Abstract
The harsh environments of desert areas lead to natural selection of resistant creatures with obvious characteristics. This experiment looked for salt-tolerant fungi from native halophyte plants. Forty fungi isolated from three halophyte plant families that were collected from desert areas of Yazd Province in Iran, and the most tolerant isolates were selected at concentrations of 1, 2, 3, 3.5 and 4 molar sodium chloride. Five selected superior isolates were assigned to the phylum Ascomycota based on internal transcribed spacers sequences and β-tubulin gene, as well as morphological characteristics of the genus and species. Aspergillus terreus showed superiority in terms of enzymes and antibacterial properties than other isolates. Other isolates were Acremonium, Paecilomyces, Microascus and Monosorascus. Aspergillus terreus also showed antifungal effects against Aspergillus fumigatus, a human pathogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity and drought are the most important abiotic stresses (Chen et al. 2009). It is estimated that more than 900 million hectares (more than 6%) of agricultural land and 30 percent of the irrigation water worldwide are affected by salt (http://www.unesco.org/water; Zhang et al. 2010). Iran has vast saline soils and about 15.2% of the country (approximately 33 million hectares) and 55% of the agricultural lands are affected by salinity (Martinez et al. 2004; Honarjoo et al. 2010). Salinity affects the growth of the plant directly through the toxicity of ions and indirectly by increasing osmotic stress (Bromham 2014). Halophytes are able to maintain high concentrations of electrolytes. In addition to other types of salts such as Na2SO4, MgSO4, CaSO4, MgCl2, KCI and Na2CO3 (Flowers et al. 2010), NaCl is mostly present in saline environments. Over the past 30 years, the term endophytic fungi has been used in microbiological studies to describe fungi that live inside healthy plants (Stone et al. 2004; Soltani 2017). The association of endophytic fungi with plants improves plant growth, its tolerance to environmental stresses like dryness, salinity, temperature, heavy metals, etc., and resistance to pathogens (Kirch et al. 2000; Giri and Mukerji 2004; Waller et al. 2005; Soltani 2017; Golparyan et al. 2018). Endophytes include microorganisms that live inside tissues of higher plants without causing symptoms of intracellular or intercellular growth and are rich in bioactive compounds. Nearly all plant species host one or more endophytes (Li et al. 2008). Endophytes isolated from plants growing in warm soils and coastal saline soils indicate a high commercialization potential by proving increased yield in hot and salty water environments (Flowers and Yeo 1995; Lucero et al. 2008; Yuan et al. 2016).
The purpose of this study was to isolate and identify salt-tolerant, bioactive and enzyme-producing endophytic fungi associating halophyte plants of Amaranthaceae, Rubiaceae and Asteraceae families in Iran. The beneficial potentials of these fungi in dealing with salinity and drought stress would be valuable in salty and drought areas. This experiment started in 2016 by collecting samples from Yazd and screening on salty treatments at Bu-Ali Sina University.
Materials and methods
Collecting halophyte plants
In September 2016, different plants including Anabasis iranica, Seidlitzia rosmarinus, Salsola tomentos and Salsola yazdiana in Amaranthaceae family; Rubiatinctorum in Rubiaceae and Artemisia annua in Asteracea family were collected from Chah Afzal, central district of Ardakan county in Yazd, a desert region in Iran, with the geographical coordinates of 32° 30′ 30″ N 53° 52′ 08″ E.
Isolation of endophytic fungi from halophyte plants
Root, stem and leaf of each plant specimen were first washed in tap water. The surface disinfection of the samples was performed with washing with 70% ethanol (volume/volume) for 5 min, disinfecting in sodium hypochlorite 2%(V/V) for 15 min, and ethanol 96% (V/V) for 2 min followed by three times washing with sterile water. Disinfected tissue segments were transferred to 8-cm-diameter plates, containing sterile potato dextrose agar (PDA) medium (QueLab), and incubated at 25 ± 1 °C for 4 weeks. Growing fungi around plant tissue segments were purified by hyphal tipping method and transferred to the new culture media for further identification.
Screening of salt-tolerant endophytic fungi
In order to evaluate salt tolerance of fungal strains, PDA medium with three different concentrations of 1, 2 and 3 molar sodium chloride is used. For each concentration of NaCl, three replications were used. Fungi that were able to grow on 1 M NaCl were tested for growing in 2 M and so on in 3 M NaCl concentrations. Fungi were incubated at 25 ± 2 °C for 7 days, and the ability of fungal isolates to grow in the presence of NaCl was monitored daily.
