Abstract
Oogenesis of Siberian sturgeon Acipenser baerii is studied on farm fish using light and electron microscopy. We have identified five stages correlated with physiological state of the ovarian follicle constituted by the oocyte surrounded by its cellular (theca and granulosa) and a-cellular (zona radiata) layers: Stages I and II before vitellogenesis, Stages III and IV during vitellogenesis, and Stage V during maturational processes.
Following the oogonial stage, Stage I presents an elevated nucleoplasmic index, and the nucleus contains only one nucleolus. In this early stage, lipid globules were identified. Stage II is characterized by nucleoli multiplication and their migration toward the nuclear periphery. During this stage, the number of lipid globules increases in the oocyte cytoplasm. At the end of this stage, cortical alveoli get synthesized. The beginning of Stage III, called sub-Stage IIIa, corresponds to the first features of vitellogenin incorporation simultaneously with the elaboration of the zona radiata externa. During sub-Stage IIIb the zona radiata interna 1 is built. Stage IV is characterized by the first apparition of pigment granules and the elaboration of the zona radiata interna 2. Yolk accumulation increases and the oocyte volume grows considerably. Stage V corresponds to maturation with the beginning of nucleus migration toward the oocyte membrane. The zona radiata is completely synthesized, and a “jelly coat” is deposited on the outer surface of the zona radiata externa by synthesis of granulosa cells. Yolk accumulation ends at this stage.
In conclusion, ultrastructural data allow accurate determination of oogenesis stages.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Most studies on oogenesis in sturgeon species have been performed using light microscopy (Ginzburg and Detlaf 1969; Caloianu-Yordachel 1971; Kornienko 1975; Trusov 1975; Fedorova 1976; Kondrat’yev 1977; Akimova et al. 1979; Badenko et al. 1981; Veshchev 1982; Kijima and Maruyama 1985; Flynn and Benfey 2007). A few investigations used electron microscopy (Markov 1975; Raïkova 1976; Cherr and Clark 1982, 1984; Grandi and Chicca 2008; Rzepkowska and Ostaszewska 2013). Nevertheless we have not found studies dealing with Acipenser baerii gametogenesis of fish living in natural conditions in Siberian rivers. This species, which arrived in France in 1976 and later in 1982, is reared in fish farms to produce caviar and in experimental fish farms as a biological model to help restore the European sturgeon (Acipenser sturio) (Williot and Brun 1982a, b).
This paper deals with the description of the oogenesis of the Siberian sturgeon reared in French fish farm conditions until the end of vitellogenesis. This study was done to identify the different stages of the reproduction process comparing the data provided by samplings of the same tissue treated with the two photonic and ultrastructural technologies.
Oogenesis was compared in teleostean and chondrichtian, with first a rapid review of initial events from evolution of primordial germ cells (PGC) toward differentiated oogonia. Meiosis of oogonia gave rise to a primary oocyte surrounded by its cellular and a-cellular layer, constituting a primary follicle. This first meiotic division is stopped at the end of the prophase in the diplotene stage for a longer time in sturgeon compared with teleostean. During this period the development of primary follicle was divided into four stages: Stage I and Stage II before vitellogenesis and Stage III and Stage IV during vitellogenesis. In these stages we presented the results dissociating the events occurring in the oocyte from those occurring in its surrounding layers. The Stage V corresponded to maturational events when metaphase of the first meiotic division occurred.
2 Material and Methods
Ovarian samples were taken from female sturgeon born in the former USSR in 1982, which arrived into France the same year, and reared at the INRA fish farm-CEMAGREF (now IRSTEA) hatchery, Donzacq (southwest of France). The fish farm was supplied with underground water at a constant temperature of 17 °C. Fish was fed with trout compound diets.
Biopsies were performed for light microscopy. Fixations were made with Bouin’s fluid for 72 h and tissue embedded in cytoparaffin. Serial sections 7 μm thick were stained with Groat eosin-hematoxylin and periodic acid-Schiff. Immunocytochemical staining using a specific antibody raised against Siberian sturgeon vitellogenin was achieved using peroxidase-antiperoxidase complex. This showed the time of incorporation of exogenous yolk into oocytes at light microscopy level (Pelissero 1988).
Very few females taken from the same 4- to 8-year-old age batch were sacrificed for electron microscopy every 3 months for 4 years. Gonads of stunned but live fish were perfused for 30 min with 3% glutaraldehyde in sodium cacodylate buffer 0.04 M, 0.05% CaCl2, with an osmotic pressure of 300 mosm at a pH of 7.4. Gonads were then cut in small pieces and immersed for 90 min in the same fixative and washed three times for 15 min in a washing buffer of the same composition as the fixative fluid except that glutaraldehyde was replaced by sucrose to obtain a similar osmolarity. Osmic postfixation by 2% OsO4 in sodium cacodylate buffer 0.04 M for 30 min was followed by washing in graded ethanols for dehydration and immersion in propylene oxide before embedding in Epon 812. Semi-thin sections were stained with 0.1% toluidine blue; lipids were revealed by azure blue 2 (Parry 1973) and slides submitted to photonic observations. Ultrathin sections were contrasted by uranyl acetate and lead citrate (Reynolds 1963) and observed on a 100S JEOL microscope (Département de Microscopie Electronique, Bordeaux University, France).
3 Oogenesis Description
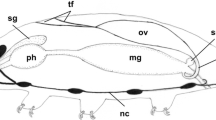
We chose a classification of oocyte stages based upon the distinction of the successive physiological steps in the process of the oocyte development (Fig. 14.1).
Schematic representation of the main development stages of the Siberian sturgeon oocytes reared in French conditions (Credit: F Le Menn). Abbreviations: BL, basement lamina; CA, cortical alveoli; EM, extracellular matrix; GC, granulosa cell; GJ, gap junction; LG, lipid globule; OMV, oocyte microvilli; PG, pigment granule; ThC, thecal cell; YG, yolk globule; zre, zona radiata externa; zri, zona radiata interna; zrie, zona radiata interna externa; zrii, zona radiata interna interna
3.1 Short Review of Initial Events
During embryogenesis, primordial diploid germ cells (PGC) of the germinal line are singled out very early and migrate toward the genital crests from which ovaries or testes derive. As soon as the PGC colonize the ovary, they connect with somatic cells. In fish as in other vertebrates, the development of oocytes in the ovary is inevitably linked with that of the surrounding somatic cells. An oocyte and its specialized somatic cell layers constitute an ovarian follicle (Wallace and Selman 1981; Guraya 1986; Selman and Wallace 1989; Selman et al. 1993; Matova and Cooley 2001).
Undifferentiated PGC give rise to differentiated oogonia. After a number of mitotic divisions, diploid oogonia undergo their first meiotic division differentiating into primary oocytes (Fig. 14.2). This first meiotic division stops at the end of the prophase in the diplotene stage. This arrest lasts for a few days or months in most Teleostei but for years in Chondrostei as for sturgeons. During this period, the female germinal cells accumulate informational components and nutritional reserves needed for embryo development in a process called vitellogenesis and complete differentiation of its cellular and a-cellular envelopes.
