Abstract
Follicular atresia is a common feature of the vertebrate ovary that occurs at different stages of folliculogenesis and ovarian regression. It has physiological significance to maintain homeostasis and control fecundity, and ensure removal of post-ovulatory follicular remnants for preparing the ovary for the next cycle. Pigments appear late in the atretic process as indigestible waste formed out of the degradation of the oocytes, follicle wall and granulocytes. In the present study, pigment accumulation was demonstrated by Schmorl’s and Perls’ staining methods in the atretic ovarian follicles of Heteropneustes fossilis during follicular development and regression. Melanins were characterized spectrophotometrically for the first time in fish ovary. The predominant form is eumelanin, followed by pheomelanin and alkali-soluble melanin. Melanins showed significant seasonal variations with levels low in gonad resting phase, increasing to the peak in the post-spawning phase. The concentration of melanins increased time-dependently in post-ovulated ovary after human chorionic gonadotropin treatment. In the spawning phase, in vitro incubation of ovary slices with estradiol-17β or dexamethasone for 8 or 16 h decreased both eumelanin and pheomelanin levels time-dependently. The alkali-soluble melanin showed a significant decrease only in the dexamethasone group at 16 h. The results show that melanin assay can be used as a biomarker of follicular atresia in fish ovary, natural or induced by environmental toxicants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follicular atresia involving oocytes and surrounding follicular envelope is a common feature in vertebrate ovaries and occurs under natural or induced under conditions such as stress, temperature, starvation, hormonal imbalances and by endocrine-disrupting chemicals (Saidapur 1978; Nagahama 1983; Guraya 1986; Miranda et al. 1999). Atresia is characterized by global changes in the follicles such as disintegration of germinal vesicle (GV), breakdown of follicular envelope, hypertrophy and hyperplasia of granulosa, invasion of blood cells (eosinophilic granulocytes), macrophages, liquefaction of yolk, yolk resorption and accumulation of pigments of various chemical nature, and ultimately removal of the atretic mass of cells, leaving empty space (Miranda et al. 1999). Atresia occurs at any stage of the follicular development and growth, such as primary, vitellogenic and post-vitellogenic follicles. It helps to maintain physiological homeostasis in the ovary and fecundity. On the other hand, upon ovulation, the post-ovulatory follicles (POFs) undergo atresia regularly during the reproductive cycle of seasonally breeding animals. In post-spawning phase of the fish, the ovaries are filled with the POFs and unovulated follicles (Besseau and Faliex 1994). The removal of the POFs is essential for the recruitment of new follicles for the next breeding cycle. These structures have been described as preovulation corpus luteum atreticum, corpus atreticum, macrophage aggregates, chromatophore brown bodies (Bretschneider and Duyvene-De Wit 1947; Sathyanesan 1961; Lambert 1970; Ziengenfuss and Wolke 1991; Ravaglia and Maggese 1995; Blazer 2002). The atretic cell masses contain pigments such as melanin, hemosiderin and lipofuscin/ceroid (oxidized lipid). In histochemical methods, melanin stains black, hemosiderin stains blue and lipofuscin/ceroid gives yellowish tan (Lambert 1970; Besseau and Faliex 1994; Blazer 2002). Pigment cell masses are indicators of atresia, containing indigestible waste formed out by the degradation of the oocytes, follicle wall and granulocytes. The pigments appear late at the γ-stage of the atretic process and remain for a long time at the δ-stage in the ovary of Poecilia reticulata (Lambert 1970). These pigment cell masses are compared to melanomacrophage aggregates of liver, spleen and kidney (Agius 1985). In oviparous teleosts, the POFs are not recognized as ‘corpora lutea’ though some steroidogenic activities have been reported (Fostier et al. 1983; van den Hurk and Peute 1985). They collapse and become pyknotic, followed by rapid resorption (Guraya 1976). Kasuya (1997) observed two types of granulosa cells, one ingesting neighboring cells and the other large round cell, identified as macrophages. Immune cells such as macrophages and eosinophilic granulocytes, devoid of their granules, invade the atretic areas and contribute to the formation of oocyte remnant waste in the form of yellowish-brown bodies (Besseau and Faliex 1994). Kennedy (2002) noted a profound metamorphosis of the granulosa from endocrine to phagocytic function, accompanied by loss of steroidogenic potential. Follicular atresia involves cellular processes such as heterophagy, autophagy and apoptosis in teleosts (Janz and Kraak 1997; Wood and Van Der Kraak 2001; Santos et al. 2008; Ucuncu and Cakici 2009). It has been shown that apoptosis is a major contributor of follicular atresia, and there is much in common between atresia and apoptosis (Hughes and Gorospe 1991; Tilly et al.1991; Guthrie et al. 1995).
