Abstract
The mechanisms and reaction pathway of UV photo-assisted Fenton-like degradation of progesterone in water and wastewater were investigated. The reaction followed the pseudo-first-order kinetics for both the dark Fenton-like and UV photo-Fenton-like processes. The reaction kinetics of the UV photo-assisted process improved with the presence of humic acid (HA) in wastewater, due to the formation of Fe(III)-HA complexes which enhanced Fe(II) production by a ligand-to-metal charge transfer. The UV photo-assisted process reduced the amount of ferric required to completely degrade progesterone by 60 % and lowered the activation energy to 42 kJ/mol compared to 104 kJ/mol for the dark Fenton-like process. Complete degradation of progesterone was achieved through a multi-step process involving several intermediate compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endocrine disrupting chemicals (EDCs), particularly the steroid hormones, have been detected in wastewater effluent around the world (Ko et al. 2007; Kueh and Lam 2008; Xu et al. 2014). They have generated considerable research on the potential global threat these chemicals pose to both human health and the environment. These chemicals, particularly the oestrogens and progestogens, disrupt the natural functioning of the endocrine system. The principal sources of release into the environment are through wastewater treatment plants (WWTPs) (Basile et al. 2011; Sin et al. 2011; Ifelebuegu 2011). Other sources of EDCs to waterways include agricultural run-offs and steroidal hormones excreted by livestock (Gadd et al. 2010; Bartelt-Hunt et al. 2012; Lim et al. 2013). Conventional biological wastewater treatment processes used in the wastewater industry are not adequate to completely eliminate these chemicals, hence the search for more efficient processes (Nakada et al. 2006; Kraigher et al. 2008; Balabanic et al. 2012).
Various advanced oxidation processes (AOPs) have been applied successfully for the removal or degradation of toxic pollutants for conversion into biodegradable compounds, which can then be treated by conventional biological methods. The efficiency of AOPs is dependent on the generation of reactive free radicals, especially the hydroxyl radicals (Klavarioti et al. 2009). The hydroxyl radical is responsible for the oxidation and mineralisation of organic compounds to water and carbon dioxide (Matilainen and Sillanpaa 2010; Ifelebuegu et al. 2014). Hydroxyl radicals possess strong reactive electrophilic properties that make them effective for destroying organic compounds by reacting non-selectively and rapidly with almost all electron-rich organic compounds (Fan et al. 2012).
Fenton oxidation is widely considered and used for the treatment of highly contaminated waters amongst other AOPs in wastewater treatment. It is an advanced oxidation process that generates a highly reactive HO˙ by the combination of Fe2+ and hydrogen peroxide (H2O2). The photo-Fenton is a typically enhanced Fenton reaction with a higher rate and faster mineralisation of recalcitrant organics than the dark reaction process and can take advantage of utilising UV irradiation (Kavitha and Palanivelu 2004; Navarro et al. 2011; Vilar et al. 2012). The Fenton process is dependent on conditions such as pH, reaction time, temperature, ratio of Fe2+ to H2O2, and iron concentration.
Fenton-like oxidation is an AOP that is based on ferric (Fe3+) and H2O2, in which H2O2 plays the role of an oxidative agent, and ferric ion plays that of a catalytic agent (Tamimi et al. 2008). This is usually carried out at an acidic pH level (pH 3) to prevent the occurrence of iron hydroxide precipitation, for the degradation of organic compounds (Mohapatra et al. 2011). The reaction between Fe3+ and H2O2 leads first to the formation of Fe2+ and hydroperoxide radical (Eq. 1), and the Fenton reaction between the formed Fe2+ and H2O2 gives rise to the generation of hydroxyl radicals (Eq. 2) responsible for the mineralisation of organic compounds in aqueous solutions.
