Abstract
The jelly coat is the gelatinous layer surrounding the oocyte follicles in most aquatic animals, and it helps in fertilization and provides nutrition and protection to developing oocytes and embryos. In Heteropneustes fossilis, the oocyte jelly coat consists of three layers, outer, middle and inner, as inferred from the optical density measurements. Histochemically, the jelly coat showed positive reactions for carbohydrate, lipid, protein, sialic acid and acidic sulfated mucosubstances. Hexosamine and sialic acid are the aminosugars present in the jelly coat. The gonadotropin treatment resulted in swelling up (hydration) of the jelly coat. The administration of gonadotropin (in vivo) increased the hexosamine and sialic acid concentrations of the jelly coat time-dependently. However, in vitro, incubation of the follicles with gonadotropin resulted in decreases in the hexosamine and sialic acid contents. The differences in the dynamics of the aminosugars after the gonadotropin treatment suggested immediate impact of surrounding and internal environment to the follicles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most aquatic oviparous animals, the ovarian follicles are surrounded by a gelatinous layer called jelly coat. The origin of the jelly coat is a matter of debate. It is secreted by the posterior portion of the ovary (oviduct) during the time of ovulation (Arranz et al. 1997; Maes et al. 1999) or synthesized in the liver (choriogenesis) along with vitellogenesis under the influence of estrogen and accumulated in oocytes as yolk (Ravaglia and Maggese 2003; Sano et al. 2017). Fish oocytes generally have three distinct layers, namely an outermost follicular layer, a median zona radiata (ZR) and an inner oolemma or oocyte plasma membrane (Shabanipour and Heidari 2004). The Bufo oocytes from ovisac are covered by four jelly layers, named as J1 to J4 from innermost to outermost (Arranz et al. 1997). Ravaglia and Maggese (2003) showed that the first vitelline envelope is seen at the perinuclear stage of the oocytes. And in mature oocytes, vitelline envelope becomes a multilaminar structure with 11 µm in width. They also described three layers, Z1, Z2 and Z3. Efforts have been carried out to find out the component of the jelly substance responsible for the biological activities. Egg jelly coat consists of a mixture of fucose-rich polysaccharides and sialic acid-rich glycoproteins, a fibrillar component that contains carboxylic (uronic/sialic) and sulfated groups and a loose element composed of neutral or weakly acidic polysaccharides, diffusible oligopeptide and mucin glycoproteins (Hotta and Kurokawa 1973; Maes et al. 1999; Vilela-Silva et al. 1999). The granulose layer is involved in the secretion of enzymes that participate in the lysis and reorganization of specific zones of the egg chorion (Shabanipour and Heidari 2004).
Egg jelly coat has essential roles in the fertilization process including capacitation, agglutination and activation of sperm, acrosome reaction, polyspermy blocking, recognition of homologous species and providing a protective environment for the developing embryos from chemical, physical and biological pressure (Salustri et al. 2018). It acts as a microbiological barrier to the environment (Gusseck and Hedrick 1971; Maes et al. 1999; Sano et al. 2017; Vilela-Silva et al. 1999).
Heteropneustes fossilis is an air-breathing catfish, which spawns during monsoon rainfall in vegetation-rich freshwater bodies like ponds, rivers and lakes. The eggs are released into the water for the fertilization and are enclosed by a thick jelly coat for protection against predators and infection. In the present study, we investigated the histochemical and biochemical nature of the catfish egg jelly and the effects of gonadotropin on egg jelly composition during late prespawning phase (June).
Materials and methods
Chemicals
Human chorionic gonadotropin (hCG, Corion, IBSA, Switzerland) was purchased from local medical stores. Other chemicals used in the experiment and analysis were of analytical grade and purchased from local scientific suppliers.
Animal collection and maintenance
The experiments were performed as per national/institutional guidelines for experimentation in test animal (fish) and all care taken to prevent cruelty of any kind.