Melanins are generally synthesized in melanocytes/melanophores. Chemically, melanins are classified broadly into two major groups: eumelanin and pheomelanin (Ito et al. 2000). Eumelanin is composed of brown to black pigment, whereas pheomelanin is yellow to red in color. Eumelanin and pheomelanin are biopolymers composed of different monomer units from the same precursor dopaquinone, an intermediate for the formation of both eumelanin and pheomelanin (Fig. 1). Eumelanin is a highly heterogenous polymer containing 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) units (Ito 2003). Eumelanin and pheomelanin occur in tissues of human, mouse, birds and fish, and are quantified by HPLC or spectrophotometric method (Ito and Fujita 1985; Ito et al. 1993; Fan et al. 2010). The occurrence of melanin in the atretic follicles, among other pigments, is not conclusively described though the presence of lipofuscin (PAS-positive) and hemosiderin (Perls’ reaction) has been demonstrated in previous studies (Besseau and Faliex 1994; Miranda et al. 1999; Blazer 2002).
Atresia is regulated by estrogens and androgens in laboratory mammals (Billig et al. 1993). Estradiol benzoate (EB) inhibited atretic process, and testosterone prevented the antiatretogenic property of EB. The estrogen secretion is under gonadotropin control, thereby pituitary elicits an indirect control on atresia. Cortisol inhibits phagocytosis in head kidney (Watanuki et al. 2002), and dexamethasone, the synthetic analogue of cortisol, suppresses melanin formation in melanomacrophage centers. In this study, we characterized ovarian atretic pigment masses histochemically and measured different types of melanins biochemically. Further, the dynamics of melanin formation was followed up in post-ovulated follicles after hCG induction of ovulation, and the effects of both estradiol-17β (E2) and dexamethasone on melanin levels were demonstrated. Our results show that the melanin assay can be used as a marker of atretic follicular activity during folliculogenesis and follicular regression.
Materials and methods
Chemical and reagents
Sepia melanin, dexamethasone and E2 were purchased from Sigma-Aldrich, New Delhi, India. Human chorionic gonadotrophin (hCG, HUCOG-2000 HP, Barat Serum and Vaccines Limited, India) was purchased from a local medical store. Leibovitz L-15 medium and syringe-driven filter (nylon hydrophilic membrane, pore size 0.45 µm, 30 mm diameter) were purchased from Hi Media Laboratories Pvt. Ltd. (Mumbai, India). All other chemicals were of analytical grade and purchased either from E. Merck (New Delhi, India) or from Ranken (RFCL Limited, New Delhi, India).
Animals
The experiments were conducted as per the guidelines of the animal ethics committee of Banaras Hindu University. Care was taken to prevent cruelty of any kind. The annual reproductive cycle of the catfish Heteropneustes fossilis can be divided into five phases: resting (December–January), preparatory (February–April), prespawning (May–June), spawning (June–July) and post-spawning (September–November). In each phase, 20 adult mature female catfish (30–40 g) of the first sexual cycle were collected from a local fish market in Varanasi. The fish were used for seasonal histological, histochemical and melanin measurement studies. For in vitro experiments, 50 fish were purchased in the spawning phase. The fish were sampled randomly to ascertain the maturity of the ovary. The ovaries were filled with mature dark green follicles of about 1 mm diameter. They were maintained in the laboratory for 48 h under normal photoperiod (13.5L: 10.5D) and temperature (25–26 °C) to overcome stress due to transportation and fed daily with egg white ad libitum. The pH of water was 7.2 ± 0.1, and hardness was 293 ppm.