Nuclear stages correlated with primary oocyte development during the prophase of the first meiotic division. (Credit: F Le Menn). Synaptonemal complex structure schemas during nuclear stages are indicated in the upper figure, while corresponding primary follicle development stages are indicated in the lower par. (a) Oogonial nest in Acipenser baerii (Chondrostei, Acipenseridae). (b) Stage I ovarian follicle of A. baerii. (c) nucleus detail of Gobius niger Stage II ovarian follicle (Teleostei, Gobiidae). (d) Oreochromis niloticus Stage III ovarian follicle (Teleostei, Cichlidae). Abbreviations: bl, basement lamina; bv, blood vessel; ca, cortical alveoli; gc, granulosa cell; lgl, lipid globule; N, nucleus; n, nucleolus; oog, oogonium; oopl, ooplasm; poopl, peripheral ooplasm; pgrc, pre-granulosa cell; ygr, yolk granule; zre, zona radiata externa; tc, thecal cell. Scale bars = 30 μm in (a), 50 μm in (b), 100 μm in (c, d). Data (a–c) from F. Le Menn, University Bordeaux, France, and (d) from P. Ndiaye, IFAN, Dakar, Senegal, respectively
This first meiotic division gives rise to two cells differing greatly in size: a very small first polar body which degenerates and a large secondary oocyte.
Ovulation occurs once maturation is completed. The secondary oocyte separates from its envelopes (Fig. 14.3) and drops into the lumen of the ovary. In fish, as in most vertebrates, the second meiotic division proceeds to metaphase and pauses until fertilization, which activates the end of the division. This leads to the formation of a transient haploid female gamete, called ovum, and of a second polar body which degenerates like the first one. Fertilization occurs immediately by fusion of the haploid ovum nucleus with the haploid spermatozoon nucleus to form a diploid egg.
Photonic microscopy of Siberian sturgeon ovarian follicle at various development stages (Credit: F Le Menn). (a) Oogonia nest. (b, c) Early Stage I ovarian follicle. In French fish farm conditions this Stage I exhibits lipid globules. (d) Stage II ovarian follicle. (e): Stage III ovarian follicle. (f) Stage IIIb ovarian follicle yolk. Abbreviations: bl, basement lamina; gc, granulosa cell; lg, lipid globule; N, nucleus; n, nucleoli; ooc, oocyte; oogn, oogonia nest; tc, thecal cell; yg, yolk globule
3.2 From PGC to Oogonia
In several fish species including the sturgeon Acipenser baerii, the gradual transformation of PGC into oogonia has been observed using electron microscopy (Bruslé and Bruslé 1978; Le Menn et al. 2007; Grandi and Chicca 2008; Zelazowska et al. 2015). PGC are larger than somatic cells. They exhibit heavy electron density and a high nucleus-to-cytoplasm ratio. Nuclei are round and eccentrically located. They contain generally only one nucleolus and have few nuclear pores. The cytoplasm contains few organelles and some relatively large mitochondria located near the nucleus. They are intimately linked by dense material termed “ciment” (Clérot 1976) or germinal dense bodies (Hamagushi 1985). A dense material of the same kind, termed “nuages,” appears scattered through the perinuclear cytoplasm. These electron-dense “ciment” and “nuages” come from the nucleus (Mazabraud et al. 1975) and are often observed passing through the nuclear pores. They constitute excellent germinal cell markers on electron micrographs (Hogan 1978; Bruslé 1980).
In sturgeon, oogonia vary in size between 8 and 15 μm. Their nucleoplasmatic ratio is greater than one. They occur in clusters of 10–20 cells surrounded by adipose tissue. Each cluster is enclosed by a common single layer of pre-granulosa cells with a basal lamina (Figs. 14.2a and 14.3a).
Ultrathin sections show numerous lipid globules and mitochondrial aggregates intermingled with a granular endoplasmic reticulum. The oogonium exhibits a large, centrally located ovoid nucleus with a prominent spherical single nucleolus. The perinuclear ooplasm shows some “nuages” near the outer nuclear membrane and a lot of mitochondria aggregates around the “ciment.”
The oogonia multiply rapidly by successive divisions before the start of meiosis. Formed oogonia remain linked together with few surrounding somatic cells constituting the pre-granulosa cells. Each oogonia nest (Figs. 14.2a and 14.3a) is separated from the ovarian stroma by a basal lamina, extracellular matrix secreted by the granulosa epithelium at its base.
3.3 From Oogonium to Primary Oocyte and Ovarian Folliculogenesis
Normally, in diploid fish each oogonium is a diploid cell, with a nucleus containing two copies of each chromosome, called homolog, one from the father and one from the mother (Fig. 14.2). During the interphase following the last mitotic division of a diploid oogonium and preceding the first meiotic division of the primary oocyte, each homolog is duplicated by DNA replication, giving rise to a pair of identical chromatids, known as a sister chromatid pair. For each chromosome, a sister chromatid pair from the mother and one from the father are present in the nucleus of the primary oocyte. As a result, the development of the primary oocyte occurs in the presence of tetraploid chromatids, i.e., a double amount of DNA for RNA synthesis. This feature is unique to primary oocytes. Oogonia begin meiosis in the oogonial nest (Grier 2000) before separating from it. The karyotype evolution of germinal cells follows the same principle in sturgeons although the evolution of Acipenseriformes (sturgeon and paddlefish) is linked with polyploidization events. Indeed, Acipenser baerii with 249 ± 5 chromosomes is considered to be tetraploid (Fontana et al. 2008) even though hexaploid A. baerii were reported recently in fish farm conditions (Havelka et al. 2014).
When each oogonium gave rise to a primary oocyte, the granulosa epithelium and the basal lamina invaginated the cluster and surrounded each cells. Thecal cells were present outside the basal lamina (Figs. 14.2b and 14.3c). The oocyte and its surrounding layers formed a follicle (Fig. 14.3b, c). Thecal cells, as granulosa cells, are involved in sexual steroidogenesis during oocyte growth and maturation (Nagahama et al. 1995; Nagahama 1997). We advise using the term “granulosa cells” as opposed to “follicle cells,” because thecal cells and oocyte are also part of the follicle.
In sturgeon, as in all species studied, the development of the primary follicle is divided into five stages, which reflect the characteristic physiological steps in the development of the oocyte and the development of its surrounding somatic cell layers (Fig. 14.1).
The Stage I follicle is characterized by the nuclear genetic recombination of chromosomal chromatids at synaptonemal complexes. During Stage II, or pre-vitellogenesis, organelles and molecules used during the later stages are synthesized. Stage III, or vitellogenesis, is characterized by yolk deposition in the oocyte and the formation of the zona radiata (ZR), a protective structure surrounding the future egg. Stage IV is characterized by the rapid growth phase of the oocyte preceding Stage V: the maturation stage.
3.4 Ovarian Follicle Development Before Vitellogenesis
During the prophase of the first meiotic division, the nucleus of the oocyte passes through five successive stages: leptotene, zygotene, pachytene, diplotene, and diacinesis (Fig. 14.2). The first three nucleus stages occur during Stage I of primary oocyte development.
3.4.1 Stage I Follicle
3.4.1.1 Oocyte Events
We observed this stage for the first time in the gonad of Acipenser baerii taken in a 1-year-old batch with a mean size of 40 cm and a mean of weight of 800 g.
On semi-thin sections, a large centrally round nucleus contained a few nucleoli close to the smooth nuclear envelope (Figs. 14.2b and 14.3b). The cell diameter varied between 20 and 80 μm. In the vicinity of the nucleus, a crown of lipid granules was observed (Fig. 14.3b).
At the ultrastructural level, numerous mitochondrial aggregates (Fig. 14.4a), intimately associated with “ciment,” are spread in the peripheral ooplasm. These structures correspond to an intense multiplication of mitochondria (Clérot and Wegnez 1977). In the vicinity of the outer nuclear membrane, a crown of spotted highly electron-dense “nuages” seems to emerge from the nuclear envelope. The oocyte membrane, or oolemma, is smooth.