Adult H. fossilis (30–40 g) were collected from local fish markets, in the late prespawning phase (June). They were maintained in the laboratory conditions under natural photoperiod (12:12; L/D) and temperature (23 ± 2 °C) for acclimatization and fed goat liver ad libitum. A few female fish were sampled randomly to check the maturity of the gonad. The ovaries were dissected out and kept in incubation medium to ascertain the maturation stage of oocytes. The incubation medium was prepared as follows: NaCl 3.74, KCl 0.32, CaCl2 0.16, NaH2PO4·2H2O 0.10, MgSO4·7H2O 0.16, glucose 0.40 and phenol red 0.008 (in g) were dissolved in 1 l triple distilled water. The pH was adjusted to 7.5 with 1 N sodium bicarbonate and autoclaved. Penicillin (2,00,000 U) and streptomycin sulfate (200 mg) were added and filtered. The medium was stored at 4 °C and prepared fresh every week. The rounded dark green oocytes of diameter 1.03 ± 0.01 mm were used for the study.
Determination of jelly coat layers
Approximately 40 oocytes each from five fish were taken in duplicate in 4 ml of Tris-DeBoer’s solution (110 mM NaCl; 1.3 mM CaCl2; 1.3 mM KCl; 10 mM Tris–HCl; pH 8.8; 60 mM mercaptoethanol). At different intervals (0, 0.2, 0.3, 0.4, 0.5, 0.6, 1.1, 1.2, 1.3, 2, 3, 4, 5, 6, 7, 24, 30, 48 h), the optical density (OD) of the medium was taken at 280 nm against the DeBoer’s solution as blank.
Determination of biochemical nature of jelly coat
Experimental set 1 Five H. fossilis were primed to reach uniform maturity by injecting intraperitoneally (ip) with hCG (100 IU/fish; Mishra and Joy 2006a, b) and maintained for 4 h. The fish were killed, and the ovaries were removed and placed in the incubation medium. Dark green post-vitellogenic follicles were separated with the help of fine brush and watchmaker’s forceps. About 40 follicles in duplicate from each fish were incubated in plain incubation medium for 0, 8 or 24 h.
Experimental set 2 Five H. fossilis were killed, the ovaries were dissected out, and post-vitellogenic follicles were collected, as described above. About 40 follicles in duplicate from each fish were incubated in an incubation medium containing hCG (20 IU/ml; Mishra and Verma 2017) for 0, 8 or 24 h.
Experimental set 3 Each of the five H. fossilis was given a single ip injection of hCG (100 IU/fish). After 16-h post-injection, the fish were stripped and the eggs were collected in a sterilized petri dish. The eggs were incubated in aerated well water for 0, 15, 30, 45 and 60 min.
Experimental set 4 Each of the twenty adult female catfish was injected with a single i.p. injection of hCG (100 IU/fish). Each of the five fish was killed at 0, 4, 8 or 16 h. The follicles were collected, as described above.
Extraction of oocyte jelly coat
At the end of the respective treatments in each experiment, the follicles/eggs were incubated in DeBoer’s solution for 8 h (from the first experiment data). After the incubation, the medium was centrifuged at 12,000g for 20 min at 4 °C. The supernatant containing jelly substance was lyophilized and stored at − 20 °C till further use.
Assay of hexosamine
Hexosamine was measured according to the method of Elson and Morgan (1933), modified by Davidson (1966). In brief, lyophilized jelly coat preparations from different experiments were hydrolyzed in 2 ml 3 N HCl for 4 h at 100 °C. After cooling, the samples were neutralized with 1 ml 6 N NaOH. One milliliter of sample was mixed with 1 ml of freshly prepared acetyl acetone (1 ml redistilled acetyl acetone in 50 ml 0.5 N sodium carbonate solution). Tubes were closed and kept in a boiling water bath for 45 min. After cooling, 4 ml 95% ethanol was added and mixed. One milliliter of freshly prepared Ehrlich’s reagent (0.4 g p-dimethyl amino benzaldehyde in 15 ml methanol and 15 ml concentrated HCl) was added to the reaction mixture. The tubes were allowed to stand at room temperature for 1 h, and optical density (OD) of the pink color developed was recorded at 530 nm against blank in a UV–Vis spectrophotometer (Systronics) taking glucosamine and galactosamine as standard. The concentration was expressed in µg/100 mg oocyte.
