Abstract
Bioengineering approaches provide cost-effective ways to protect slopes against surface erosion and shallow mass movements. Indeed, vegetation is an excellent way to control slope erosion and instability of slopes. Plants play an active role both on the surface, protecting and holding soil particles, and at deeper layers, reducing pore pressure and increasing soil shear strength. The use of vegetation is particularly appropriate where soil conservation measures are needed. In the paper, a series of laboratory tests are described, together with the equipment used, to better understand plant root effects on soil shear strength and slope stability. Two species of Mediterranean plants, such as Asparagus acutifolius and Spartium junceum, were tested in the laboratory. More than 170 tensile tests have been performed on dry and saturated samples. In order to evaluate the effect of soil moisture, most several roots were also tested at different saturation ratios. Laboratory tests also included direct shear tests on root-reinforced and unreinforced samples. Comparison between reinforced and non-reinforced samples confirms the contribution of roots to improve the soil strength.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The interaction between plants and soil has been studied in different research areas. From the engineering point of view, the contribution of plants to the soil improves its mechanical characteristics. The naturalistic approach for slope stabilization on preventing erosion and shallow mass movements involves the use of live plants or parts of them (such as seeds, roots, cuttings), alone or in combination with natural inert materials (wood, rocks or soil), artificial biodegradable materials (biomats, geojuta) and non-biodegradable materials (geonetworks, geogrids, geotextiles).
An extensive study of the role that plants play in slope stabilization can be found in specialized papers [1,2,3,4,5,6,7,8,9,10,11].
The focus on the literature in recent decades can be divided into three main topics: theoretical modelling, experimental activity and numerical analysis. For theoretical modelling, an important contribution was given by Wu [10] who described the state of the art on modelling until 2013. Wu divided the theoretical activities into models dedicated to the volume element and finite applications [9, 10, 12]. Further contributions to theoretical modelling were provided by Chen et al. [13] and Schwarz et al. [12]. Experimental activity consists of finite problems [14, 15] and volume element that were carried out by both on-site tests [16, 17] and laboratory tests [17,18,19,20]. Experiments confirm the improvement in mechanical characteristics of the soils and the different contribution to the shear resistance offered by the different types of roots [21]. Other experimental activities have been aimed to the characterization of existing models [12, 22,23,24,25,26,27] or of the individual roots [20, 26, 28,29,30]. Finally, numerical studies have focused on the analysis of finite problems [31,32,33]. The synthesis of theoretical modelling introduced by Wu [10] and related to the volume element indicates that the soil–root macroelement can be described with an increased shear strength expressed by the Mohr–Coulomb failure criterion. The increase in resistance is due to an apparent cohesion of the soil in the presence of roots and quantified in an interval between 2 and 15 kPa depending on the species considered and on the tensile strength of the single root. Finally, other authors have performed several numerical simulations. Mickovski et al. [34] simulated, through a 2D and 3D FEM analysis, direct shear tests on rooted and non-rooted soil. Roots have been inserted within the volume of soil modelled at Mohr–Coulomb failure criterion, and the roots were modelled by linear elastic behaviour with an appropriate soil–root interface.
