the value with main welding only (480 HV). It is believed that the maximum temperature in the reproducible thermal-cycle tests exceeded AC1, and carbides dissolved. From Fig. 9, CTS was not improved at approximately 380 HV, but was improved significantly at approximately 330 HV.
Thus, the Vickers hardness was decreased to approximately 330 HV to improve the CTS by tempering with post-heating. The appropriate temperature range was 630–720 °C. The post-heating maximum temperature may change depending on the contact condition between the electrode and the steel sheet. Therefore, the post-heating maximum temperature deviates from the narrow appropriate temperature range, and the variation of CTS shown in Section 3.2 is considered to be large.
3.4 EPMA measurement results in reproducible thermal-cycle test specimens
The amount of solidification segregation relaxation required to improve CTS was investigated. P is a segregation element that affects the CTS [2,3,4]. Thus, we estimated the effective temperature range for solidification segregation relaxation with post-heating, based on the segregation state of P.
Figure 10 shows the EPMA measurement results for the reproducible thermal-cycle test specimens. Figure 11 shows the relationship between the area above 0.05% P (P-enriched area) based on the EPMA measurements and CTS. The segregation area was defined as the region with 0.05% P-enrichment or greater; 0.05% is approximately three times the amount of P in the base metal. The red line in Fig. 11 indicates the CTS results in Fig. 7, with a maximum temperature at the nugget edge during post-heating between 800 and 1550 °C. In Fig. 11, the P-enriched area fraction varies from 9 to 12% up to a maximum temperature of 860 °C in the reproducible thermal-cycle test. Above 860 °C, the P-enriched area fraction decreased to approximately 8.5%; above 1200 °C, the P-enriched area fraction decreased significantly. The diffusion distance of P increased as the temperature increased. The estimated maximum post-heating temperatures producing a high CTS in Fig. 11 are 1050 °C and 1450 °C; thus, solidification segregation relaxation occurred from 1050 to 1450 °C in the reproducible thermal-cycle tests. It is believed that CTS was improved by solidification segregation relaxation from 860 to 1100 °C, where the P-enriched area fraction was equivalent to that at 1050 °C. However, CTS did not improve when the maximum temperature at the nugget edge during post-heating was approximately 1500 °C, the melting point of iron. Most of the nugget was re-melted by post-heating; solidification segregation occurred during cooling of the nugget after post-heating. Thus, it is assumed that 1450 °C is the maximum temperature at which solidification segregation relaxation occurs.
To improve CTS by solidification segregation relaxation, it was necessary to decrease the P-enriched area fraction compared to that of the main welding-only area. The appropriate temperature range for solidification segregation relaxation with post-heating was 860–1450 °C.
4 Discussion
The Vickers hardness of the main welding-only joints at the nugget edge was approximately 480 HV. The CTS was improved when the Vickers hardness decreased to approximately 330 HV with tempering, possibly due to the reduction in solute carbon or dislocations of the martensitic microstructure by post-heating. However, as the post-heating time was approximately 2 s, the dislocations were not expected to recover. Thus, the decrease in hardness can be attributed to the reduction in solute carbon in the matrix. To clarify the mechanism of the hardness decrease associated with post-heating, the amount of solute strengthening due to carbon was calculated in the martensite.
First, the amount of solute carbon in the martensite was determined. Maruyama et al. reported the amount of segregated or precipitated C in water-quenched martensite steel [28]. Using this reference, we found that 45% of the carbon segregated or precipitated in 0.17% C martensite steel; we assumed that the martensite had 0.094% solute C. Next, the amount of solute strengthening due to carbon was estimated using Eq. (1) [29]. In Eq. (1), σ0 represents the friction stress and [mass% C] is the amount of solute carbon in the steel. Equations for solute strengthening by carbon have not been reported. Thus, the friction stress for which the solute strengthening was formulated was considered the amount of solute strengthening.
From Eq. (1), the amount of solute strengthening by carbon was estimated to be 0.423 GPa. Equations (2) [30] and (3) were used to convert to the Vickers hardness, where HVP is the Vickers hardness expressed in terms of pressure, σ is the tensile strength, and HV is the Vickers hardness, where σ is the amount of solute strengthening.
From the calculation, the amount of solute strengthening by carbon was estimated to be 135 HV. This value was similar to the decrease in hardness (150 HV) required to improve CTS, suggesting that all solute carbon in martensite must be segregated or precipitated to improve CTS.