Assay of sialic acid
Sialic acid was measured according to the method of Warren (1959). In brief, lyophilized jelly coat powder was dissolved in 0.1 N cold H2SO4 (1 ml/10 mg tissue) and kept in a water bath at 80 °C for 1 h. The samples were kept in a refrigerator for 2 h to allow the tissue fragments to sediment. 0.5 ml sample was oxidized with 0.25 ml periodate reagent (25 mM periodic acid in 0.125 N H2SO4, pH 1.2) for 30 min at 37 °C. Excess of periodate was reduced by adding 0.2 ml sodium arsenite (2% solution of sodium arsenite in 0.5 N HCl). When the yellow color of the liberated iodine disappeared, 2 ml of thiobarbituric acid reagent (0.1 M solution of thiobarbituric acid in water, pH adjusted by NaOH at 9.0) was added. Tubes were tightly plugged and kept in boiling water for 7.5 min. The samples were cooled in ice water, and 5 ml acid butanol (butan-1-ol containing 5% of 12 N HCl) was added and mixed, and OD was taken from the upper separated butanol layer in a spectrophotometer against a blank solution of distilled water at 549 nm and 532 nm. The concentration of sialic acid was expressed as µmoles N-acetyl neuraminic acid/100 mg oocyte and calculated according to Warren’s equation: 0.09 × OD549 − 0.033 × OD532.
Histochemical analysis of jelly coat
Five H. fossilis were injected with 100 IU hCG/fish. After 12 h of injection, the fish were killed, and hydrated post-vitellogenic follicles were fixed in Bouin’s fluid, and paraffin blocks were prepared. The sections (5 µm) were cut and processed according to the following staining procedures: PAS-hematoxylin (for carbohydrate containing 1,2 glycol groups) (Leblond et al. 1957), Sudan black B (for lipid) (Tas et al. 1980), bromophenol blue (for protein) (Mazia et al. 1953), Alcian blue (for acidic sulfated mucosubstances) (Sorvari and Sorvari 1968), Alcian blue-PAS (for mucosubstance) (Wilson et al. 1983) and sialic reaction (for sialic acid) (Ueda et al. 1995). After staining, the slides were examined under the microscope and photographed.
Statistical analysis
The data were expressed as mean ± SEM and analyzed by one-way analysis of variance (ANOVA; P < 0.001), followed by Newman–Keuls’ test (P < 0.05) for multiple group comparisons.
Results and discussion
The jelly coat plays a significant role in the reproductive and developmental processes: firstly as an interface between the egg and sperm and secondly between the embryo and its environment. The jelly coat is rich in mucus. Mucus aggregates and maintains the embryos in close contact with each other, and presumably prevents jelly coat materials surrounding each embryo from diffusing away (Simmons et al. 2009).
Spectrophotometric analysis of jelly coat layers
The jelly coat preparations showed sharp changes in the OD at 280 nm at three time intervals: 0–0.5 h, 0.5–1.5 h, and 1.5–7 h, and the OD plot plateaued until 48 h (Fig. 1). These three changes in the OD were related to the dissolution of three different layers: outer, middle and inner layers of the jelly coat. It is clear from the study that H. fossilis oocytes possess a three-layered jelly coat of different viscosity and characteristics, as they showed variations at three time points in OD absorption at 280 nm. The outermost layer dissolved fast followed by the middle layer, and the innermost layer took a long time to dissolve. After the dissolution of the innermost layer, a minimal change in the OD was noticed, suggesting no more dissolution. The denuded egg remains surrounded by the vitelline membrane as indicated by its spherical shape. The de-jellied eggs are somewhat delicate and continued contact with other de-jellied eggs, and the DeBoer’s solution eventually led to the rupture of the vitelline membrane, judged from the flattening of the egg due to a lack of physical support and lysis of the plasma membrane. The mechanism of jelly coat solubilization is attributed to the reduction in disulfide bonds, which maintained the jelly coat structure (Gusseck and Hedrick 1971; Salustri et al. 2018; Sano et al. 2017).