Histology
The fish were weighed and killed by decapitation. Both left and right ovaries were used for experiments. Samples were collected from the middle part of the ovaries. Ovary segments were fixed in Bouin’s fluid for 24 h and processed for paraffin embedding. Serial transverse sections were cut at 6 µm and stained with Ehrlich’s hematoxylin and eosin. The sections were dehydrated in alcohol grades, cleared in xylene and mounted in DPX. The sections were examined under Leica DM 2000 microscope, and images were taken with a Leica digital camera DFC 295 with 3 megapixel.
Identification of pigment cell masses
In each phase, segments from middle part of the ovary were removed and fixed in Orth’s fluid for 4 h and embedded in paraffin. Serial transverse sections were cut at 6 µm. The sections were deparaffinized and hydrated using serial downgrades of alcohol. The hydrated sections were immersed for 10–15 min in freshly prepared ferric-ferricyanide reagent (Schmorl’s reaction) or Perls’ Prussian blue reaction with slight modifications (Bancroft and Gamble 2008). Thirty milliliter of 1 % ferric chloride and 10 ml of freshly prepared potassium ferricyanide were mixed to form ferric-ferricyanide solution. Perls’ solution was prepared by mixing 2 % potassium ferrocyanide and 2 % HCl in equal ratio. The sections were washed, dehydrated in ethanol, cleared in xylene and mounted in DPX. Photoimages were taken as described above.
Experiments
Seasonal study
In different reproductive phases, the acclimated fish (n = 5 per phase) were killed by decapitation, and ovaries were sampled and stored at −80 °C for 1–2 days and processed for melanin assays, as described below.
hCG treatment
In the spawning phase, 20 fish were injected with human chorionic gonadotrophin (hCG, HUCOG-2000 HP, Barat Serum and Vaccines Limited, India) intraperitoneally in a concentration of 100 IU per fish (Singh et al. 2010). After 16 h, the fish were stripped to remove the eggs. Five fish were immediately sampled (0 week control). The remaining fish were maintained for 1, 2 and 4 weeks. The fish were weighed and killed by decapitation (n = 5). Ovaries were collected and stored at −80 °C for 1–2 days for melanin assays.
In vitro experiments
Ovary slices were incubated in Leibovitz’s L-15 medium (AT011A-1L, Hi Media Laboratories Pvt. Ltd., Mumbai, India), supplemented with 10 % fetal bovine serum and 100 µg/mL streptomycin. The medium was filtered in sterilized syringe-driven filter having a nylon hydrophilic membrane of pore size 0.45 µm and 30 mm diameter. In the spawning phase, fish were dissected and ovaries were collected in phosphate saline (PBS) buffer (pH 7.4) and cut into slices. The ovary slices (n = 5) in duplicate were incubated in the medium for 24 h for conditioning. One milligram each of dexamethasone or E2 was dissolved in 50 μL of 90 % ethanol, then diluted in propylene glycol to make up 1 mL solution and further diluted to make 100 nM working solution. After 24-h conditioning, 100 nM of dexamethasone or 100 nM of E2 was added to the medium. The control groups were incubated in plain medium or the medium containing ethanol plus propylene glycol. After the incubation, the ovary slices were collected at 8 or 16 h and processed for melanin assays.
Assay of eumelanin, pheomelanin and alkali-soluble melanin
Melanins were measured by the spectrophotometric methods of Ozeki et al. (1995) and Fan et al. (2010) with slight modifications. Sepia melanin was used as a standard for analysis of eumelanin (EM), pheomelanin (PM) and alkali-soluble melanin (ASM). A suspension of sepia melanin in water was prepared at a concentration of 1 mg/mL by sonicating for 5 min to make a homogenous suspension.
Sample preparation
The ovaries collected in different seasons and experiments were excised into about 40 mg samples. The samples were sonicated on ice in 500 μL of phosphate buffer saline (PBS) at pH 7.4. The homogenate was centrifuged at 5000 rpm for 5 min at 4 °C. Supernatant was collected for the assay of melanins.
Assay of eumelanin (EM)
Fifty microliters of supernatant was hydrolyzed in 450 μL of hot 30 % hydroiodic acid (HI). After HI hydrolysis, melanin was solubilized in hot 100 μL of 1 M NaOH and 15 μL of 1.5 % H2O2. The mixture was cleared by centrifugation at 10,000 rpm for 5 min at 4 °C, and the supernatant was analyzed for absorbance at 350 nm (A350) in a microplate reader (EPOCH, BioTek Instruments Inc, USA).