Enzyme activity of fungal isolates
To evaluate extracellular enzymatic activity of fungal isolates, amylase (Hankin and Anagnostakis 1975; Simair et al. 2017), protease (Hankin and Anagnostakis 1975; Razzaq et al. 2019), cellulose (Samanta et al. 1989; El-Said et al. 2014), keratinase (Joshi et al. 2007), and pectinase (Khairnar et al. 2009) production assays were applied in the medium base containing appropriate substrate. The treatments were incubated for 72 h at 25 °C, and the enzymatic activity was measured by eye estimation of diameter of the zones with an opalescent halo (Hankin and Anagnostakis 1975; Legodi et al. 2019).
Antimicrobial properties of fungi
The superior fungi were grown in potato dextrose broth (PDB) on a shaker at 120 revolutions per minute (rpm) for 1 week; then, they were placed in dark for two more weeks. PDB media containing fungal mycelia were filtered (Hosseini Moghaddam et al. 2013). Chloroform was added to the resultant solution (1:1; V:V), and the mixture was placed on a shaker with 120 rpm for 24 h. Two formed phases were separated by separator funnel. The medium including the chloroform solvent and metabolites was poured into the glass and dried in an oven at 45 °C. In total, 100 mg of each extracts dissolved in 1 ml of 100% dimethyl sulfoxide (DMSO) solvent. Five human pathogenic microbes including Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhimurum and Candida albicans were selected to evaluate antimicrobial effect of isolated fungi. Antibiotic disks containing 100 µg of gentamicin, kanamycin and erythromycin were used as positive controls of inhibition. A volume of 15 microliters of microbial suspension (optical density: 0.8) was poured into each petri dishes containing Mueller–Hinton medium. An aliquot of 20 μl of each fungal extract added to the wells was made in each petri. An aliquot of 20 μl of DMSO was used as the control. The plates were placed in a refrigerator for 2 h and then incubated at 37 °C for 48 h (Smania et al. 1999).
Isolate 1 was tested in a dual culture assay against Aspergillus fumigates. For this test, a disk in 4 mm diameter from the colony margin of actively growing cultures of each fungus was placed on PDA. Disks were placed 5 cm far from each other in the same petri. Petri dishes were incubated in dark at 25 °C for 7 days according to the growth rate of fungi (Demirci et al. 2011). Amount of growth was compared and evaluated with control.
Identification of fungal isolates
A combination of morphological, physiological and molecular characteristics was used to identify fungal isolates. Isolates were classified based on morphological characteristics including color, shape, texture or hyphae and spore characteristics like size, shape and reproductive structures. Genomic DNA of spores was extracted by cetyl trimethyl ammonium bromide (CTAB) method (Doyle and Doyle 1987; Healey et al. 2014). The internal transcribed spacer (ITS) of the nuclear ribosomal DNA (rDNA) was amplified using the universal ITS1 and ITS4 primers (White et al. 1990; Martin and Rygiewicz 2005). In addition, the β-tubulin gene was amplified using βt2a and βt2b primer pairs (Glass and Donaldson 1994). The ITS1 and ITS4 primers amplify 550 to 750 base pair of the gene, and βt2a and βt2b primers amplify the 500–600-bp region through the PCR (Table 1). PCR products were sequenced by Bioneer Company, and data were obtained in FASTA format, edited by Chromas software 2.6.6. and compared to GenBank sequences at the National Center for Biotechnology Information (NCBI).
Results and discussion
Results
In total, 40 fungi were isolated and colony purified using hyphal tipping culture. Out of 40 isolates, 23 recovered from roots, 15 from stems and 2 from leaves. Thus, about 57% of the isolates were isolated from root tissues. Eleven fungi were isolated from A. iranica, whereas only two fungi were isolated from S. rosmarinus and A. annua (Table 2).
Salt tolerance screening
From 40 fungal isolates, 32 grew on 1 M, 20 on 2 M, 11 on 3 M, 5 on 3.5 M and 1 on 4 M NaCl. As isolates no. 1, 2, 3, 7 and 31 were all morphologically the same, then isolate 1 was used for further experiments. Isolates no. 1, 6, 11, 14 and 19 were able to grow on 3.5 M NaCl. However, only isolate 1 was capable of growing on 4 M NaCl (Fig. 1).