Hexosamine and sialic acid contents in jelly coat
Hexosamine is 6-carbon sugars with an amino group and is present in high concentrations in male reproductive organs of mammals and is essential intermediates in the biosynthesis of sialic acid (Mann and Lutwak-Mann 1981). Hexosamine promotes sperm survival and fertilization ability. Sialic acid is a complex class of amino sugars and a major constituent of the seminal fluid. The sialic acid content in the jelly coat is higher (three times) as compared to the hexosamine concentration. Sialic acid is reported in the egg jelly coat of different vertebrates, and the sialoprotein induces agglutination of spermatozoa (Hotta and Kurokawa 1973; Vilela-Silva et al. 1999). The sialic acid-rich polysaccharide is present in both egg and sperm with difference in composition to facilitate species specificity in echinoderm and sea urchin (Valle et al. 2015; Yessilyurt et al. 2015).
Priming the catfish with hCG in vivo for 4 h resulted in significant changes in the hexosamine and sialic acid contents of the jelly coat (hexosamine, F = 18.07; sialic acid, F = 393.47, P < 0.001, one-way ANOVA; Fig. 2). The hexosamine content decreased significantly both at 8 and 24 h (P < 0.05, Newman–Keuls’ test). The sialic acid content showed a significant decrease only at 24 h.
Hexosamine and sialic acid concentration in oocyte (around forty) of 4-h hCG (100 IU/fish)-primed fish at different intervals in plain medium. N = 5 for each interval data. Data were expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls’ test (P < 0.05). Asterisk (*) shows the significant difference with their respective control
The incubation of the post-vitellogenic follicles with hCG (20 IU/mL) produced an overall significant effect on the hexosamine and sialic acid contents (F = 35.55, F = 219.95, respectively; P < 0.001, one-way ANOVA; Fig. 3). The decrease was significant both at 8 and at 24 h (P < 0.05, Newman–Keuls’ test).
Hexosamine and sialic acid concentration in oocyte (around forty) with hCG (20 IU/ml) at different intervals. N = 5 for each interval data. Data were expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls’ test (P < 0.05). Asterisk (*) shows the significant difference with their respective control
The maintenance of the eggs in aerated well water for 0, 15, 30, 45 and 60 min, following stripping of the eggs after 16 h of the injection of hCG (100 IU/fish), produced an overall significant effect on hexosamine and sialic acid contents (hexosamine, F = 32.00; sialic acid, F = 154.42; P < 0.001, one-way ANOVA; Fig. 4). The hexosamine content did not show any significant change at 15 and 30 min but increased significantly at 45 and 60 min in water (Newman–Keuls’ test, P < 0.05). The sialic acid concentration dropped initially at 15 min and recovered subsequently, but all values were significantly lower than the concentration at 16 h.
Hexosamine and sialic acid concentration in stripped oocyte (around forty) at different intervals after 16 h of hCG (100 IU/fish). N = 5 for each interval data. Data were expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls’ test (P < 0.05). Asterisk (*) shows the significant difference with their respective control
The injection of fish with an ovulatory dose of hCG (100 IU/fish) showed significant periovulatory changes in hexosamine and sialic acid contents (hexosamine, F = 398.63; sialic acid, F = 719.38; P < 0.001; one-way ANOVA; Fig. 5). Both the hexosamine and sialic acid contents decreased significantly at 4 h but recovered and increased significantly at 6 and 16 h (Newman–Keuls’ test, P < 0.05). The sialic acid increase was about two times higher compared to the 4-h concentration.