Theoretical Background
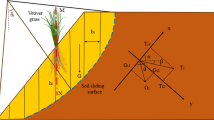
One of the most effective models proposed to quantify the effects of root systems on soil stability is based on the limit equilibrium method, assuming that the single roots are cylindrical fibres that extend through the sliding surface. When the soil moves along the sliding plane, shear stress is generated which induces tensile stresses on the deforming roots as shown in Fig. 1. The roots are stretched until the shear stress mobilized at the root–soil interface and the confining stress are sufficient to prevent the root from pull-out or until the tensile strength of the root is reached. Usually, the maximum tensile strength or pull-out resistance of roots, together with an assessment of root size and distribution, can be used to evaluate the appropriate root reinforcement values to be used in the stability analysis of a slope. Shear strength increment due to roots can be represented as follows (see Fig. 1):
where τ is the soil shear strength and Δτ the increment in strength due to the root, that is (see Fig. 1),
Model of root-reinforced soil reinforced by perpendicular roots (after Wu [9], modified)
The factor of safety of an infinite slope, including the stabilizing effect due to the root apparatus, can be expressed as follows (see Fig. 2):
where Fs0 is the factor of safety with no stabilizing system, that is,
σR the design root tensile stress, ϕ′ the friction angle of the soil, kh and kv the horizontal and vertical seismic coefficients and AR the total area of the roots in a sliding base area A; Fs0 is the factor of safety without taking the root stabilizing effects into account. Thus, Fs depends on σR and on the root area AR. From Eq. (5), the root area ratio AR/A required to obtain a given factor of safety Fs and for a given design root tensile stress σR is:
For a static analysis of slope stability, the seismic coefficients kh and kv must be assumed zero. The determination of the eradicated area ratio is quite complex depending on the value of tensile strength σr and the depth of the effective root apparatus. Thus, a preparatory analysis to be done for a given slope is the determination of the areal distribution of roots and their depths. Referring to the angle α, Waldron [35] suggests values in the range 40°–50°. According to Wu [36], α can vary between 45° and 70°. However, the function f(α) has a limited range of values as shown in Fig. 3. Just for an example, Fig. 4 shows the root area ratio derived from Eq. (6), to obtain an increase in safety factor ΔFs = (Fs − Fs0) = 0.3, for a horizontal seismic coefficient kh = 0.125 and for a vertical seismic coefficient kv = 0.5 kh. The root area is determined as a function of the non-dimensional parameter γH/σR.
In order to evaluate an appropriate root area ratio AR/A to obtain a given factor of safety Fs, the design tensile stress σR acting on the roots must be determined. This choice depends on the failure mechanism occurring in the soil–root system. The design tensile stress σR must be deduced from the minimum ultimate value determined among all the possible failure mechanisms, that is,
- 1.
Ultimate limit state for achieved tensile strength in the root;
- 2.
Ultimate limit state for achieved pull-out of the root;
- 3.
Ultimate limit state for the achieved failure of the soil arching between two adjacent roots.
In this study, we refer only to the ultimate state achieved for the reached tensile strength in the root and whose design values can be determined by experimental tests.
Laboratory Tests
The experimental activity carried out in the laboratory represents a further development of previous work son the tensile strength of roots of native Mediterranean plants [37, 38]. Here, the presented tests are much more numerous and were performed at different saturation ratios of the root to evaluate the contribution to tensile strength of the water content inside the root. Root samples of two typical Mediterranean plant species were collected from the sites located near Catania and Ragusa in Sicily. Different sets of plants have been used: Black Asparagus from Motta S. Anastasia (Catania), Mirabella Imbaccari (Enna) and Cava d’Ispica (Ragusa) and Spartium junceum from Motta S. Anastasia (Fig. 5).
These plants have been removed with their root balls from their sites. The Spartium junceum roots are usually deeper than the Asparagus acutifolius ones. Indeed, roots of Asparagus samples were taken from a depth of 0.20–0.40 m (Fig. 6).
The Spartium junceum roots were taken at a depth of about 0.50–1.50 m from the ground surface. The experimental activity consisted in the evaluation of tensile strength of 128 root samples of Asparagus acutifolius and 44 roots samples of Spartium junceum. Qualitative information about the increase in soil shear strength due to root reinforcement was achieved by performing a series of direct shear tests at the laboratory of the University of Catania.
Tensile Tests
A root tensile test is used to estimate the mechanical properties of a root in terms of maximum tensile strength and elastic modulus. Tensile tests are carried out in the laboratory, usually using a universal testing machine [26, 28, 32, 39]. A testing machine has a stationary part and a moving part that pulls the root. The tensile force is recorded with a load cell usually coupled to a displacement sensor. Different solutions were used by Operstein and Frydman [26] who used a simple dead-load system. The quality of results from tensile tests depends on a number of factors, some related to the experimental set-up of the device such as clamping and pull-out speed and others to the root specimen, e.g. diameter, length and moisture, that can affect data significantly.