Stage II ovarian follicle (Credit: F Le Menn). (a) Mitochondrial aggregate, (b) peripheral ooplasm of early Stage II, (c) perinuclear area. Abbreviations: bl, basement lamina; ca, cortical alveoli; exom, extra-oocyte matrix; Gc, Golgi complex; gcr, granulosa cell; lg, lipid globule; m, mitochondrium; N, nucleus; n, nucleoli; ne, nuclear envelope; nex, nuclear extrusions; omv, oocyte microvilli; oopl, ooplasm; pnoopl, perinuclear ooplasm
In the leptotene phase (Fig. 14.2), both maternal and paternal sister chromatid pairs attach each of their ends to the inner membrane of the nucleus envelope, and, recognizing each other at a distance, they move closer together. In the following zygotene stage, the maternal and paternal homologous sister chromatids join together. A like-with-like recognition is mediated by a long central proteinous ladderlike core that gradually forms a synaptonemal complex with a maternal sister chromatid pair on one side and a paternal homologous sister chromatid pair on the other side. This tetrad chromatid structure named bivalent is observable by electron microscopy. During the pachytene stage, chromosomal exchanges occur between opposite non-sister chromatids with the help of recombination modules located along the central protein core of the synaptonemal complex.
3.4.1.2 Surrounding Events
The somatic granulosa cells, associated with the germinal cells since the PGC stage, multiply forming a regular epithelium of flattened cells. They complete the secretion of the basement lamina from their basal membrane. Outside the basement lamina, multilayers of unorganized meso-epithelial cells constitute the thecal layer, or theca, irrigated by numerous blood vessels.
Granulosa epithelium, closely adjacent to the oolemma, exhibit ultrastructural features of metabolic activity (mitochondria, rough endoplasmic reticulum, Golgi complexes, and coated vesicles) (Fig. 14.3c). Coated pits are present on the outer basal granulosa membrane against the basement lamina.
3.4.2 Stage II Follicle
In this stage, the oocyte diameters vary between 80 and 120 μm. The Stage II appeared for the first time in gonad of 3-year-old fish with an average weight of 2 kg.
3.4.2.1 Oocyte Events
This is the pre-vitellogenesis stage, i.e., preceding the entry of yolk precursors into the oocyte (Le Menn and Burzawa-Gerard 1985). Semi-thin observations showed, by azure blue coloration, an increase of the number of lipid globules (Fig. 14.3d).
The nucleus exhibited a new feature, characteristic of the diplotene stage of the prophase of the first meiotic division. Two kinds of processes occurred. The first was the de-structuring of the synaptonemal organization by disassembling the lateral protein axes of the ladderlike central core (Fig. 14.2 higher part). Compared to somatic cells, each sister chromatid pair was highly active in RNA synthesis, due to the double amount of DNA. The newly transcribed RNAs, packed into dense RNA/protein complexes, were visible on electron micrographs on large chromatin loops emanating from the linear chromatid axis. At this pre-vitellogenic stage, the so-called lampbrush chromosome structure appeared and persisted throughout the diplotene phase, i.e., during vitellogenic Stages IIIa and IIIb. The second process was an increase in nucleoli number due to a huge amplification of the nucleolar organizer genes. They were located in the concavities of the wrinkles of the nuclear envelope near the inner nuclear membrane (Figs. 14.2c and 14.4c). At the same time, both ribosomal RNA and mRNAs were transported toward the ooplasm, appearing in the perinuclear ooplasm as electron-dense nuclear extrusions (Fig. 14.4c). For example, large quantities of vitellogenin receptor mRNA were accumulated in the ooplasm during this Stage II (Davail et al. 1998; Perazzolo et al. 1999; Agulleiro et al. 2007).
The ooplasm was very electron dense, due to numerous tightly packed ribosomes. It exhibited a scattered smooth endoplasmic reticulum. Mitochondria migrated toward the peripheral ooplasm where few Golgi complexes were observed. The remaining part of the cytoplasm also contained Golgi complexes with numerous Golgian vesicles.
The oolemma separated from some areas of the apical granulosa cell surface. The intervening space was filled with a flocculent, electron-clear material, corresponding to an extra-oocyte matrix (Fig. 14.4b). In this matrix, oocyte microvilli protruded from the oocyte surface toward the granulosa epithelium (Fig. 14.4b). In numerous species, microvilli are grouped together in batches. In Acipenser baerii, circular oocyte microvilli batches are under umbrellalike structures formed by the apex of the granulosa epithelium (Fig. 14.5b). This apical granulosa membrane adhered to the oolemma as gap junctions around each batch of microvilli (Fig. 14.5c). Then, at the end of this Stage II, as soon as these junctions disappeared, oocyte microvilli appeared in the extra-oocyte matrix in a parallel radial position toward the granulosa cells (Fig. 14.6a), as observed on cryofracture preparations (Le Menn, personal observations).
Stage II ovarian follicle (Credit: F Le Menn). (a) Ovarian follicle envelopes, (b) relationship granulosa cell/oocyte, (c) gap junction granulosa cell/oocyte, (d) gap junction granulosa cell/granulosa cell. Abbeviations: bl, basement lamina; cp, endocytotic coated pit; erc, erythrocyte in thecal blood vessel; exom, extra-oocyte matrix; gc, granulosa cell; gp, gap junction; m, mitochondrium; N, nucleus; omv, oocyte microvilli; oopl, ooplasm; tc, thecal cell; arrows, gap junction
Early Stage IIIa ovarian follicle (credit: F Le Menn). (a) Disappearance of gap junction granulosa cells/oocyte. (b) Detail of VG endocytosis. Abbreviations: bl, basement lamina; exom, extra-oocyte matrix; gc, granulosa cell; omv, oocyte microvilli; oopl, ooplasm; arrow, coated pit and coated vesicles
3.4.2.2 Surrounding Events
Granulosa cells multiplied to form an epithelial coating of cubic cells, joined together by gap junctions (Fig. 14.5d). In species such as the Siberian sturgeon, the basement lamina might be exceptionally thick up to five times thicker than as the granulosa epithelium (Fig. 14.5a). The thecal layer consisted of a few cells close to blood vessels just outside the basement lamina (Fig. 14.5a).
At the end of pre-vitellogenic Stage II, the oocyte contained all the molecules and organelles needed for its subsequent endocytotic and exocytotic activities during oocyte vitellogenesis. It was surrounded in a centripetal pattern by cellular and a-cellular layers, i.e., theca, basement lamina, granulosa, and extra-oocyte matrix. The oocyte started forming microvilli which became, at the next stage, the essential means for managing exchanges with the general blood flow.
3.5 Ovarian Follicle Development During Vitellogenesis
Ovarian follicle enlargement occurred in Stage III, while the nucleus remained in the diplotene stage (Fig. 14.2). During this stage the oocyte accumulated, from the blood stream, the yolk containing nutritional reserves needed for embryo development. It also completed the differentiation of its cellular and a-cellular envelopes. In sturgeon as in other oviparous vertebrates, the sexual cycle and ovarian follicle development are controlled by environmental factors. In response to these factors, the central nervous system induces a cascade of neurohormones leading to the secretion of gonadotropin-releasing hormone (GnRH) by specialized hypothalamic neurons. The GnRH acts on pituitary cells, which secretes the undifferentiated gonadotrophin hormones (GTH). The GTH signal, mediated by specific ovarian receptors located on thecal and granulosa cells, leads to the synthesis of sexual steroid hormones, such as 17ß-estradiol (E2). E2 is secreted into the theca blood vessels to reach the blood stream. In response specific receptors in hepatocytes mediate the synthesis and release into the blood of vitellogenins (Vtgs), the main yolk precursors in plasma. Vtgs are specifically incorporated by the oocytes via receptor mediated endocytosis (Stifani et al. 1990; Chan et al. 1991; Davail et al. 1998; Hiramatsu et al. 2004). The term vitellogenesis has a double meaning: strictly speaking the hepatic synthesis of Vtgs but also the incorporation of Vtgs by the oocyte and further processing into yolk proteins.