Hexosamine and sialic acid concentration in oocyte (around forty) of hCG-induced fish (100 IU/fish) at different intervals. N = 5 for each interval data. Data were expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls’ test (P < 0.05). Asterisk (*) shows the significant difference with their respective control
Human CG injections influenced the concentrations of both hexosamine and sialic acid in the catfish oocyte jelly coat. Under in vitro conditions, hCG inhibited the levels of the aminosugars in contrast to in vivo conditions in which the concentrations of sialic acid and hexosamine increased after an initial drop. The decrease in the aminosugars may be due to their utilization associated with oocyte final maturation and jelly coat differentiation, and the increases may be due to synthesis or accumulation (Gallo and Costantini 2012; Mishra and Joy 2004). Sialic acid promotes sperm agglutination and activation, thereby increasing the fertilization ability. In the jelly coat, it is present in proper amounts and also showed its turnover by hCG induction (Goto and Iritani 1992). Jelly coat with mucus and high viscosity facilitates agglutination of spermatozoa in even a much diluted concentration. The jelly coat also facilitates the grouping of yeast cells in 4–6 cells groups. It suggests the jelly role in the protection of egg first and later embryo (Hirohashi et al. 2008).
Histochemical analysis of jelly coat
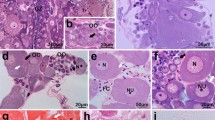
A single injection of hCG (100 IU/fish) resulted in hydrated follicles when sampled at 12-h post-injection (Fig. 6a). The follicular layer of the oocytes showed positive reactions to PAS for carbohydrate (Fig. 6b), Alcian blue-PAS for mucosubstance (Fig. 6c), sialic reaction for sialic acid (Fig. 6d), Sudan black B for total lipids (Fig. 6e), Alcian blue pH 2.0 for acidic sulfated mucosubstance (Fig. 6f) and bromophenol blue for total protein (Fig. 6g). From the histochemical analysis, the follicular layer of the oocytes contains proteins, lipids, carbohydrates, sialic acid and sulfated mucosubstances. The jelly coat formation is facilitated by the dissolution of theca and granulosa cell (Ravaglia and Maggese 2003). The epithelial organization of the granulosa cells disappeared, and a full mucus envelope is formed around the maturing oocytes. During vitellogenesis, the granulosa cells surrounding the oocytes proliferate and synthesize large amounts of spherical aggregates containing mucus grains. The formation of the jelly coat is differently described among teleost. The egg envelope has two compartments: a ZR interna and a ZR externa, with multifunctional properties (Riehl and Patzner 1998). Its primary function is the protection of the embryo by the ZR interna. Another critical function of the egg envelope is the attachment of deposited eggs to a substrate, which is primarily mediated by the ZR externa. Abraham et al. (1993) in the catfish Silurus glanis reported that at the end of vitellogenesis, the follicle cells are filled with mucosomes and cytoplasmic residua are only sparingly observed among them. After spawning, the mucosomes are released and become responsible for the extreme adhesive ability of the catfish eggs. A similar mucus coating has also been reported for other siluriformes (Riehl and Appelbaum 1991; Rizzo et al. 1998). In Clarias gariepinus, there is an attachment disk around the micropyle, consisting of numerous tiny attaching filaments embedded in a cementing substance containing acidic mucopolysaccharides (Riehl and Appelbaum 1991). In other teleosts like perch, stickleback and dwarf lionfish, the eggs are arranged in threads produced by the jelly layer which surrounds the egg (Riehl and Patzner 1998).
Histochemistry to study jelly coat nature; a hydrated egg (after 12 h of 100 IU hCG/fish); b PAS-hematoxylin for carbohydrate detection; c Alcian blue-PAS for mucosubstance detection; d sialic reaction for sialic acid detection; e Sudan black B for lipid detection; f Alcian blue (pH 2) for acidic sulfated mucosubstance detection; g bromophenol blue for protein detection. JL jelly layer; Th theca; Gr granulose; Pv perivitelline space; Ov body of ovum [magnification × 20]
Conclusion
Heteropneustes fossilis oocytes possess a three-layered jelly coat (outer, middle and internal jelly coat). The jelly coat contains carbohydrate, protein, lipid, sialic acid and sulfated mucosubstances. The changes in the concentrations of sialic acid and hexosamine in response to hCG treatment are associated with final oocyte maturation and jelly coat differentiation.