The diffusion distance of P during post-heating is considered to be the dominant factor in segregation relaxation. From Fig. 11, the diffusion distance of P was determined at a maximum temperature of 720 °C, where segregation relaxation was considered insufficient, and at 860 °C or higher, where good CTS improvement by segregation relaxation was achieved. The diffusion distances of P were obtained using Eqs. (4)–(6) [31, 32], where D is the diffusion coefficient, R is the gas constant, T is the temperature, Q is the activation energy of diffusion, C and C0 is concentration, x is displacement, t is time, α is the width of the diffusion source, and \(l\) is the diffusion distance. In this paper, the following points were considered in determining the diffusion distance. First, atoms one-dimensionally diffuse from the diffusion source on the thin film (in this case, the P segregation area). Then, the diffusion of the atoms was solved using Fick’s second law, and a solution representing a normal distribution was obtained (Eq. (5)). The mean-squared displacement is equal to the variance; therefore, the average diffusion distance, which is the square root of the mean squared displacement, is equal to the standard deviation (Eq. (6)). Table 4 presents the physical properties in the calculations [33]. For simplicity in calculation, 0.2 s was used as time t to account for the thermal history in the reproducible thermal-cycle tests (0.2 s is the average time to remain within 50 °C of the maximum temperature). Considering the phase transformation in the nugget during post-heating, diffusion was assumed to occur in ferrite at 720 °C and 860 °C, and in austenite at higher temperatures.
Table 5 shows the relationship between the fraction of P-enriched area and the diffusion distance of P at each temperature. The P-enriched area fraction was set to the values shown in Fig. 10; the diffusion distance of P was set as the calculated value. The P-enriched area fraction at a maximum temperature of 720 °C was equivalent to that for main welding only; the diffusion distance of P was 17 nm. The P-enriched area fraction at a maximum temperature of 860 °C decreased compared with that for main welding only; the diffusion distance of P increased to 64 nm. However, the diffusion distance of P at a maximum temperature of 1000 °C was 47 nm, which was less than that at 860 °C, probably because diffusion of P is more difficult in austenite than in ferrite. At higher temperatures (1100 °C and 1200 °C), the diffusion distance of P increased, and the P-enriched area fraction decreased.
The results suggest that a diffusion distance of P of approximately 50 nm or more is necessary to achieve increased CTS by segregation relaxation.
5 Conclusion
Resistance spot-weld joints using 1.5-GPa-class hot-stamped steel sheets were subjected to cross-tension tests, Vickers hardness tests, and EPMA measurement. The findings are summarized as follows.
-
1)
CTS was improved in two regions by varying the current ratio. In the tempering region, CTS varied in a wide range, and the current ratio varied in a narrow range. In the solidification segregation relaxation region, CTS did not vary, and the current ratio varied in a wide range.
-
2)
To improve CTS by tempering with post-heating, all solute carbon in martensite must be segregated or precipitated; the temperature for tempering with post-heating should be between 630 and 720 °C.
-
3)
To improve CTS by solidification segregation relaxation with post-heating, the P-enriched area fraction must be reduced; the temperature range for solidification segregation relaxation with post-heating is 860–1450 °C.
References
Sato A (2005) The problem of the high strength steel and that countermeasure. Journal of the JSTP 46(534):548–551
Clare G (2008) Welding advanced high-strength steel is pushing welding technology. Penton's Weld Mag. 81(3):PP.14–16
Oikawa H, Murayama G, Sakiyama T, Takahashi Y, Ishikawa T (2007) Resistance spot weldability of high strength steel (HSS) sheets for automobile. Nippon Steel Tech Rep 95:39–45
Hirai T, Takahashi Y (1979) Resistance spot welding of high strength, IIW Doc. III-612–79
Beevers A, French EJ (1962) Post weld heat treatment of spot welds in hardenable steels. Brit Weld J pp 523–532
Defourny J, Bragard A (1984) Chemistry of high strength steel sheets for automotive industry and the criteria to define the resistance spot weldability. Revue de la Soudure 1(17):17–24
Saito T (1982) Welding technology 30:34–38
Hamatani H, Watanabe F, Miyazaki Y, Tanaka T, Maki J, Oikawa H, Nose T (2011) Characterization of cross tension strength in resistance spot welding ultrahigh strength steel sheets. Preprints of the National Meeting of JWS 89:44–45
Matsuyama K, Takahashi Y, Hasegawa K (2011) Fundamentals and practices of resistance welding, SANPO (Textbook)
Watanabe F, Furusako S, Hamatani H, Miyazaki Y, Nose T (2012) Fracture mechanical analysis of cross tension test for high-strength steel spot welded joints. Math Model Weld Phenom 10:653–667