Assay of pheomelanin (PM)
One hundred microliters of supernatant was solubilized in 400 μL PBS buffer (pH 10.5) for 10 min at room temperature. The mixture was centrifuged at 10,000 rpm for 10 min at 4 °C, and the supernatant was collected. An equal volume of chloroform was added to the supernatant and mixed by vortexing. Chloroform was added to the supernatant to remove fatty acids. Pale yellow-colored aqueous phase supernatant was collected in fresh tubes by centrifugation at 4000 rpm for 10 min at 4 °C to remove all impurities. The process was repeated again to remove all the trace impurities by centrifuging at 10,000 rpm for 10 min at 4 °C, and the supernatant containing pheomelanin was analyzed for absorbance at 400 nm (A400) in the microplate reader.
Assay of alkali-soluble melanin (ASM)
This method is very similar to the spectrophotometric analysis of pheomelanin. Fifty microliters of supernatant was dissolved in 8 M urea/1 M NaOH solution, which was prepared by dissolving 8 M urea in 1 M NaOH. Pale brown-colored aqueous phase was analyzed for absorbance at 400 nm (A400) in the microplate reader.
Calculation
Standard curves were prepared for EM, PM and ASM by taking different concentrations of sepia melanin and processed like the tissue samples. The standard curves were linear over the concentrations used (Fig. 2). The minimum detection limit was 2 μg. Using the standard graphs, concentrations of melanins were measured in ovary samples and expressed in μg/mg tissue weight.
Validation
Recovery was above 90 % for the melanins. However, no correction was made for the tissue samples. The coefficients of intra- and inter-assay variations were 6.66 and 6.68 %, respectively, for EM, 7.69 and 8.69 %, respectively, for PM and 7.89 and 6.39 %, respectively, for ASM.
Statistical analysis
The data were expressed as mean ± SEM and checked for homogeneity by the Shapiro–Wilk normality test. The data showed a normal distribution and were further analyzed by one-way analysis of variance (ANOVA), followed by Newman–Keuls’ test (p < 0.05) for multiple group comparisons.
Results
Atretic follicles
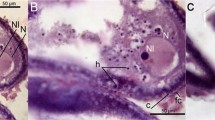
In the prespawning and spawning phases, the ovaries contained all developmental stages of follicles, viz. previtellogenic primary follicles, vitellogenic follicles and post-vitellogenic follicles (Fig. 3a, d). Atretic follicles were observed along the thin ovarian lamellae, close to normal healthy ones (Fig. 3b–f). In the preovulated ovary, the atretic follicles were characterized by epithelial cell proliferation, invasion by blood cells, phagocytotic activity engulfing yolk and other cellular debris, and fibrosis transforming them into pigmented structures. These bodies induced atresia in nearby follicles. In the post-spawning phase, the ovaries were filled with oogonial nests, primary follicles and a large number of atretic follicles of different size and shape containing pigments (Fig. 3g–i). The pigments were stained positively with Schmorl’s (Fig. 3b, e, h) and Perl’s reagents (Fig. 3c, f, i). The pigments imparted yellow–brown shades in hematoxylin–eosin preparations (Fig. 4a–c), and Prussian blue–black or yellow–green shades in Schmorl’s or Perls’ reaction (Figs. 3b, e, h; 4d, f). In early POFs, the follicular epithelium was collapsed, and the granulosa cells were stained blue in Perls’ reaction (Fig. 4e).
Transverse sections of ovary of the catfish H. fossilis in prespawning, spawning and post-spawning phases. a, d and g were stained with hematoxylin–eosin (bar 200 μm). b, e and h were stained with ferric-ferricyanide reaction (bar 50 μm). c, f and i were stained for Perl’s reaction (bar 200 μm). GV germinal vesicle, YG yolk granule, CA cortical alveolus, A atretic oocyte, LPN late perinucleolar stage, P pigmented group of cells
Seasonal changes in melanins
In the ovary, different forms of melanins were identified spectrophotometrically (Fig. 5). The principal type is eumelanin, followed by pheomelanin and total alkali-soluble melanin. The melanins showed significant seasonal variations (p < 0.001, one-way ANOVA; F = 192.357 for EM, F = 43.278 for PM and F = 7.027 for ASM. The concentration of EM was low in the resting phase and increased through preparatory, prespawning and spawning phases, to the peak value in the post-spawning phase. The levels in the resting, preparatory and prespawning phases were not significantly different from each other. The levels in the spawning and post-spawning phases were significantly different and were also different from other phases (p < 0.05, Newman–Keuls’ test). Pheomelanin was also low in the resting phase and increased significantly to the peak value in the post-spawning phase. The levels in the resting, preparatory and prespawning phases did not vary significantly. The spawning and post-spawning values were significantly different with each other and also from those in other phases. The concentration of ASM was low from resting to the prespawning phases and increased significantly in the spawning and post-spawning phases. The levels in the resting, preparatory and prespawning phases did not vary significantly. The levels in the spawning and post-spawning phases were also not significantly different from each other but were significantly higher from other phases.