References
Abraham M, Hilge V, Riehl R, Iger Y (1993) Muco-follicle cells of the jelly coat in the oocyte envelope of the sheatfish (Silurus glanis L.). J Morphol 217:37–43
Arranz S, Albertali IE, Carada MO (1997) Bufo arenarum egg jelly coat: purification and characterization of two highly glycosylated proteins. Biochem J 323:307–312
Davidson EA (1966) Analysis of sugars found in mucopolysaccharides. In: Neufeld EF, Ginsburg V (eds) Methods in enzymology, vol VIII. Academic Press, New York, pp 52–60
Elson LA, Morgan WTJ (1933) A colorimetric method for the determination of glucosamine and galactosamine. Biochem J 27:1824–1828
Gallo A, Costantini M (2012) Glycobiology of reproductive processes in marine animals: the state of the art. Mar Drugs 10(12):2861–2892
Goto K, Iritani A (1992) Oocyte maturation and fertilization. Anim Reprod Sci 28(1–4):407–413
Gusseck DJ, Hedrick JL (1971) A molecular approach to fertilization, Disulfide bonds in Xenopus laevis jelly coat and a molecular hypothesis for fertilization. Dev Biol 25:337–347
Hirohashi N, Kamei N, Kubo H, Sawada H, Matsumoto M, Hoshi M (2008) Egg and sperm recognition systems during fertilization. Dev Growth Differ 50(1):S221–S238
Hotta K, Kurokawa M (1973) A novel sialic acid and fucose-containing disaccharide isolated from the jelly coat of sea urchin eggs. J Biol Chem 248:629–631
Leblond CP, Glegg RE, Eidinger D (1957) Presence of carbohydrates with free 1,2-glycol groups in sites stained by the periodic acid-Schiff technique. J Histochem Cytochem 5(5):445–458
Maes E, Florea D, Coppin A, Strecker G (1999) Structural analysis of 20 oligosaccharide-alditols released from the jelly coat of Rana palustris eggs by reductive β-elimination. Characterization of the polymerized sequence [gal(β1,3)GalNAc(a1-4)]n. Eur J Biochem 264:301–313
Mann T, Lutwak-Mann C (1981) Male reproductive function and semen: themes and trends in physiology, biochemistry and investigative andrology. Springer, Berlin, p 495
Mazia D, Brewer PA, Alfert M (1953) The cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol Bull 104(1):56–67
Mishra A, Joy KP (2004) Ovarian monosaccharides (glucose and fructose): hormonal effects and their role in final oocyte maturation and egg quality in catfish Heteropnuestes fossilis, Bloch. Indian J Exp Biol 42:1084–1090
Mishra A, Joy KP (2006a) Effects of gonadotrophin in vivo and 2-hydroxyoestradiol-17β in vitro on follicular steroid hormone profile associated with oocyte maturation in the catfish Heteropneustes fossilis. J Endocrinol 189:341–353
Mishra A, Joy KP (2006b) HPLC-Electrochemical detection of ovarian estradiol-17β and catecholestrogens in the catfish Heteropneustes fossilis: seasonal and periovulatory changes. Gen Comp Endocrinol 145:81–95
Mishra A, Verma S (2017) In vitro effects of chlorpyrifos on gonadotropin-induced oocyte maturation and steroidal interplay of freshwater catfish, Heteropneustes fossilis. Int J Pharma Bio Sci 8(3):912–920
Ravaglia MA, Maggese MC (2003) Ovarian follicle ultrastructure in the teleost Synbranchus marmoratus (Bloch, 1795), with special reference to the vitelline envelope development. Tissue Cell 35:9–17
Riehl R, Appelbaum S (1991) A unique adhesion apparatus on the eggs of the catfish Clarias gariepinus (Teleostei, Clariidae). Jpn J Ichthyol 38(2):191–197