3.5.1 Stage IIIa Follicle
This substage was characterized by the first discrete entry of Vtgs into the oocyte ooplasm, only detectable in electron microscopy (Figs. 14.3e and 14.6b). As in many fish species, a huge accumulation of lipid globules was seen in the Siberian sturgeon Stage IIIa oocytes. This was previously described as Type I vitellogenesis (Breton et al. 1983).
Oocyte diameter varied from 120 to 600 μm. This sub-Stage IIIa appeared for the first time in the gonad of 3.5-year-old females with a mean weight of 4 kg.
3.5.1.1 Oocyte Events
The Vtgs reached the oolemma by passing first between thecal cells, through the basement lamina, between the granulosa cells, and, finally, along the oocyte microvilli in the surrounding oocyte matrix (Selman and Wallace 1982; Abraham et al. 1984; Le Menn et al. 2007) (Fig. 14.7a).
Schematic representation of Stage III ovarian follicle (Credit: F Le Menn). (a) Stage IIIa, (b) Stage IIIb. Abbreviations: bl, basement lamina; bv, blood vessel; ca, cortical alveoli; cp, endocytotic coated pit; cv, coated vesicle; gc, Golgi complex; grc, granulosa cell; lgl, lipid globule; mvb, multivesicular body; poopl, peripheral ooplasm; tc, theca cell; Vg, vitellogenin; ygl, yolk globule; ygr, yolk granule; zre, zona radiata externa; zri, zona radiata interna; arrow, Golgian exocytotic vesicle
It is now well established that Vtgs is selectively sequestered by growing ovarian follicles via specific receptors in the oolemma, which become effective at this early stage of vitellogenesis. Numerous endocytotic clathrin-coated pits (Pearse 1976) developed in the oolemma leading to the formation of coated vesicles that moved into the peripheral ooplasm (Fig. 14.7a). These Vtg-containing coated vesicles are fused with lysosomes, as multivesicular bodies (MVB), originating from the Golgi apparatus. The MVB contain lysosomal enzymes, including cathepsin D, that possibly cleave the Vtgs into smaller yolk proteins (Sire et al. 1994; Carnaveli et al. 1999).
In the Siberian sturgeon oocytes, the MVB increased in size and were gradually transformed into small yolk granules, then in larger yolk globules (Fig. 14.7a). Spots of electron-dense “nuages” were still located near nuclear pores, but the patches of “ciment” had disappeared. Mitochondria were scattered throughout the ooplasm. It contained an abundant smooth endoplasmic reticulum. In the nucleus, nucleoli nested in crenelated nuclear envelope concavities and exhibited a granulated feature. Exocytosis events occurred in the peripheral ooplasm and oolemma, associated with a first outer deposit of a protective layer (Fig. 14.7a).
3.5.1.2 Surrounding Events
This deposit occurred in the oocyte matrix, between oocyte microvilli. In ultrathin tangential sections, this zona radiata externa (ZRE) appeared as an amorphous structure perforated by channels containing the oocyte microvilli (Fig. 14.8a–c). This was correlated with the first entry of Vtgs into the oocyte and indicated the beginning of Type I vitellogenesis (Fig. 14.6b) (Le Menn et al. 1999). In all species studied, the ZRE was visible using high-magnification light microscopy. It appeared as a thin, neutral glycoprotein, periodic acid-Schiff positive line located at the oolemma surface. In electron microscopy, some cells of the thecal layer had irregularly shaped nuclei, well-developed smooth endoplasmic reticulum, and characteristic crested mitochondria. These steroidogenic cells were involved in androgen synthesis induced by GTH. The androgens were converted into estrogens in the underlying granulosa cells. E2 passing through basement lamina and thecal cells was discharged into the blood stream via thecal vessels (Kagawa et al. 1981; Kagawa 1985; Nakamura and Naghama 1885; Nakamura et al. 1993).
3.5.2 Stage IIIb Follicle
At this substage, the acting molecules and organelles were identical as in the previous sub-Stage IIIa. However, the proportion of lipid and yolk globules was inverted, and the follicles increased rapidly in size (Fig. 14.7b). This stage was previously described as Type II vitellogenesis (Breton et al. 1983).
3.5.2.1 Oocyte Events
The Vtgs endocytosis was very active at this stage, inducing a rapid growth of the follicle from 600 to 900 μm in sturgeon. All yolk globules and lipid globules were intermingled in the ooplasm (Fig. 14.3f).
In light microscopy, a clear crown of lipid globules was observed in the medium cytoplasm. With PAS treatment, a very thin glucidic layer was observed. This was the first visualization of the ZRE located between the oolemma and the granulosa epithelium. Small granules located in the peripheral ooplasm were PAS positive and corresponded to the cortical alveoli.
Cortical alveoli appeared clustered in groups of three or four near a Golgi complex and mitochondria on electron micrographs (Fig. 14.9a–c). They contained fibrillar material. These organelles were not used by the embryo and cannot be considered as part of the yolk. At the end of vitellogenesis, they were distributed in a single layer underlying the oolemma. At fertilization, they fused with the oolemma. They discharged their glycoprotein content at the oocyte surface by exocytosis during the cortical reaction of the female gamete.
Cortical alveoli formation (Credit: F Le Menn). (a) Transversal section, (b) longitudinal section, (c) peripheral ooplasm in Stage IV ovarian follicle. Abbreviations: ca, cortical alveolus; ca (ls), in longitudinal section; ca (ts), in transversal section; gc, Golgi complex; m, mitochondrium; mvb, multivesicular body; ooml, oolemma; oomv, oocyte microvilli; pg, pigment granule; ygl, yolk globule; zri, zona radiata interna
In the peripheral ooplasm, a huge amount of endocytotic vesicles of Vtgs moved centripetally toward the MVB. In the meantime, numerous dense-cored vesicles originating from the Golgi apparatus reached the oolemma to deposit the zona radiata interna (ZRI) at the oocyte surface by exocytosis (Fig. 14.7b). These transfers were probably helped by the presence of a cytoskeleton network underlying the oolemma. This network, known as the terminal web, was formed by actin filaments, where the internal actin skeleton of each microvillus was anchored. A great number of endocytotic vesicles budded off from the oolemma to the peripheric ooplasm near the base of the oocyte microvilli, in correlation with the enormous amount of yolk stored. These coated pits and coated vesicles have not previously been observed in the oolemma of the microvilli, probably due to bundles of axial actin filaments forming an internal skeleton preventing any distortion of the oolemma (Fig. 14.6a, b).
In sturgeon as in numerous other species, the formation of crystals in yolk globules (yolk platelets) seems to enhance the capacity of the oocyte to store yolk (review in Wallace and Selman 1981). Yolk platelets appeared as regularly or irregularly shaped crystalline cores distributed in an apparently amorphous matrix (Fig. 14.10) (Karasaki 1967).