Melanin levels in the ovary of the catfish H. fossilis during different reproductive phases. Significant seasonal variations are found in eumelanin, pheomelanin and alkali-soluble melanin contents. Values are mean ± SEM of five fish each. Data were analyzed by one-way ANOVA (p < 0.001) and Newman–Keuls’ test (p < 0.05). Groups bearing the same letter or number are not significantly different, and those bearing different letters or numbers are significantly different
Melanins in post-ovulated ovary
The injection of hCG-induced ovulation and eggs was stripped out at 16 h. The post-ovulated ovary was examined for melanin determination (Fig. 6). Eumelanin level increased significantly at week 1, 2 and 4 with the peak rise at week 2 in comparison with the 0 h week (16 h post-hCG injection). Both PM and ASM levels increased at week 2 but decreased at week 4 (p < 0.001, one-way ANOVA; p < 0.05, Newman–Keuls’ test).
Eumelanin, pheomelanin and alkali-soluble melanin contents in the post-ovulated ovary of the catfish H. fossilis were measured after 16 h (0 week) 1, 2 and 4 weeks of ovulation by hCG. Values are ± means SEM of five fish each. Data were analyzed by one-way ANOVA (p < 0.001) and Newman–Keuls’ test (p < 0.05). Groups bearing the same letters are not significantly different, and those bearing different letters or numbers are significantly different
In vitro effects of E2 and dexamethasone on melanins
The incubation of ovary slices with 100 nM of E2 (Fig. 7) or 100 nM dexamethasone (Fig. 8) for 8 h or 16 h decreased significantly the concentrations of EM and PM (p < 0.001, one-way ANOVA; p < 0.05, Newman–Keuls’ test). The concentration of ASM did not show any significant effect at 8 h or 16 h after the E2 treatment. The dexamethasone treatment decreased the ASM level only at 16 h.
In vitro effects of estradiol-17β on ovarian melanin levels in the catfish H. fossilis. Values are mean ± SEM of five fish each. Data were analyzed by one-way ANOVA (p < 0.001) and Newman–Keuls’ test (p < 0.05). Groups bearing the same letters or numbers are not significantly different, and those bearing different letters or numbers are significantly different
In vitro effects of dexamethasone on ovarian melanin levels in the catfish H. fossilis. Values are mean ± SEM of five fish each. Data were analyzed by one-way ANOVA (p < 0.001) and Newman–Keuls’ test (p < 0.05). Groups bearing the same letters or numbers are not significantly different, and those bearing different letters or numbers are significantly different
Discussion
This is the first study reporting the presence of melanins in ovary of a fish species associated with follicular atretic activity. Yellow to brown pigments have been described in the atretic follicles in hematoxylin–eosin staining, as shown in the catfish. Histochemically, the pigment masses contain lipofuscin/ceroid, which appears sudanophilic (Lambert 1970), PAS-positive and Perls-positive (yellow-tan) (Besseau and Faliex 1994; Blazer 2002). Perls’ Prussian blue reaction is specific for ferric iron (Bancroft and Gamble 2008), indicating sites of hemolysis or iron deposits. Perls’ stain imparted different colors, black for melanin, blue for hemosiderin and yellowish-brown for lipofuscin/ceroid in macrophage aggregates of spleen (Blazer 2002). In the catfish atretic follicles, Perls’ staining resulted in shades of yellow to blue-black hues, suggesting the presence of lipofuscin/ceroid, hemosiderin and melanin pigments. However, the PAS test was weak in the atretic follicles but stained intensely the follicular layer of normal follicles (not shown here). Lillie’s ferric-ferricyanide (Schmorl’s reaction) gave strong positive reaction in the pigment masses in the catfish ovary. The Schmorl’s reaction is used to demonstrate melanins, argentaffin cells, chromaffin cells, some lipofuscin, thyroid colloid and bile (Bancroft and Gamble 2008). Many of these pigments like melanins and chromaffin substance share a common origin from tyrosine and hence similar staining. The specific histochemical tests are useful to demonstrate qualitatively the presence of melanins in the atretic follicles.