Riehl R, Patzner RA (1998) Minireview: the modes of egg attachment in teleost fishes. Ital J Zool 65(S1):415–420
Rizzo E, Moura TFC, Sato Y, Bazzoli N (1998) Oocyte surface in four teleost fish species postspawning and fertilization. Braz Arch Biol Technol 41(1):37–48
Salustri A, Campagnolo L, Klinger FG, Camaioni A (2018) Molecular organization and mechanical properties of the hyaluronan matrix surrounding the mammalian oocyte. Matrix Biol. https://doi.org/10.1016/j.matbio.2018.02.002
Sano K, Kawaguchi M, Katano K, Tomita K, Inokuchi M, Nagasawa T, Hiroi J, Kaneko T, Kitagawa T, Fujimoto T, Arai K, Tanaka M, Yasumasu S (2017) Comparison of egg envelope thickness in teleosts and its relationship to the sites of ZP protein synthesis. J Exp Zool B Mol Dev Evol 328(3):240–258
Shabanipour N, Heidari B (2004) A histological study of the zona radiata during late oocyte developmental stages in the Caspian sea mugilid, Liza aurata (Risso 1810). Braz J Morphol Sci 21(4):191–195
Simmons LW, Roberts JD, Dziminski MA (2009) Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. J Evol Biol 22:225–229
Sorvari TE, Sorvari R-M (1968) The specificity of Alcian blue ph 1.0-alcian yellow ph 2.5 staining in the histochemical differentiation of acidic groups in mucosubstances. J Histochem Cytochem 17(4):291–293
Tas J, Frederiks WM, Frank JJ (1980) A new approach to the staining of lipids with Sudan Black B: a study by means of polyacrylamide model films containing liposomes. Acta Histochem Suppl 21:123–129
Ueda T, Fujimori O, Yamada K (1995) A new histochemical method for detection of sialic acids using a physical development procedure. J Histochem Cytochem 43(10):1045–1051
Valle GM, Cinelli LP, Todeschini AR, de Brito-Gitirana L, Vilela-Silva AES, Mourão PAS (2015) Sperm and egg jelly coat from sea urchin Lytechinus variegatus collected in Rio de Janeiro contain distinct sialic acid-rich polysaccharides. Braz Arch Biol Technol 58(4):617–627
Vilela-Silva AC, Alves AP, Valente AP, Vacquier VD, Mourao PA (1999) Structure of the sulfated α-L-fucan from the egg jelly coat of the sea urchin Strongylocentrotus franciscanus: patterns of preferential 2-O- and 4-O-sulfation determine sperm cell recognition. Glycobiology 9(9):927–933
Warren L (1959) The thiobarbituric acid assay of sialic acids. J Biol Chem 234:1971–1975
Wilson TS, McDowell EM, Trump BF (1983) An Alcian blue (pH 2.5)-PAS-keratin immunoperoxidase method for the simultaneous demonstration of keratin and neutral and acidic mucosubstances. Stain Technol 58(4):225–229
Yessilyurt B, Ssahar U, Devec R (2015) Determination of the type and quantity of sialic acid in the egg jelly coat of the sea urchin Paracentrotus lividus using capillary LC-ESI-MS/MS. Mol Reprod Dev 82:115–122
Acknowledgements
Manuscript is duly checked with Grammarly software to avoid errors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Rupali Datta.
Rights and permissions
About this article
Cite this article
Mishra, A., Joy, K.P. Characterization of gonadotropin-induced oocyte jelly coat from the catfish Heteropneustes fossilis (Bloch). Int. J. Environ. Sci. Technol. 17, 287–294 (2020). https://doi.org/10.1007/s13762-019-02299-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02299-2