The experiment consisted in the evaluation of the root tensile strength of 172 samples, that is, 21 samples of Asparagus acutifolius from Mirabella Imbaccari, three root samples of Asparagus acutifolius from Motta S. Anastasia, 104 root samples of Asparagus acutifolius from Cava d’Ispica and 44 roots of Spartium junceum from Motta S. Anastasia (Sicily). The root samples were classified, according to their diameter, into five groups for the Asparagus and four groups for the Spartium (Fig. 7 and Tables 1, 2).
In order to evaluate the effect of moisture content on the tensile strength of roots [38], in most samples imbibition and drying tests were performed. Typical examples of imbibition and drying curves are shown in Fig. 8. Imbibition was obtained by immersing the roots in water and measuring the weight variation at fixed time intervals until the roots did not absorb water anymore. The roots were dried using an oven.
The device utilized to measure tensile strength of samples combines three functions: tensile force generation up to 40 kN, load and displacement measuring and data acquisition. The ends of the roots were clamped using cork and polystyrene as shown in Fig. 9. This clamping system did not affect the outcome of the test, since the root failure occurred in the middle of their length, distant from the clamps (Fig. 10).
Results of tensile tests for Asparagus samples are shown in Fig. 11. The failure load is plotted versus the diameter at various saturation ratios ranging from 100 to 25% (Fig. 11a–c). The tensile strength versus the root diameter at saturation ratios Sr from 100 to 25% is plotted in Fig. 11d–f. The failure load obviously increases with the root diameter, but the results indicated that the tensile strength at failure σR roughly decreases with the increase in the root diameter. A same trend was observed for the Spartium junceum tensile tests, as shown in Fig. 12. This behaviour could be explained if one assumes that the tensile strength of the root cuticle is greater than the tensile strength of the internal flesh, so that the less is the diameter of the root, the greater is the relative contribution of the cuticle to the root tensile strength. This phenomenon can be considered similar to the specific surface concept. It was found that the tensile strength of Asparagus root increases for saturation ratio ranging from 50 to 100% while it decreases in dry roots. Even for the broom samples, it was found that dry roots were weaker than roots with a saturation ratio ranging from 50 to 100%. Finally, all the results were put together, regardless of the saturation ratio, as illustrated in Fig. 13 which shows the root tensile strength for both the species of plants. There are significant differences in the tensile strength of the two species analysed. Generally speaking, it has been found that the tensile strength of Spartium junceum was much greater than the tensile strength of Asparagus.
Results obtained from Asparagus acutifolius samples: a failure load versus root diameter at saturation = 100%; b failure load versus root diameter at saturation = 50%; c failure load versus root diameter at saturation = 25%; d tensile strength versus root diameter at saturation = 100%; e tensile strength versus root diameter at saturation = 50%; f tensile strength versus root diameter at saturation = 25%
Results obtained from Spartium junceum samples: a failure load versus root diameter saturation = 100%; b failure load versus root diameter saturation = 50%; c failure load versus root diameter saturation = 0%; d tensile strength versus root diameter saturation = 100%; e tensile strength versus root diameter saturation = 50%; f tensile strength versus root diameter saturation = 0%
Direct Shear Tests
The behaviour of a rooted soil is similar to that of a composite material, consisting of a matrix in which elastic fibres are inserted. A series of direct shear tests were carried out in order to verify the contribution of plant roots on soil shear strength. Reinforcements were realized with Asparagus roots. The Kaolin Speswhite and the Montelupo clay were used in the tests. Shear tests were carried out on 60 mm × 60 mm reconstituted samples. Tests were performed on both unreinforced and reinforced soil. For the reinforced Kaolin samples, a single root was disposed as shown in Fig. 14a, while for reinforced Montelupo clay samples five roots were disposed as shown in Fig. 14b.