In order to confirm the presence of melanins, we employed a spectrophotometric method that not only identified the different types of melanins but also quantified them under different physiological states. In the ovary, eumelanin is the principal form of melanin, followed by pheomelanin and alkali-soluble melanin. The melanin contents varied seasonally with the lowest level in the resting phase and the highest level in the post-spawning phase. In the resting phase, the ovary contains only stage 1 primary follicles in a dormant state. It implies that atretic activity is low during the gonadal quiescence. The melanin contents increased in the preparatory, prespawning and spawning phases, parallel with stepped up folliculogenesis. These increases can be attributed to the increased atretic activity of the follicles, concurrent with folliculogenesis. Although we did not investigate the causes or mechanisms of atresia, it is perceived that normally the growth pressure within the ovary for space as a result of intense hormonal/paracrine activities may be a limiting factor for many follicles to grow fast, and these become pathological, infective and ultimately demised. However, there are some primary follicles, which are resistant to atretic changes and look normal, healthy and survive till spawning or beyond. In that sense, atresia that occurs in the prespawned ovary is considered normal or physiological to maintain homeostasis, leading to fecundity control in nature (ecological advantage).
In the post-spawned ovary, the unovulated follicles and follicular envelopes of ovulated follicles undergo degenerative changes referred to as ovarian or follicular regression. It has been reported to be mediated by the interplay of heterophagy, autophagy and apoptosis (Janz and Kraak 1997; Wood and Van Der Kraak 2001; Santos et al. 2008; Ucuncu and Cakici 2009; Morais et al. 2012). The post-spawned ovary is characterized by a large number of pigment masses, as shown in this study, along with new crop of follicles. The removal of the remnants and cleaning of the ovary are important for the maintenance and survival of the primary follicles during the long quiescence phase. Proportionate to the pigment masses, the melanin pigment content was the highest in the post-spawning ovary. The increase in the melanin content was experimentally replicated in the ovary of the fish induced to spawn by hCG. The eumelanin content increased over 4-week time, the peak was achieved on week 2. It is not clear whether hCG has a direct role in pigmentation. Our in vitro study shows that both E2 and dexamethasone inhibit melanin levels. E2 is the female hormone that stimulates folliculogenesis and the atretogenesis may be a sequel to lack of sufficient estrogen level. Janz and Kraak (1997) reported that E2 suppressed apoptotic fragmentation of DNA in a concentration-dependent manner in rainbow trout preovulatory follicles. The E2 inhibition of melanin may be a manifestation of a general cytotropic effect, preventing degenerative changes. Steroids such as E2, progesterone, 11-ketotestosterone and cortisol inhibited phagocytotic functions of head kidney macrophages (Watanuki et al. 2002). Dexamethasone is a synthetic analogue of cortisol and is immunosuppressive. Melanins are accumulated by macrophages, forming melanomacrophage centers (MMCs) in head kidney, spleen and liver ((Meseguer et al. 1994). The dexamethasone-induced inhibition of melanins may be related to the immunosuppressive effect of the steroid on the follicular atretic cells. More work in this direction is required in future. The atretic follicles may be considered analogous with MMCs, sharing some of the properties.
Melanogenesis is generally a property of skin melanocytes or melanophores. However, there are other tissues that form pigments including melanins with different functional attributes. In the salamander Hynobius nigrescens, ovarian eggs deposit melanin pigment in the cortical region during growth and development, which may help to camouflage them with the surroundings (Hasumi 1996). The present study shows that the ovarian atretic follicle is another tissue with a different function. In hematolymphopoietic organs (spleen and kidney), thymus and liver, the MMCs contain pigments such as melanin, hemosiderin and lipofuscin/ceroid (Agius and Roberts 2003; Haugarvoll et al. 2006; Ribeiro et al. 2011). Several functions have been proposed for the MMCs such as immune reaction, phagocytosis of cell debris and capture and storage of cations. Like the MMCs, melanin pigment-bearing atretic follicles are useful biomarkers for the assessment of environmental contaminants and stress.