3.5.2.2 Surrounding Events
The ZR interna was formed in the extra-oocyte matrix by successive deposits of inner electron-clear reticulated layers, gradually displacing the ZR externa toward the granulosa cells (Fig. 14.8c, d). Unlike the amorphous structure of the ZR externa, the ZR interna had reticulated deposits with twisted arrangements, giving it an arched appearance of polymerized fibrillar secretion in a matrix. The parabolic lamellar pattern appeared clearly on oblique ultrathin sections (Fig. 14.8b). The ZR interna structure was common to all oviparous Teleostei and Chondrostei species studied. Many other biological polymers produce these twisted fibrous arrangements by deposition of microfibrils in regular helicoidal arrays, providing pliant structures, having generally a protective function (Bouligand 1972; Giraud-Guille 1996). These formations showed a pattern similar to the well-defined molecular state known as the cholesteric phase, as first described in microscopic preparations of cholesterol derivatives (Fridel 1922). These supramolecular arrangements appeared in a viscous state with some fluidity, as molecules are free to move with respect to each other (Besseau and Giraud-Guille 1995). In ray-finned fish, this particular structure might gradually stretch, due to very great increase of oocyte volume during its rapid growth phase in this Stage IIIb. The basement lamina very thick (over 10 μm) at the previous stage decreased to 1 μm.
The micropyles were fully formed during sub-Stage IIIb (Fig. 14.11b). The micropylar cells originating from the granulosa epithelium induced a local reorganization of the zona radiata into one or more funnels, depending on the species. In the Siberian sturgeon, the number of micropyles was reported to be 8.2 ± 3.2 (Ginzburg and Detlaf 1969). Micropyles constitute the only entrance into the egg for the spermatozoa (Dumont and Brummet 1980; Cherr and Clark 1982; Amanze and Lyengar 1990). The location of the micropyles on the oocyte surface delineates the future animal pole (Fig. 14.11a). ZR glycoproteins were reported to have an affinity for spermatozoa and to guide sperm into the micropyle (Amanze and Lyengar 1990; Iwamatsu et al. 1997).
Stage V ovarian follicle (Credit: F Le Menn). (a) Migration of the germinal vesicle of a fully grown ovarian follicle after boiling and medial cutting, (b) one micropyle of A. baerii, observed with scanning microscope after ovulation. Abbreviations: ap, animal pole; jc, jelly coat; N, germinal vesicle; pg, pigment granules accumulated beneath the oolemma; zr, zona(s) radiata(s)
3.6 From Primary Oocyte Undergoing Vitellogenesis to Secondary Oocyte: Stage IV and Maturational Processes
3.6.1 Stage IV Follicle
During Stage IV, the primary oocyte left the diplotene stage and underwent the first meiotic division. Unfortunately, the term “maturation” has very often been used improperly to describe primary oocyte size enlargement during arrest in the prophase of the first meiotic division. This has led to misunderstandings between scientists and fish farmers. Maturation processes were induced by gonadotropic hormones and occurred in the ooplasm and nucleus of primary oocytes. The maturation signal consisted of a new pulse of gonadotrophic hormones, stimulating ovarian somatic follicle cells and triggering synthesis of the maturation-inducing steroid (MIS). MIS acted on specific receptors located on the oolemma, which, in turn, triggered the synthesis and activation of maturation-promoting factor (MPF) in the ooplasm (Yamashita 1998; Senthilkumaran et al. 2004).
This stage corresponded to the rapid vitellogenic growth phase leading to a considerable increase in oocyte diameter (900–2800 μm). It was found for the first time in a 5-year-old fish with a mean weight of 5 kg.
In light microscopy, yolk globules were first observed in the outer part of the cytoplasm, then in the whole cytoplasm intermingled with lipid globules (Fig. 14.3f). In the surrounding layers, the basement lamina became too thin to be observable.
On semi-thin sections, yolk globules were ovoid and lipid globules were spheric (Fig. 14.3f). The zona radiata interna exhibited two layers, numerous narrow stratifications, and a new zona radiata interna interna (ZRII) deposited under the zona radiata interna externa (ZRIE) (Fig. 14.8a–c). Electron micrographs showed that the number of yolk globules increased considerably compared to those of the cortical alveoli and lipid globules. The yolk globules showed yolk platelets (Fig. 14.10) inside a granular matrix. Mitochondria and rough endoplasmic reticulum were scattered throughout these inclusions. Pigment granules appeared underneath the peripheral ooplasm and a very thick layer of mitochondria. These pigments showed a very electron-dense matrix inside a vacuole with dark granulations (Fig. 14.10).
3.6.2 Stage V Follicle or Maturational Events
This stage corresponds to the maturation process. We observed these features in gonads of 6-year-old females with an average weight of 5.5 kg, and the size of oocyte can increase to 3200 μm. These fish were maintained in the INRA fish farm (Donzacq) at a constant temperature of 17 °C during the whole year.
On semi-thin sections, the nucleus migrated to the periphery of the oocyte toward the animal pole, associated with the micropyles in the granulosa layer (Fig. 14.11a).
The morphological events during maturation generally followed a similar sequence in all Teleostei and Chondrichthyes. However, in sturgeon it can take several months. For Acipenser baerii the phenomena took up to 6 months under the conditions of the Donzacq fish farm.
On ultrathin sections, at the end of this stage, just before the germinal vesicle break down (GVBD), the microvilli had retracted. The structure of the zona radiata was denser (Fig. 14.12). Yolk granules could fuse with lipid globules to form a liquid yolk mass located at the vegetative pole of the oocyte. Above the ZRE, a new electron-clear spongy layer, the “jelly coat,” was deposited by granulosa cells (Fig. 14.12). This layer partially entered the empty pores of the ZRE. The thickness of the jelly coat varied from 0.5 to 0.9 μm (Le Menn and Pelissero 1991).
MPF acted on the lamina covering the inside of the internal nuclear membrane, phosphorylating lamina serines, and leading to de-structuring of the nuclear envelope, setting in motion the GVBD.
Metaphase of the first meiotic division occurred. It produced two cells, a large secondary oocyte and a small first polar body which degenerated. The secondary oocyte separated from its somatic surrounding layers (Fig. 14.13) during ovulation. In the wild, spawning occurred spontaneously. In fish farm, sturgeon’s spawning required carp or mammalian LH injections as well as manual stripping. This was a gentle pressure on the female’s abdomen. In all cases, the spawn cells were matured secondary oocytes or ova. Fusion with a spermatozoon produced fertilized egg, i.e., the starting point of embryo development.
Dissection after boiling of a Stage V ovarian follicle (Credit: P Williot). In the upper left part is a heap which corresponds to the two granulosa and theca externa layers irrigated by blood vessels. Underneath and fold to the left is the jelly coat. The most complete and thicker layer is the zona radiata externa. Inside and fold to the right is the zona radiata interna
4 Discussion
Oogenesis in Acipenser baerii resembles oogenesis in numerous fresh water teleosts (Nagahama 1983). Nevertheless some synthetic and structural particularities were worth noting.
4.1 Acipenser baerii Synthetic and Endocytotic Features
4.1.1 Type 1 and Type 2 Vitellogenesis
Type 1 vitellogenesis corresponds to the formation of cortical alveoli and lipid globules in the oocyte. It is usually called endogenous vitellogenesis. Type 2 vitellogenesis corresponds to the concomitant incorporation of vitellogenin, a hepatically secreted phosphoglycoprotein which is the plasma yolk precursor (review of De Vlaming et al. 1980; Wallace et al. 1983). In Type 2 vitellogenesis, endocytotic events predominate greatly compared with synthetic events. Pre-vitellogenesis corresponds to Type 1 vitellogenesis (Jalabert, Le Bail and Le Menn, unpublished data).
In Acipenser baerii lipid globules are identified by histochemical staining from the oogonial stage onward (Pelissero et al. 1985). This has not been observed in other species (Wallace and Selman 1981), even in freshwater species where lipid synthesis occurs for a very long time before Type 2 vitellogenesis (Droller and Roth 1966, for Anguilla anguilla; Ulrich 1969, for Brachydanio rerio or Busson-Mabillot 1969, for Lebistes reticulatus). However, we should recall that Acipenser baerii is reared in France under specific temperature and feeding conditions, and no firm conclusion can be reached until gametogenesis in wild populations living in natural conditions in the Russia has been studied.