The identification of fungi
The proper fragment in the range of 500 to 750 bp was amplified by PCR using ITS1 and ITS4 primers. Also sequence of beta-tubulin gene using primers βT2a and βT2b amplified the fragment of 500 bp by PCR (Fig. 2).
Antimicrobial properties of fungal extract
Antimicrobial properties of extracts of Aspergillus terreus (isolate 1), Acremonium sclerotigenum (isolate 6), Paecilomyces formosus (isolate 11), Monosporascus ibericus (isolate 14) and Microascus pyramidus (isolate 19) were assessed on 5 pathogenic microbes including B. cereus, S. aureus, P. aeruginosa, S. typhimurum and C. albicans by agar diffusion assay. Inhibitory zone data of the fungi extracts were analyzed by a one-way ANOVA and compared with Tukey’s test in SPSS software (Fig. 4). The inhibition zone diameter was very different between fungal extracts. Based on inhibitory zone data A. terreus and A. sclerotigenum had the most antimicrobial effect compared with other isolates and antibiotics, whereas the extracts from M. ibericus and M. pyramidus had no inhibitory effect (Fig. 4).
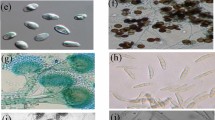
Figure 3 shows the antagonistic interactions between two species of aspergillus, one is plant endophyte and the other is a human pathogen. Dual culture assay for A. terreus showed inhibition of pathogen growth. Evaluation of inhibition zone was difficult due to spreading of A. terreus spores, but finally A. terreus covered whole plate.
Enzymatic activities of fungi
The enzymatic activity of 5 fungi A. terreus, A. sclerotigenum, P. formosus, M. ibericus and M. pyramidus with a higher salt tolerance ability than other screened isolates, was evaluated (Table 2). Diameter of the zones with an opalescent halo was comparatively measured.
Discussion
As halophyte plants can survive in high salt conditions, the study and evaluation of their symbiotic fungi would be very beneficial for crop production in salty areas. In the present study, 40 fungal isolates were purified from domestic halophyte plants growing in one of the saltiest regions of Iran, Chah Afzal village in Yazd Province. Obtained data showed that more than half of isolates were inhabited on the roots. Young et al. (2012) focused on roots of six halophyte plants, looking for endophytic fungi with plant growth ability. In addition, the involvement of root microbiome of Suaeda salsa, a halo-tolerant plant, has been studied (Yuan et al. 2016). The association of endophytic microorganisms with halophyte plants may enhance plant salt tolerance. Out of forty collected fungal isolates in this research, half of them could survive on 2 M NaCl and about 12% of them on 3.5 NaCl. There was variation among isolates for salt tolerance screening. For example, isolate 34, Penicillium, had ability to grow on 1 and 2 M but stopped at 3 M, while Aspergillus (isolate no. 1) survived on 4 M NaCl. The five halo-tolerant isolates growing on 3.5 M NaCl were identified as A. terreus, A. sclerotigenum, P. formosus, M. ibericus and M. pyramidus, all in Ascomycota phylum based on morphological, physiological and molecular data (Tables 2, 3 and Fig. 2). To determine the genus and species of the fungus, ITS and β-tubulin amplification were used. The isolated A. terreus superior halo-tolerant fungus from R. tinctorum roots was reported here for the first time. It was also isolated before from plant species at sandy soils and marshes at southeast Spain (Maciá-Vicente et al. 2008). The superior halo-tolerant fungus A. terreus (isolate no. 1) was capable of decomposing cellulose. The commercial application of this species in production of organic acids and enzymes has been reported (Okabe et al. 2009). Also soil-isolated A. terreus MS105 has been introduced as a cellulase production strain by Sohail et al. (2016). Fungal isolates like Alibertia macrophylla and Uncaria gambier Roxb (Rubiaceae) with a broad range of biologically active compounds were introduced by Oliveira et al. (2009). In this study A. terreus also showed keratinase, protease, pectinase and amylase activities. In addition A. terreus and Acremonium isolates exhibited in vitro activity against five human pathogens and almost were placed in one group with antibiotics based on Tukey’s test (Fig. 4). The antibacterial effect of the fungal extracts in case of Salmonella was particularly interesting. This pathogen showed resistance to erythromycin and gentamicin but was sensitive to extract of isolates 1, 6 and kanamycin. The antimicrobial-resistant strains of Salmonella are a great threat to public health (Liao et al. 2019); therefore, finding new antibiotics seems necessary. Marine A. terreus var. africanus showed antimicrobial activity against virulent fish pathogens (Barakat and Gohar 2012). Aspergillus fumigatus is an airborne fungal pathogen and shows resistance to environmental invasion (Valsecchi et al. 2019). Here the endophytic fungus A. tereus was able to inhibit the growth of this pathogen.