In conclusion, melanin pigments were characterized in the atretic follicles of the catfish. The melanin content showed seasonal variation and increased in the post-ovulated ovary. E2 and dexamethasone decreased the major melanin (EM and PM) contents. The melanin assay can be used as a bioindicator of atresia in fish.
References
Agius C (1985) The melano-macrophage centres of fish: a review. In: Manning MJ, Tatner MF (eds) Fish immunology. Academic Press, London, pp 85–105
Agius C, Roberts RJ (2003) Melano-macrophages centres and their role in fish pathology. J Fish Dis 26:499–509
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, London
Besseau L, Faliex E (1994) Resorption of unemitted gametes in Lithognathus mormyrus (Sparidae: Teleostei): a possible synergic action of somatic and immune cells. Cell Tissue Res 276:123–132
Billig H, Furuta I, Hsueh AJW (1993) Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 133:2204–2212
Blazer VS (2002) Histopathological assessment of gonadal tissue in wild fishes. Fish Physiol Biochem 26:85–101
Bretschneider LH, Duyvene-De Wit J (1947) Sexual endocrinology of non-mammalian vertebrates, vol 11. Elsevier, Amsterdam
Fan R, Yang G, Dong C (2010) Study of hair melanins in various hair color Alpaca (Lama pacos). Asian Aust J Anim Sci 23:444–449
Fostier A, Jalabert B, Billard R, Breton B, Zohar Y (1983) The gonadal steroids. In: Hoar WS, Randall DL, Donaldson EM (eds) Fish physiology, vol 9A (reproduction). Academic Press, New York, pp 277–372
Guraya SS (1976) Recent advances in the morphology, histochemistry, and biochemistry of steroid-synthesizing cellular sites in the non-mammalian vertebrate ovary. Int Rev Cytol 44:365–409
Guraya SS (1986) The cell and molecular biology of fish oogenesis. In: Sauer HW (ser. ed) Monographs in developmental biology, vol 18. S Karger AG, Basel, pp 1–223
Guthrie HD, Grimes RW, Cooper BS, Hammond JM (1995) Follicular atresia in pigs: measurement and physiology. J Anim Sci 73:2834–2844
Hasumi M (1996) Seasonal fluctuations of female reproductive organs in the salamander Hynobius nigrescens. Herpetologica 53:598–605
Haugarvoll E, Thorsen J, Laane M, Huang Q, Koppang EO (2006) Melanogenesis and evidence for melanosome transport to the plasma membrane in a CD83+ teleost leukocyte cell line. Pigment Cell Res 19:214–225
Hughes FM Jr, Gorospe WC (1991) Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology 129:2415–2422
Ito S (2003) A chemist’s view of melanogenesis. Pigment Cell Res 16:230–236
Ito S, Fujita K (1985) Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal Biochem 144:527–536
Ito S, Wakamatsu K, Ozeki H (1993) Spectrophotometric assay of eumelanin in tissue samples. Anal Biochem 215:273–277
Ito S, Wakamatsu K, Ozeki H (2000) Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res 13:103–109
Janz DM, Kraak GVD (1997) Suppression of apoptosis by gonadotropin, 17β-estradiol, and epidermal growth factor in rainbow trout preovulatory ovarian follicles. Gen Comp Endocrinol 105:186–193
Kasuya K (1997) Elimination of apoptotic granulosa cells by intact granulosa cells and macrophages in atretic mature follicles of the guinea pig ovary. Arch Histol Cytol 60:175–184
Kennedy AM (2002) Reproduction of striped bass Morone saxatilis; a structural, biochemical and functional characterization of atresia. http://www.lib.ncsu.edu/theses/available/etd-06162002-210408/
Lambert JGD (1970) The ovary of the guppy, Poecilia reticulata. The atretic follicle, a corpus atreticum or a corpus luteum praeovulationis. Z Zellforsch 107:54–67
Meseguer J, Lopez-Ruiz A, Esteban MA (1994) Melano-macrophages of the seawater teleosts, sea bass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata): morphology, formation and possible function. Cell Tissue Res 277:1–10