4.1.2 Cortical Alveoli Synthesis
In the oocyte at the end of Stage 2, at the beginning of oocyte microvillosities protrusion, we observed a very considerable Golgian synthetic activity in the medium and peripheral ooplasm with numerous Golgi complexes (Fig. 14.4b). In the vicinity of each Golgi complex, several spherical aggregates of Golgian vesicles led to the formation of small vesicles by deposition of the Golgian secretions in central smooth endoplasmic reticulum vesicles. These vesicles were filled with granular material resembling material contained by cortical alveoli in the next stage (Figs. 14.9 and 14.10). We suppose that these features are the first visualization of synthesis of cortical alveoli. Further investigations are necessary to confirm this hypothesis, by identifying the chemical nature of their content on ultrathin sections.
4.2 Specific Structural Features
4.2.1 Basement Lamina
This extracellular matrix was located between the granulosa epithelium and the thecal cells. The basement lamina is generally secreted by epithelium (Hay 1981). In this case, a granulosa source was indicated by observations of exocytotic features on the plasmic granulosa membrane related to the matrix. Its secretion occurred until the follicle reached Stage III where the thickness could be 10 μm. During the rapid growth phase, the stretching of the follicle caused a decrease of the thickness to 1 μm making it invisible in light microscopy.
This matrix was identified in Huso huso, Acipenser gueldenstaedtii, Acipenser ruthenus, and Acipenser stellatus (Caloianu-Yordachel 1971; Kornienko 1975). In Acipenser baerii, it is a polysaccharidic layer stained with PAS. In this species electron, micrographies showed a simple organization of small circular electron-clear conglomerates in an amorphous matrix, unlike the usual three-layered structure of a true basement lamina (Vrackq 1974) with a lamina lucida between two lamina densa. The literature leads us to expect a biochemical composition of glycosaminoglycans, elastin, and collagen and the function of a semipermeable filter (Hay 1981). This function would be interesting to observe in an investigation of the pathway of plasma vitellogenin from thecal capillaries to the oocyte (Selman and Wallace 1982; Abraham et al. 1984). Another explanation could be the protection of early oocyte stages from shocks. As most cartilaginous fish, the sturgeon species are known to be fragile to manipulation and therefore to any shock which could occur in rapid water in the wild. Because the early vitellogenic stage lasts for a long time in this species at a time when the fish is still of modest size, this protection may help in ensuring the preservation of future progeny.
4.2.2 Extra-Oocyte Matrix
This matrix deposited between the oolemma and the granulosa epithelium is particularly emphasized in Acipenser baerii. Few authors mention its presence in other fish: its electron-clear fibrillar material mentioned without specification by Caporiccio and Connes (1977) has been interpreted as a cell coat by Busson-Mabillot (1973), as the first deposited zone of the primary envelope (Anderson 1967; Wourms 1976), as a follicular matrix by Abraham et al. (1984), and as an extra-oocyte matrix by Le Menn (1984) and Nunez Rodriguez (1985). In all fish we studied, ultrastructural features gave no indication about its cellular origin.
This matrix was deposited as soon as the granulosa epithelium separated from oolemma at the end of Stage 1 follicle. It is in this environment that the zona radiata externa is deposited. The chemical composition of this matrix is not defined, but it probably contains proteoglycans (Hascall and Hascall 1981) well known for their ability to capture and release free ions. This structure could influence the pH in the vicinity of the oocyte and act on the incorporation of exogenous vitellogenin (VTG). Indeed this molecule reaches the oolemma by passing through this matrix along the oocyte microvilli. It has been demonstrated in birds that the binding of the VTG to its receptors is pH dependent with the best binding for acid pH values (Woods and Roth 1980; Yusko et al. 1981). So, such a structure may interfere in the process of internalization by providing an adapted microenvironment. As for the basement lamina, a protective function of this extracellular matrix toward the future progeny cannot be simply ruled out.
4.3 Zona Radiata
This proteoglycan matrix is built up between the oocyte and the granulosa epithelium synthesized apparently by the oocyte. The first part deposited is then in an outer position compared with the later parts deposited. At the end of vitellogenesis, at Stage 4, just before process of maturation, the zona radiata is formed by three layers: we observed in order of deposition the zona radiata externa (ZRE), the zona radiata interna externa (ZRIE), and the zona radiata interna interna (ZRII). During the formation of this matrix, we observed numerous dense-cored Golgian vesicles associated with Golgi complexes located in the peripheral ooplasm just under the oolemma. These features seem to confirm as in other fish species the oocyte origin of this matrix (Tesoriero 1977; Le Menn 1984; Nunez Rodriguez 1985). These three layers have been observed in other sturgeon species (Magnin 1967; Ginzburg 1968; Caloianu-Yordachel 1971; Markov 1975; Cherr and Clark 1982), but the interpretations of Russian authors are not in agreement with ours. They consider the ZRE as the outer layer which allows the eggs to stick on the substrate after laying, and they call it the “jelly coat.” In fact at the end of vitellogenesis during maturation, this new spongy layer is deposited on the zona radiata externa (Pelissero et al. 1985) causing the fixation of the egg when it is spawned in water. This layer is described in Acipenser transmontanus (Cherr and Clark 1982, 1984). Such specific secretions involved in the fixation of eggs have been described in teleosts: Cichlasoma nigrofasciatum (Busson-Mabillot 1977), Cynolebias melanotoenia and Cynolebias ladigesi (Wourms and Sheldon 1976), Pomatoschistus minutus (Riehl 1978), and Gobius niger (Le Menn 1984). In all these species, as in Acipenser baerii, ultrastructural features indicated that the sticking material was secreted by the granulosa cells.
Until the end of vitellogenesis, only one microvillus is observed in each channel of the zona radiata. Granulosa cells never put out microvilli toward the oocyte. From the beginning of Stage III onward, the follicular epithelium is completely separated from the surface of the oocyte as soon as the junctions with oolemma disappeared. We have never observed on this species junctions between the apical part of oocyte microvilli and the apical plasmic membrane of the granulosa epithelium as shown in Plecoglossus altivelis (Toshimori and Yasuzuni 1979).