The contribution of reinforcing roots is clearly evidenced by the comparison between reinforced and unreinforced soil tests. Shear stress–displacements curves for the Kaolin Speswhite are plotted in Fig. 15. Figure 15a concerns the reinforced soil with a single root; Fig. 15b concerns the unreinforced soil. In the reinforced soil, an increase of about 30% in strength was observed. Shear stress–displacement curves of the Montelupo clay are plotted in Fig. 16. Figure 16a concerns the reinforced soil with five roots; Fig. 16b concerns the unreinforced soil. Despite the major number of the roots, the increase in soil shear strength in the reinforced soil was not proportional to the number of roots. The increase in shear strength was also dependent on the vertical stress applied since this one increases the pull-out resistance. Indeed, because of the limited thickness of the soil samples, in such tests, the ultimate shear strength was conditioned by the root pull-out resistance rather than the root tensile strength. A typical failure in reinforced samples is shown in Fig. 17.
Finally, Fig. 18a, b shows the failure envelopes for both unreinforced and reinforced soil. The contribution of root manifests in some apparent cohesion and in a slight increase in friction angle. The results show an improvement in the shear strength parameters in both tests.
Conclusions
An experimental laboratory investigation about the tensile strength of the roots of two species of Mediterranean plants has been presented. The main findings can be used as a reference for further studies and are summarized as follows:
In the tensile tests that were carried out, the failure load increased with the root diameter; however, the root diameter has a significant effect on tensile strength σR which tends to decrease as the diameter increases. This behaviour can be explained if one considers that the tensile strength of the root cuticle is greater than the tensile strength of the internal flesh, so that the less the diameter of the root, the greater the relative contribution of the cuticle to the root tensile strength;
It was found that the tensile strength of Asparagus roots roughly increases for saturation ratios ranging from 50 to 100%, while the tensile resistance decreases in dry roots. Even for the brooms it was found that dry roots were weaker than roots with a saturation ratio from 50 to 100%;
The kind of species may have a great effect on tensile strength of roots. Significant differences were found in tensile strength between the two species analysed. The tensile strength of Spartium junceum was much greater than the tensile strength of the Asparagus;
A verification of the role of the root in the increase in soil shear strength was also achieved with direct shear tests by comparing the resistance of unreinforced and root-reinforced samples. The tests performed on the direct shear box confirm the contribution to soil shear strength of root reinforcement. An increase of about 30% of soil shear strength was found, but the increase was not proportional to the number of roots utilized as reinforcements. It has been observed that, due to the small size of the soil samples, the ultimate shear strength was achieved with a pull-out mechanism of failure, so that the increase in shear strength was also dependent on the applied confining pressure. In this case, the increase in shear strength can be taken into account by an apparent cohesion and a greater friction angle.