Taniguchi K, Ikeda R, Endo S (2012) Development of resistance spot welding with pulsed current pattern for high strength steel sheets. Preprints of the National Meeting of JWS 90:240–241
Ito C, Hiratsuka K (1970) Determination of heat treatment conditions of spot welds in anti-corrosive high tensile steels. J Jpn Weld Soc 39:3
Wakabayashi C, Yasuyama M, Furusako S, Miyazaki Y (2015) Spot-welded joint-strength improvement by acceleration of auto-tempering at HAZ. Preprints of the National Meeting of JWS 96:38–39
Ramachandran DC, Figueredo B, Sherepenko O, Jin W, Park YD, Biro E (2022) A study on improving the mechanical performance by controlling the halo ring in the Q&P 980 steel resistance spot welds. J Manuf Process 75:320–330
Liu XD, Xu YB, Misra RD, Peng F, Wang Y, Du YB (2019) Mechanical properties in double pulse resistance spot welding of Q&P 980 steel. J Mater Process Tech 263:186–197
Eftekharimilani P, van der Aa EM, Hermans MJ, Richardson IM (2017) The microstructural evolution and elemental distribution of a 3rd generation 1 GPa advanced high strength steel during double pulse resistance spot welding. Weld World, pp.691–701
Sherepenko O, Juttner S (2019) Transient softening at the fusion boundary in resistance spot welded ultra-high strengths steel 22MnB5 and its impact on fracture processes. Weld World, pp.151–159
Taniguchi K, Matsuda H, Ikeda R (2016) Heat distribution in welds by short-time high-current post-heating and its improving effect on cross tension strength: development of resistance spot welding with pulsed current pattern for ultrahigh-strength steel sheets. Weld Int 30:817–825
Jahandideh A, Hamedi M (2011) An experimental study on effects of post-heating parameters on resustance spot welding of SAPH440 steel. Sci Technol Weld Joining 16:669–675
Yuanfang W, Kai D, Bingge Z, Yuanheng Z, Guanzhi W, Tao W, Hua P, Yulai G (2020) Highly enhanced cross tensile strength of the resistance spot welded medium manganese steel by optimized post-heating pulse, TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, pp.1871–1880
Wakabayashi C, Furusako S, Miyazaki Y (2015) Strengthening spot weld joint by autotempering acceleration at heat affected zone. Sci Technol Weld Joining 20:468–472
Wei S, Liu D, Lin L, Guo Y (2014) Similar and dissimilar resistance spot welding of advanced high strength steels: welding and heat treatment procedures, structure and mechanical properties. Sci Technol Weld Joining 19:427–435
Jahandideh A, Hamedi M, Mansourzadeh S, Eisazadeh H, Rahi A (2010) Investigating the effects of post heating parameter on the quality of automotive body joints in resistance spot welding,” 63rd Annual Assembly & International Conference of the International Institute of Welding, pp.733–737
Taniguchi K, Okita Y, Ikeda R (2015) Development of next generation development of next generation improving the weld properties of advanced high strength steel sheets. JFE Tech Rep 20:85–91
Hiroshi M, Koichi T, Rinsei I, Kenji O (2020) Microstructure and its hardness estimation of resistance spot weld of ultra-high strength steel sheets: development of resistance spot welding with pulsed current pattern for ultra-high strength steel sheets. Weld Int 34:166–175
Koichi T, Hiroshi M, Rinsei I (2019) Influence of the pulsed current pattern on cross-tension strength of spot-welded joint with nugget diameter variation. Development of resistance spot welding with a pulsed current pattern for ultra-high-strength steel sheets. Weld Int 33:211–222
Yuanfang W, Kai D, Bingge Z, Yuanheng Z, Guanzhi W, Tao W, Hua P, Yulai G. Effect of the cooling time on the cross tensile strength of the resistance spot welded medium manganese steel, TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings, pp.515–523, 2020.
Maruyama N, Tabata S (2018) Atom prove analysis of carbon distribution in low and medium carbon as-quench martensite, CAMP-ISIJ Vol.31:943
Cracknell A, Petch N (1955) Frictional force on dislocation arrays at the lower yield point in iron. Acta Metall 3:186–189
Takaki S (2019) Work hardening in ferritic steel. Tetsu-to-Hagané 105:10
Paul SG (1976) Diffusion in solids, CORONA PUBLISHING CO., LTD
Furusako S, Miyazaki Y, Hamatani H, Akiniwa Y (2015) Consideration of decision guidelines for welding condition by analytic solution of heat conduction equation. Quarterly J Jpn Weld Soc 33(2):160–170
Oikawa H (1982) Lattice diffusion in iron. Tetsu Hagane 68:10
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Recommended for publication by Commission III - Resistance Welding, Solid State Welding, and Allied Joining Process
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taniguchi, T., Furusako, S. & Kodama, S. Examination of post-heating conditions to improve CTS in resistance spot-weld joints. Weld World 67, 1359–1366 (2023). https://doi.org/10.1007/s40194-023-01477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-023-01477-8