Antagonistic interaction of two Aspergillus isolates: A. terreus (brown) and A. fumigates (white). Aspergillus terreus (isolate 1), Acremonium sclerotigenum (isolate 6), Paecilomyces formosus (isolate 11), Monosporascus ibericus (isolate 14) and Microascus pyramidus (isolate 19). E = erythromycin, K = kanamycin, G = gentamicin. The different letters (a, b, c, d and e) indicate statistically significant difference between the groups (p < 0.01)
Secondary metabolites of endophytic fungi were active against pathogens and founded an important noticeable source of biocontrol agents (Kongue Tatong et al. 2014; Lo Piccolo et al. 2015). Acremonium sp. isolated from Garcinia tree had maximum zone of inhibition against Salmonella typhi, S. aureus and Klebsiella (Ruma et al. 2013). Acremonium sp. that exist in Taxus baccata could produce leucinostatin, a peptide antifungal–anticancer agent (Strobel et al. 1997). We isolated A. sclerotigenum from R. tinctorum (isolate 6). This isolate had the highest gelatinase and pectinase activity among five studied isolates. These enzymes are capable of hydrolyzing sugarcane bagasse usable in industry (de Almeida et al. 2011; Bischoff et al. 2009). Endophytes required enzymes for degradation of host cell wall and penetration (Schulz et al. 2002). P. formosus (isolate 11) also showed enzymatic and antimicrobial activity. It is previously reported that P. formosus isolated from the cucumber, Boswellia sacra and Eugenia jambolana produced bioactive compounds such as carbohydrates, alkaloids, phenols, amino acids, hormones and extracellular enzymes (Khan et al. 2016; Yadav et al. 2014; Khan et al. 2012). There is no report on A. iranica endophytes, and two salt-tolerant fungi, P. formosus and M. ibericus, are reported for the first time. The latter was already isolated from roots and stems of three halophyte plants in the Ebro Delta in Spain salt marshes in 2002 (Collado et al. 2002). Although M. pyramidus was already isolated from desert soil (Barron et al. 1961) and animal (Woudenberg et al. 2017), we identified that as a salt-tolerant isolate from plant, S. tomentosa.
Conclusion
The study on salt tolerance and enzymatic activity of fungal community in halophyte plants resulted in isolation of two species, A. terreus and A. sclerotigenum as halo-tolerant fungi with strong enzymatic and antibacterial activities. In addition, two species of M. ibericus and M. pyramidus were detected as salt-tolerant isolates in A. iranica and S. tomentosa for the first time. This study also reveals that Ascomycota phylum includes strains, which may be involved in plant salt tolerance.