Abbreviations
- α :
-
Angle between the root and the normal to the sliding surface
- A R :
-
Roots area
- A :
-
Considered area for stability analysis
- A i :
-
Root area in the class i
- β :
-
Angle of the sliding surface to the horizontal
- c′:
-
Cohesion
- D b :
-
Root diameter into the stable soil
- Δτ L :
-
Increment in strength due to the root
- ϕ :
-
Friction angle
- F s :
-
Safety factor with root contribution
- F s0 :
-
Safety factor without root contribution
- γ :
-
Soil unit weight
- H w :
-
Height of the water table
- H :
-
Height of the sliding slope
- k h :
-
Horizontal seismic coefficient
- k v :
-
Vertical seismic coefficient
- L b :
-
Average length of the root apparatus into the stable zone
- m :
-
Number of classes of roots having a same diameter
- n :
-
Number of roots with a same diameter
- p m :
-
Value of distributed load
- σ R :
-
Tensile strength of the root
- σ Ri :
-
Average root tensile strength in the class i
- τ *L :
-
Strength increment due to roots
- τ L :
-
Soil shear strength
- τ lb :
-
Average value of the ultimate shear stress at the interface along the root into the stable zone
References
Bischetti GB (2000) Quantificazione dell’effetto dell’apparato radicale sulla stabilità dei versanti. Riv Ing Agrar 2:70–81
Cazzuffi D, Cardile G, Gioffrè D (2014) Geosynthetic engineering and vegetation growth in soil reinforcement applications. Transp Infrastruct Geotechnol 1(3–4):262–300
Gray DH, Leiser AT (1989) Biotechnical slope protection and erosion control. Krieger, Malabar
Gray DH, Sotir RB (1996) Biotechnical and soil bioengineering slope stabilization. J. Wiley and Sons Inc., New York, USA
Lawrance CJ, Rickson RJ, Clark JE (1996) The effect of grass roots on the shear strength of colluvial soils in Nepal. Advances in hillslope processes. Wiley, Chichester, pp 857–868
Mattia C, Bischetti GB, Gentile F (2005) Biotechnical characteristics of root systems of typical Mediterranean species. Plant Soil 278(1–2):23–32
Noorasyikin MN, Zainab M (2016) A tensile strength of Bermuda grass and Vetiver grass in terms of root reinforcement ability toward soil slope stabilization. In: IOP conference series: materials science and engineering, vol 136, no. 1. IOP Publishing, p 012029
Wang K, Lee C (1998) Brief mechanical analysis of bioengineering techniques for slope protection. Chin J Rock Mechan Eng 17(6):687–691
Wu TH (1976) Investigation on landslides on Prince of Walles Island, Alaska Geotech. Rpt. No 5, Dpt. Of Civil Eng., Ohio State Univ., Columbus OH
Wu TH (2013) Root reinforcement of soil: review of analytical models, test results, and applications to design. Can Geotech J 50(3):259–274
Schwarz M, Giadrossich F, Cohen D (2013) Modeling root reinforcement using a root-failure Weibull survival function. Hydrol Earth Syst Sci 17(11):4367–4377
Schwarz M, Rist A, Cohen D, Giadrossich F, Egorov P, Büttner D, Stolz M, Thormann JJ (2015) Root reinforcement of soils under compression. J Geophys Res Earth Surf 120(10):2103–2120
Chen L, Wang P, Yang Y, He J (2014) Constitutive model of single root system’s resistance to tensile stress-taking Pinus tabulaeformis, Betula platyphylla, Quercus mongolica and Larix gmelinii as experimental objects. PLoS ONE 9(4):e93066
Cazzuffi D, Corneo A, Crippa E (2006) Slope stabilisation by perennial “gramineae” in southern Italy: plant growth and temporal performance. Geotech Geol Eng 24(3):429–447
Mickovski SB, Bengough AG, Bransby MF, Davies MCR, Hallett PD, Sonnenberg R (2007) Material stiffness, branching pattern and soil matric potential affect the pullout resistance of model root systems. Eur J Soil Sci 58(6):1471–1481
Comino E, Druetta A (2009) In situ shear tests of soil samples with grass roots in Alpine environment. Am J Environ Sci 5(4):475
Docker BB, Hubble TCT (2008) Quantifying root-reinforcement of river bank soils by four Australian tree species. Geomorphology 100(3–4):401–418