References
Abraham M et al (1984) The cellular envelope of oocytes in Teleosts. Cell Tissue Res 235:403–410
Agulleiro M et al. (2007) High transcript level of fatty acid-protein but not very low-density lipoprotein receptor is correlated to ovarian follicle in teleost fish (Solea senegalensis). Biol Reprod PMID: 17554079
Akimova NB et al. (1979) Growth and gametogenesis of the Siberian sturgeon (Acipenser baerii B.) under experimental and natural conditions. Proc. 7th Japan Soviet joint Symp Aquacult Tokyo: 179
Amanze D, Lyengar A (1990) The micropyle: a sperm guidance system in teleost fertilization. Development 109:495–500
Anderson E (1967) The formation of the primary envelope during oocyte differentiation in teleosts. J Cell Biol 35:193–212
Badenko LV et al (1981) Method for evaluating the quality of sturgeon spawners as exemplified in the sevryuga (Acipenser stellatus) from the Kuban river. J Ichthyol 21:96–103
Besseau L, Giraud-Guille MM (1995) Stabilization of fluid cholesteric phases of collagen to ordered gelateed matrices. J Mol Biol 251:197–202
Bouligand Y (1972) Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 4:189–217
Breton B et al (1983) Maturational glycoprotein gonadotropin and estradiol-17ß during the reproductive cycle of the female brown tour (Salmo trutta). Gen Comp Endocrinol 49:220–231
Bruslé S (1980) Fine structure of early previtellogenic oocytes in Mugil (Lisa) auratus Risso, 1810 (Teleostei, Mugilidae). Cell Tissue Res 207:121–134
Bruslé S, Bruslé J (1978) An ultrastructural study of early germs cells in Mugil (Liza) auratus Risso, 1810 (Teleostei, Mugilidae). Ann Biol Anim Biophys 18:1141–1153
Busson-Mabillot S (1969) Données récentes sur la vitellogénèse. Ann Biol 8:199–227
Busson-Mabillot S (1973) Evolution des enveloppes de l’ovocyte et de l’œuf chez un poisson téléostéen. J Microscopie 18:23–44
Busson-Mabillot S (1977) Un type particulier de sécrétion exocrine, celui de l’appareil adhésif de l’oeuf d’un poisson téléostéen. Biol Cell 30:233–244
Caloianu-Yordachel M (1971) L’ovogénèse chez les poissons Acipenséridés, la morphogénèse et la constitution histochimique des membranes externes. Rev Roum Biol Zool 16(2):113–120
Caporiccio B, Connes R (1977) Etude ultrastructurale des enveloppes périovocytaires et périovulaires de Dicentrarchus labrax L. (Poisson téléostéen). Ann Sei Nat Zool Paris 19(12ème série):351–368
Carnaveli O et al (1999) Yolk formation and degradation during oocyte maturation in bream, Sparus aurata: involvement of two lysosomal proteinases. Biol Reprod 60:140–146
Chan L et al (1991) Vitellogenin purification and development of assay for vitellogenin receptor in oocyte membranes of the tilapia (Oreochromis niloticus Linnaeus) 1766. J Exp Zool 257:96–109
Cherr GN, Clark WH Jr (1982) Fine structure of the envelope and the micropyles of the eggs of the white sturgeon Acipenser transmontanus. Develop Growth Differ 24:341–352
Cherr GN, Clark WH Jr (1984) Jelly release in the eggs of the white sturgeon Acipenser transmontanus: an enzymatically mediated event. J Exp Zool 230:145–149
Clérot JC (1976) Les groupements mitochondriaux des cellules germinales de poissons Téléostéens Cyprinidés. 1. Etude ultrastructurale. J Ultrastruct Res 54:461–475
Clérot JC, Wegnez M (1977) Etude ultrastructurale et biochimique de l’ovocyte en prévitellogénèse de vertébrés inférieurs. Biol Cell 29:23a
Davail B et al (1998) Evolution of oogenesis: the receptor fot vitellogenin from the rainbow trout. J Lipid Res 39:1929–1937
De Vlaming VL et al (1980) Golsfish Carassius auratus vitellogenin: induction, isolation, properties and relationship to yolk proteins. Comp Biochem Physiol 67 b:613–623
Droller MJ, Roth TF (1966) An electron microscope study of yolk formation during oogenesis in Lebistes reticulatus Guppyi. J Cell Biol 28:209–232
Dumont JN, Brummet AR (1980) The viteline envelope, chorion and micropyle of Fundulus heteroclitus eggs. Gamete Res 3:25–44
Fedorova LS (1976) Physiological and biochemical characteristics of the reproductive products and larvae of sturgeons during artificial rearing. J Ichthyol 16:427–436
Flynn SR, Benfey TJ (2007) Sex differentiation and aspects of gametogenesis in shortnose sturgeon Acipenser brevirostrum Lesueur. J Fish Biol 70:1027–1044
Fontana F et al (2008) Evidence of hexaploid karyotype in shortnose sturgeon. Genome 51(2):113–119
Fridel G (1922) Les états mésomorphes de la matière. Ann Phys 18:273–474
Ginzburg AS (1968) Fertilisation in fishes and the problem of polyspermy. N O A A and National Scientific Foundation Translation, Silver Spring, p 290
Ginzburg AS, Detlaf TA (1969) Razvitie Osetrovykh Ryb. Sozrevanie yats, oplodotvorenie i embriogenez. Izdatel’stvo “NAUKA”, Moskva, p 134
Giraud-Guille MM (1996) Twisted liquid crystalline supramolecular arrangements in morphogenesis. Int Rev Cytol 166:59–101
Grandi G, Chicca M (2008) Histological and ultrastructural investigation of early gonad development and sex differentiation in Adriatic sturgeon Acipenser nacarii, (Acipenseriformes, Chondrostei). J Morphol 269:1238–1262
Grier H (2000) Ovarian germinal epithelium and folliculogenesis in the common snook Centropomus undecimalis (Teleostei, Centropomidae). J Morphol 243:265–281
Guraya SS (1986) The cell and molecular biology of fish oogenesis. In: Sauer HW (ed) Monographs in developmental biology, vol 18. Karger, Basel, pp 1–223
Hamagushi S (1985) Changes in the morphology of the germinal dense bodies in primordial germ cells of the teleost Oryzias latipes. Cell Tissue Res 240:669–673
Hascall VC, Hascall GK (1981) Proteoglycans. In: Hay ED (ed) Cell biology of extracellular matrix. Plenum Press, New York, pp 39–60
Havelka M et al (2014) Fertility of a spontaneous hexaploid male Siberian sturgeon, Acipenser baerii. BMC Genet 15:5
Hay ED (1981) Cell biology of extracellular matrix. In: Hay ED (ed) . Plenum Press, New York, p 417
Hiramatsu N et al (2004) Molecular characterisation and expression of vitellogenin receptor from the white perch (Morone Americana). Biol Reprod 70:1720–1730
Hogan JC (1978) An ultrastructural analysis of “cytoplasmic maker” in germ cells of Oryzias latipes. J Ulrastruct Res 62:237–250
Iwamatsu T et al (1997) Effect of micropylar morphology and size on rapid sperm entry into the eggs of medaka. Zool Sci 14:626–628
Kagawa H (1985) Ultrastructural and histochemical observations regarding the ovarian follicles of the amago salmon (Oncorhynchus rhodurus). J UOEH 7:27–35
Kagawa H et al (1981) Correlation of plasma estradiol-17-β and progesterone levels with ultrastructure and histochemistry of ovarian follicles in the spoted char, Salvenilus leucomaenis. Cell Tissue Res 218:315–329
Karasaki S (1967) An electron microscope study on the crystalline structure of the yolk platelets of the lamprey egg. J Ultrastruct Res 18:377–390
Kijima T, Maruyama T (1985) Histological research for the development of the gonad of the hybrid sturgeon bester (Acipenser ruthenus male x Huso huso female). Bull Nati Res Inst Aquacult 8:23–29
Kondrat’yev AK (1977) The functional morphology of oocytes in the period of previtellogenesis in the Siberian sterlet Acipenser ruthenus M. At different time of its annual biological cycle. J Ichthyol 17:769–778
Kornienko GG (1975) Early degenerative changes in the oocyte of the Kuban sevryuga Acipenser stellatus. J Ichthyol 15:503–507
Le Menn F (1984) Aspects ultrastructuraux, biochimiques et endocriniens de la vitellogénèse d’un poisson téléostéen Gobius niger L. Thèse de Doctorat d’Etat, Univ. Bordeaux I, n° 814