Change history
24 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s13762-021-03628-0
References
Barakat KM, Gohar YM (2012) Antimicrobial agents produced by marine Aspergillus terreus var. africanus against some virulent fish pathogens, Indian. J Microbiol 52:366–372
Barron GL, Cain RF, Gilman JC (1961) A revision of the genus Petriella. Can J Bot 39(4):837–845
Bischoff KM, Wicklow DT, Jordan DB, de Rezende ST, Liu S (2009) Extracellular hemicellulolytic enzymes from the maize endophyte Acremonium zeae. Curr Microbiol 58:499–503
Bromham L (2014) Macroevolutionary patterns of salt tolerance in angiosperms. Ann Bot 115:333–341
Chen L, Ren F, Zhong H, Jiang W (2009) Identification and expression analysis of genes in response to high-salinity and drought stresses in Brassica napus. Acta Biochim Biophys Sin 42:154–164
Collado J, Gonzalez A, Platas G, Stchigel AM, Guarro J (2002) Monosporascus ibericus sp. nov., an endophytic ascomycete from plants on saline soils, with observations on the position of the genus based on sequence analysis of the 18S rDNA. Mycol Res 106:118–127
de Almeida MN, Guimarães VM, Bischoff KM, Falkoski DL, Pereira OL (2011) Cellulases and hemicellulases from endophytic Acremonium species and its application on sugarcane bagasse hydrolysis. Appl Biochem Biotechnol 165:594–610
Demirci E, Dane E, Eken C (2011) In vitro antagonistic activity of fungi isolated from sclerotia on potato tubers against Rhizoctonia solani. Turk J Biol 35:457–462
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem Bull 19:11–15
El-Said AHM, Saleem A, Maghraby TA, Hussein MA (2014) Cellulase activity of some phytopathogenic fungi isolated from diseased leaves of broad bean. Int J Curr Microbiol Appl Sci 3(2):883–900
Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants: where next? Funct Plant Biol 22:875–884
Flowers TJ, Galal HK, Bromham L (2010) Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol 37:604–612
Giri B, Mukerji KG (2004) Mycorrhizal inoculant alleviates salt stress in Sesbaniaaegyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 14:307–312
Glass NL, Donaldson GC (1994) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Golparyan F, Azizi A, Soltani J (2018) Endophytes of Lippia citriodora (Syn. Aloysia triphylla) enhance its growth and antioxidant activity. Eur J Plant Pathol 152:759–768
Hankin L, Anagnostakis SL (1975) The use of solid media for detection of enzyme production by fungi. Mycologia 67:597–607
Healey A, Furtado A, Cooper T, Henry RJ (2014) Protocol: a simple method for extracting next-generation sequencing quality genomic DNA from recalcitrant plant species. Plant Methods 10:21
Honarjoo N, Mojiri A, Jalalian A, Karimzadeh HR (2010) The effects of salinity and alkalinity of soil on growth of Haloxylon sp. in Segzi plain (Iran). In: International conference on chemistry and chemical engineering (ICCCE), pp 285–288
Hosseini Moghaddam M, Soltani J, Babolhavaeji F, Hamzei J, Nazeri S (2013) Bioactivities of endophytic Penicillia from Cupressaceae. J Crop Prot 2:421–433
Joshi SG, Tejashwini MM, Revati N, Sridevi R, Roma D (2007) Isolation, identification and characterization of a feather degrading bacterium. Int J Poult Sci 6:689–693
Khairnar Y, Krishna VK, Boraste A, Gupta N, Trivedi S, Patil P (2009) Study of pectinase production in submerged fermentation using different strains of Aspergillus niger. Int J Microbiol Res 1:13
Khan AL, Hamayun M, Radhakrishnan R, Waqas M, Kang SM (2012) Mutualistic association of Paecilomyces formosus LHL10 offers thermotolerance to Cucumis sativus. Antonie Van Leeuwenhoek 101:267–279
Khan AL, Al-Harrasi A, Al-Rawahi A, Al-Farsi Z, Al-Mamari A (2016) Endophytic fungi from Frankincense tree improves host growth and produces extracellular enzymes and indole acetic acid. PLoS ONE 11:0158207
Kirch HH, Vera-Estrella R, Golldack D, Quigley F, Michalowski CB (2000) Expression of water channel proteins in Mesembryanthemum crystallinum. Plant Physiol 123:111–124
Kongue Tatong MD, Talontsi FM, Abdel Rahim HMD, IslamM Tofazzal, Oswald RB (2014) Banchromene and other secondary metabolites from the endophytic fungus Fusarium sp. obtained from Piper guineense inhibit the motility of phytopathogenic Plasmopara viticola zoospores. Tetrahedron Lett 55:4057–4061
Legodi LM, La Grange D, Jansen van Rensburg EL, Ncube I (2019) Isolation of cellulose degrading fungi from decaying banana pseudostem and Strelitzia alba. Enzyme Res 2019:1–10
Li JH, Wang ET, Chen WF, Chen WX (2008) Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean grown in Heilongjiang province of China. Soil Biol Biochem 40:238–246