Bovolenta R, Mazzuoli M, Berardi R (2018) Soil bio-engineering techniques to protect slopes and prevent shallow landslides. Ital Geotech J 52(3):44–65
Khalilnejad A, Ali FH, Osman N (2012) Contribution of the root to slope stability. Geotech Geol Eng 30(2):277–288
Mickovski SB, van Beek LPH (2018) Test data from pullout experiments on vetiver grass (Vetiveria zizanioides) grown in semi-arid climate. Data Brief 17:463–468
Comino E, Marengo P (2010) Root tensile strength of three shrub species: Rosa canina, Cotoneaster dammeri and Juniperus horizontalis: soil reinforcement estimation by laboratory tests. Catena 82(3):227–235
Bischetti GB, Chiaradia EA, Epis T, Morlotti E (2009) Root cohesion of forest species in the Italian Alps. Plant Soil 324(1–2):71–89
Chiaradia EA, Vergani C, Bischetti GB (2016) Evaluation of the effects of three European forest types on slope stability by field and probabilistic analyses and their implications for forest management. For Ecol Manag 370:114–129
Comino E, Marengo P, Rolli V (2010) Root reinforcement effect of different grass species: a comparison between experimental and models results. Soil Tillage Res 110(1):60–68
Federica G, Chiara V, Rodolfo G, Anne B, Pierre C, Sandra C, Chiaradia EA (2017) Root characteristics of herbaceous species for topsoil stabilization in restoration projects. Land Degrad Dev 28(7):2074–2085
Operstein V, Frydman S (2000) The influence of vegetation on soil strength. Proc Inst Civ Eng Ground Improv 4(2):81–89
Zhang C-B et al (2010) Triaxial compression test of soil–root composites to evaluate influence of roots on soil shear strength. Ecol Eng 36(1):19–26
Bischetti GB, Chiaradia EA, Simonato T, Speziali B, Vitali B, Vullo P, Zocco A (2005) Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant Soil 278:11. https://doi.org/10.1007/s11104-005-0605-4
Bischetti GB, Chiaradia EA, D’agostino V, Simonato T (2010) Quantifying the effect of brush layering on slope stability. Ecol Eng 36(3):258–264
Genet M, Stokes A, Salin F, Mickovski SB, Fourcaud T, Dumail JF, Van Beek R (2005) The influence of cellulose content on tensile strength in tree roots. Plant Soil 278(1–2):1–9
Cohen D, Schwarz M (2017) Tree-root control of shallow landslides. Earth Surf Dyn 5(3):451
Kokutse NK, Temgoua AGT, Kavazović Z (2016) Slope stability and vegetation: conceptual and numerical investigation of mechanical effects. Ecol Eng 86:146–153
Yang Y, Chen L, Li N, Zhang Q (2016) Effect of root moisture content and diameter on root tensile properties. PLoS ONE. https://doi.org/10.1371/journal.pone.0151791
Mickovski SB, Stokes A, Van Beek R, Ghestem M, Fourcaud T (2011) Simulation of direct shear tests on rooted and non-rooted soil using finite element analysis. Ecol Eng 37(10):1523–1532
Waldron LJ (1977) The shear stress resistance of root-permeated homogeneous and stratified soil. Soil Sci Soc Am Proc 41:843–849
Capilleri PP, Motta E, Raciti E (2016) Experimental study on native plant root tensile strength for slope stabilization. In: VI Italian conference of researchers in geotechnical engineering—geotechnical engineering in multidisciplinary research: from microscale to regional scale, CNRIG Bologna, 2016 22–23 September
Wu TH, McKinnell WP III, Swanston DN (1979) Strength of tree roots and landslides on Prince of Wales Island, Alaska. Can Geotech J 16(1):19–33
Capilleri PP, Motta E, Raciti E, Todaro M (2017) Evaluation of root tensile strength of some Mediterranean plant species for slope stabilization. Ital Geotech J 51(1):60–68
Giadrossich F, Schwarz M, Cohen D, Cislaghi A, Vergani C, Hubble T, Stokes A (2017) Methods to measure the mechanical behaviour of tree roots: a review. Ecol Eng 109:256–271
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capilleri, P.P., Cuomo, M., Motta, E. et al. Experimental Investigation of Root Tensile Strength for Slope Stabilization. Indian Geotech J 49, 687–697 (2019). https://doi.org/10.1007/s40098-019-00394-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40098-019-00394-2