Miranda ACL, Bazzoli N, Rizzo E, Sato T (1999) Ovarian follicular atresia in two teleost species: a histological and ultrastructural study. Tissue Cell 31:480–488
Morais RDVS, Thome RG, Lemos FS, Bazzoli N, Rizzo E (2012) Autophagy and apoptosis interplay during follicular atresia in fish ovary: a morphological and immunocytochemical study. Cell Tissue Res 347:467–478
Nagahama Y (1983) The functional morphology of teleost gonads. In: Hoar WS, Randall DL, Donaldson EM (ed) Fish physiology, vol 9A (reproduction). Academic Press, New York, pp 223–275
Nezirevic D, Arstrand K, Kagedal B (2007) Hydrophilic interaction liquid chromatographic analysis of aminohydroxyphenylalanines from melanin pigments. J Chromatogr A 1163:70–79
Ozeki H, Ito S, Wakamatsu K, Hirobe T (1995) Chemical characterization of hair melanins in various coat—color mutants of mice. J Invest Dermatol 105:361–366
Ravaglia MA, Maggese MC (1995) Melano-macrophage centres in the gonads of the swamp eel, Synbranchus marmoratus Bloch, (Pisces, Synbranchidae): histological and histochemical characterization. J Fish Dis 18:117–125
Ribeiro HJ, Procopio MS, Gomes JMM, Vieira FO, Russo RC, Balzuweit K, Chiarini-Garcia H, Casrro ACS, Rizzo E, Correa JD (2011) Functional dissimilarity of melanomacrophage centres in the liver and spleen from females of the teleost fish Prochilodus argenteus. Cell Tissue Res 346:417–425
Saidapur SK (1978) Follicular atresia in the ovaries of nonmammalian vertebrates. Int Rev Cytol 54:225–244
Santos HB, Thome RG, Arantes FP, Sato Y, Bazzoli N, Rizzo E (2008) Ovarian follicular atresia is mediated by heterophagy, autophagy, and apoptosis in Prochilodus argenteus and Leporinus taeniatus (Teleostei: Characiformes). Theriogenology 70:1449–1460
Sathyanesan AG (1961) The ovarian cycle in the catfish Mystus seenghala (Sykes). Proc Natl Inst Sci India 28:497–506
Singh V, Chaube R, Chourasia TK, Joy KP (2010) Temporal and periovulatory changes in ovarian catecholamines in the catfish Heteropneustes fossilis. Gen Comp Endocrinol 168:46–54
Tilly JL, Kowalski KI, Johnson AL, Hsueh AJW (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 129:2799–2801
Ucuncu SI, Cakıcı O (2009) Atresia and apoptosis in preovulatory follicles in the ovary of Danio rerio (zebrafish). Turk J Fish Aquat Sci 9:215–221
Van den Hurk R, Peute J (1985) Functional aspects of the postovulatory follicle in the ovary of the African catfish, Clarias gariepinus, after induced ovulation. Cell Tissue Res 240:199–208
Wakamatsu K, Ito S (2002) Advanced chemical methods in melanin determination. Pigment Cell Res 15:174–183
Watanuki H, Yamaguchi T, Sakai M (2002) Suppression in function of phagocytic cells in common carp Cyprinus carpio L. injected with estradiol, progesterone or 11-ketotestosterone. Comp Biochem Physiol C: Toxicol Pharmacol 132:407–413
Wood AW, Van Der Kraak GJ (2001) Apoptosis and ovarian function: novel perspectives from the teleosts. Biol Reprod 64:264–271
Ziengenfuss MC, Wolke RE (1991) The use of fluorescent microspheres in the study of piscine macrophage aggregate kinetics. Dev Comp Immunol 15:165–171
Acknowledgments
RK is a recipient of Rajiv Gandhi National Fellowship of UGC, New Delhi, which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Joy, K.P. Melanins as biomarkers of ovarian follicular atresia in the catfish Heteropneustes fossilis: biochemical and histochemical characterization, seasonal variation and hormone effects. Fish Physiol Biochem 41, 761–772 (2015). https://doi.org/10.1007/s10695-015-0044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0044-y