Liao J, Orsi RH, Carroll LM, Kovac J, Ou H (2019) Serotype-specific evolutionary patterns of antimicrobial-resistant Salmonella enterica. BMC Evol Biol 19:132
Lo Piccolo S, Alfonzo A, Giambra S, Conigliaro G, Lopez Llorca L (2015) Identification of Acremonium isolates from grapevines and evaluation of their antagonism towards Plasmopara viticola. Ann Microbiol 65:2393–2403
Lucero ME, Barrow JR, Osuna P, Reyes I, Duke SE (2008) Enhancing native grass productivity by cocultivating with endophyte-laden calli. Rangel Ecol Manag 61:124–130
Maciá-Vicente JG, Jansson HB, Abdullah SK, Descals E, Salinas J (2008) Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol Ecol 64:90–105
Martin KJ, Rygiewicz PT (2005) Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol 5:28
Martinez JP, Lutts S, Schanck A, Bajji M, Kine JM (2004) Is osmotic adjustment required for water stress resistance in the Mediterranean shrub Atriplex halimus L. J Plant Physiol 161:1041–1051
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Micribiol Biotechnol 84:597–606
Oliveira CM, Silva GH, Regasini LO, Zanardi LM, Evangelista AH (2009) Bioactive metabolites produced by Penicillium sp. 1 and sp. 2, two endophytes associated with Alibertia macrophylla (Rubiaceae). Z Naturforsch B 64:824–830
Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A, Ashraf M (2019) Microbial proteases applications. Front Bioeng Biotechnol 7:110
Ruma K, Sunil K, Prakash HS (2013) Antioxidant, anti-inflammatory, antimicrobial and cytotoxic properties of fungal endophytes from Garcinia species. Int J Pharm Pharm Sci 5:889–897
Samanta R, Pal D, Sen SP (1989) Production of hydrolases by N2-fixing microorganisms. Biochemie und Physiologie der Pflanzen 185(1–2):75–81
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106:996–1004
Simair AA, Qureshi AS, Khushk I, Ali CH, Lashari S, Bhutto MA, Mangrio GS, Lu C (2017) Production and partial characterization of α-amylase enzyme from Bacillus sp. BCC 01-50 and potential applications. Biomed Res Int 2017:1–9
Smania A Jr, Monache FD, Smania EDFA, Cuneo RS (1999) Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int J Med Mushrooms 1:325–330
Sohail M, Ahmad A, Ahmed Khan S (2016) Production of cellulase from Aspergillus terreus MS105 on crude and commercially purified substrates. 3 Biotech 6:103
Soltani J (2017) Endophytism in Cupressoideae (Coniferae): a model in endophyte biology and biotechnology. In: Maheshwari D (ed) Endophytes: biology and biotechnology. Sustainable development and biodiversity, vol 15. Springer, Cham, pp 127–143
Stone JK, Polishook JD, White JF (2004) Endophytic fungi. Biodiversity of fungi. Elsevier Academic Press, Burlington, pp 241–270
Strobel GA, Torczynski R, Bollon A (1997) Acremonium sp.—a leucinostatin A producing endophyte of European yew (Taxus baccata). Plant Sci 128:97–108
Valsecchi I, Dupres V, Michel JP, Duchateau M, Matondo M (2019) The puzzling construction of the conidial outer layer of Aspergillus fumigatus. Cell Microbiol 21:e12994
Waller F, Achatz B, Baltruschat H, Fodor J, Becker K (2005) The endophytic fungus Piriformosporaindica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci India A 102:13386–13391
White TJ, Bruns T, Lee SJWT, Taylor JL (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Woudenberg JHC, Meijer M, Houbraken J, Samson RA (2017) Scopulariopsis and scopulariopsis-like species from indoor environments. Stud Mycol 88:1–35
Yadav M, Yadav A, Yadav JP (2014) In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac J Trop Med 7:S256–S261
Young YH, Yoon H, Kang SM, Shin JH, Choo YS (2012) Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J Microbiol Biotechnol 22:1549–1556
Yuan Z, Druzhinina IS, Labbé J, Redman R, Qin Y (2016) Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci Rep 6:32467
Zhang H, Irving LJ, McGill C, Matthew C, Zhou D (2010) The effects of salinity and osmotic stress on barley germination rate: sodium as an osmotic regulator. Ann Bot 106:1027–1035
Acknowledgements
This work was supported by Bu-Ali Sina University, Hamedan, Iran. We thank Dr. Gholamreza Zarei for assistance with the collection and identification of halophyte plants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Jalili, B., Bagheri, H., Azadi, S. et al. Identification and salt tolerance evaluation of endophyte fungi isolates from halophyte plants. Int. J. Environ. Sci. Technol. 17, 3459–3466 (2020). https://doi.org/10.1007/s13762-020-02626-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02626-y