Le Menn F, Burzawa-Gerard E (1985) Effect of carp gonadotrophin (cGTH) and a fraction unadsorbed on concavalin A-sepharose obtained from c-GTH on vitellogenesis in the hypophysectomized marine teleost Gobius niger. Gen Comp Endocrinol 57:23–36
Le Menn F, Pelissero C (1991) Histological and ultrastructural studies of oogenesis of the Siberian sturgeon (Acipenser baerii). In: Williot P (ed) , vol 57. CEMAGREF, Antony, France, pp 23–36
Le Menn F et al. (1999) A new approach to fish oocyte vitellogenesis. Proc 6th Int. Symp Reprod Physiol Fish, Bergen, Norway: 281–284
Le Menn F et al. (2007) Ultrastructural aspects of the ontogeny and differentiation of ray-finned fish ovarian follicles. In: Babin PJ et al (eds) The fish oocyte: from basic studies to biochemical applications, p 1–37
Magnin E (1967) Recherches sur les cycles de reproduction des esturgeons Acipenser fulvescens Raf. de la rivière Nottaway tributaire de la Baie James. Verh Int Ver Limnol 16:1018–1024
Markov KP (1975) Scanning electron microscope study of the microstructure of the egg membrane in the Russian sturgeon Acipenser gueldenstaedtii B. J Ichthyol 15:739–749
Matova N, Cooley L (2001) Comparative aspects of animal oogenesis. Dev Biol 231:291–320
Mazabraud A et al (1975) Biochemical research on oogenesis. RNA accumulation in the oocytes of teleosts. Dev Biol 44:326–332
Nagahama Y (1983) The functional morphology of teleost gonad. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology. Acad Press, NY IX A, pp 223–275
Nagahama Y (1997) 17-20ß-dihydroxy-pregnen-3-one, a maturation inducing hormone in fish oocytes: mechanisms of synthesis and action. Steroids 62:190–196
Nagahama Y et al (1995) Regulation of oocyte growth and maturation in fish. Curr Yop Dev Biol 30:103–145
Nakamura M, Nagahama Y (1985) Steroid producing cells during ovarian differentiation of the tilapia, Sarotherodon niloticus. Dev Growth Diffr 27:701–708
Nakamura M et al (1993) Ultrastructural analysis of the developing follicle during early vitellogenesis in tilapia, Oreochromis niloticus, with special references in the steroid-producing cells. Cell Tissue Res 272:33–39
Nunez Rodriguez J (1985) Contribution à l’étude de la biologie de la reproduction de la sole (Solea vulgaris Quensel 1806). Approche ultrastructurale et physiologique. Thèse 3ème Cycle, Université de Bordeaux I, n° 2061
Parry EW (1973) Methylene blue and azure-2 as stains for lipid in osmium-fixed tissues embeded in araldite. J Clin Pathol 16:546–548
Pearse (1976) Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sc USA 73:1255–1259
Pelissero C (1988) Mise en place des bases méthodologiques pour l’étude de la reproduction chez l’esturgeon Acipenser baerii femelle. Thèse de 3ème cycle, Université de Bordeaux I, n° 2229
Pelissero C et al (1985) Ultrastructural characteristic features of the oocyte of the sturgeon Acipenser baerii B, 7th Conf Fish Culture Europ Soc Comp Physiol Biochem Barcelona, vol A3, Promociones Publicationes Universitarias, Barcelona, p 8
Perazzolo LM et al (1999) Expression and localization of messenger ribonucleic acid for the vitellogenin receptor in ovarian follicles throughout oogenesis in the rainbow trout, Oncorhynchus mykiss. Biol Reprod 60:1057–1068
Raïkova EV (1976) Evolution of the nuclear apparatus during oogenesis in Acipenseridae. J Embryol Exp Morphol 35(8):667–687
Reynolds ES (1963) The use of lead citrate at hight pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208
Riehl R (1978) Electronen mikroskopische und autoradiographische Untersuchungen an den Dotterkernen in den Oocyten von Noemacheilus barbatulus L. und Phoxinus phoxinus L. (Pisces, Teleostei). Cytobiologie 17:137–145
Rzepkowska M, Ostaszewska T (2013) Proliferating cell nuclear antigen and Vasa protein expression during gonadal development and sexual differentiation in cultured Siberian (Acipenser baerii Brandt, 1869) and Russian (Acipenser gueldenstaedtii Brandt, Ratzeburg, 1833) sturgeon. Rev Aquac 5:1–14
Selman K, Wallace RA (1982) The inter- and intracellular passage of protein through the ovarian follicle in teleosts. In: Proc. Int Symp Reprod Physiol Fish. Wageningen, The Netherlands, p 57
Selman K, Wallace RA (1989) Cellular aspects of oocyte growth in teleost. Zool Sci 6:211–231
Selman K et al (1993) Stages of oocyte development in the zebrafish, Brachidanio rerio. J Morphol 218:203–224
Senthilkumaran B et al (2004) A shift un steroidogenesis occurring in ovarian follicles prior to oocyte maturation. Mol Cell Endocrinol 215:11–18
Sire MF et al (1994) Involvement of the lysosomal system in yolk protein deposit and degradation during vitellogenesis and embryonic development in trout. J Exp Zool 269:69–83
Stifani S et al (1990) Regulation of oogenesis: the piscine receptor for vitellognin. Biochem Biophys Acta 1045:271–279
Tesoriero JV (1977) Formation of the chorion (zona pellucida) in the teleost Oryzias latipes. I. Morphology of early oogenesis. J Ultrastruct Res 59:282–291
Toshimori K, Yasuzuni F (1979) Gap junctions between microvillosities of the oocyte and follicular cells in the teleost Plecoglossus altivelis. Z Mikrosk Anat Forsch 93:458–164
Trusov VZ (1975) Maturation in the gonads of the female sevryuga Acipenser stellatus during its life in the ocean. J Ichthyol 15:61–72
Ulrich E (1969) Etude des ultrastructures au cours de l’ovogenèse d’un poisson téléostéen le Danio Brachydanio rerio. J Microsc 8:447–473
Veshchev PV (1982) Reproduction of sterlet Acipenser ruthenus (Acipenseridae) in the lower volga. J Ichthyol 22:40–46
Vrackq R (1974) Basal lamina scaffold: anatomy and significance for maintenance of orderly tissue structure. Am J Pathol 77:314–346
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
Wallace RA et al. (1983) The oocyte as an endocytotic cell. In: Molecular biology of egg maturation. Ciba Found Symp Eds Pitman Books London: p 228–248
Williot P, Brun R (1982a) Résultats sur la reproduction d’Acipenser baerii en 1982. Bull Fr Piscic 287:19–22
Williot P, Rouault T (1982b) Compte rendu d’une première reproduction en France de l’esturgeon sibérien Acipenser baerii. Bull Fr Piscic 286:255–261
Woods JW, Roth TF (1980) Selective protein transport: identity of the solubilized phosvitin receptor from chicken oocyte. J Supramol Struct 14:473–480
Wourms JP (1976) Annual fish oogenesis. I. Differentiation of the mature oocyte and formation of the primary envelope. Dev Biol 50:338–354
Wourms JP, Sheldon H (1976) Annual fish oogenesis. II. Formation of the secondary egg envelope. Dev Biol 50:355–366
Yamashita M (1998) Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin Cell Dev Biol 9:569–579
Yusko S et al (1981) Receptor-mediated vitellogenin binding to chicken oocytes. Biochem J 200:43–50
Zelazowska M et al. (2015) Ovarian nests in immature and mature sturgeons (Acipenser gueldenstaedtii) and paddlefish (Polyodon spathula) (Chondrostei, Acipenseriformes) comprise early previtellogenic oocytes. Tissue Cell
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Le Menn, F., Benneteau-Pelissero, C., Le Menn, R. (2018). An Updated Version of Histological and Ultrastructural Studies of Oogenesis in the Siberian Sturgeon Acipenser baerii . In: Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M. (eds) The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 1 - Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-61664-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-61664-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61662-9
Online ISBN: 978-3-319-61664-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)