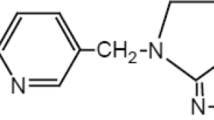
The Fenton process has been shown to be a good option for the removal of micro-contaminants from water systems (Klavarioti et al. 2009; Ifelebuegu and Ezenwa 2011; Rivera-Utrilla et al. 2013). Although the impact and treatment of oestrogens are well documented in the literature, a limited study has been conducted on the removal and treatment of progesterone (Fayad et al. 2013; Liu et al. 2009; Ifelebuegu and Onwugbuta 2016)—the most important and only naturally occurring progestogen. The aim of this paper is to determine the mechanisms of photo-Fenton-like degradation of progesterone (Fig. 1) in water and wastewater and to propose a degradation pathway. This study was carried between 2014 and 2015 at Coventry University, UK.
Materials and methods
Materials
Progesterone (99 %) was purchased from Sigma-Aldrich UK. Analytical grade ferric nitrate (Fe(NO3)3·9H2O), 30 % hydrogen peroxide (H2O2), sodium hydroxide (NaOH), hydrochloric acid (HCl), potassium permanganate (KMnO4), and methanol (CH3OH) was obtained from Fisher Scientific UK. Milli-Q water was obtained from a water purification system (Millipore, USA), resistivity ≥ 8 ΩM. Unless otherwise stated, all samples were prepared in deionised water obtained from a water purification system. Wastewater effluent was obtained from an activated sludge plant in Coventry, UK.
Fenton-like oxidation treatment
Non-photocatalytic experiments were undertaken to establish their isolated influence on the degradation of progesterone. A 150 mL of 2 mg/L progesterone solution in milli-Q water was transferred into a 250 mL batch reactor. The solution was adjusted to pH 3 using 1 M HCl to prevent iron precipitate formation (Mohapatra et al. 2013). To determine the optimum iron concentration, various ferric concentrations (4, 7.5, 15, 30, 75 and 85 mg/L as Fe) were prepared. A molar ratio of 1:10 of Fe:H2O2 was used as it is said to be effective for Fenton oxidation (Mert et al. 2010; Nakrst et al. 2010). Details of the analytical procedures have been previously reported by Ifelebuegu and Ezenwa (2011). The thermodynamic evaluation was carried out at varying temperatures of 20, 25, 30 and 35 ± 2 °C.
Photo-Fenton-like oxidation treatment
The photo-Fenton-like oxidation was carried out under the same experimental conditions as the Fenton-like oxidation. The only difference was the introduction of a UV lamp set at a wavelength of 254 nm (E = 6 J/cm2), which was placed at a distance of 10.5 cm from the surface of the reactor. The photo-Fenton-like experiments were conducted as described by Yaping and Jiangyong (2008) at temperature of 25 ± 2 °C. The experiments were also repeated using wastewater to evaluate the effects of natural organics on the degradation kinetics.
Instrumental analysis
Analytical determination of progesterone concentration was carried out on a Hewlett-Packard Series 1050 HPLC system (HP UK) equipped with a UV detector wavelength set at 254 nm). A UV lamp with BDH dual wavelength of 365/254 nm was used as the source of UV radiation; the lamp was set to 254 nm for the photo-Fenton-like experiments. The separation was done on a Thermo ODS Hypersil C18 column (150 × 4.6 mm, 5 µm) (ThermoFisher UK) fitted with a guard column. The mobile phases methanol and water (80:20) ran in an isocratic mode with a flowrate of 1.2 mL/min and a total runtime of eight minutes (Ifelebuegu et al. 2010). The analysis and identification of by-products were carried out through an LC–MS/MS (Applied Biosystems, Carlsbad, CA, USA) as previously described elsewhere (Kanda and Churchley 2008; Ifelebuegu 2011). The humic matter was measured by TOC-V CSH total organic carbon analyser. Wastewater characteristics were analysed according to established methods (APHA 2012).
Results and discussion
Effect of ferric concentration
The effect of initial ferric concentration on the removal of progesterone in Milli-Q water was studied at pH 3 (Ifelebuegu and Ezenwa 2011; Li and Zhang 2014). Various concentrations of Fe(III) were used at 60 min of treatment time and a temperature of 25°± 2 °C. The effects of Fe(III) concentration (4–85 mg/L as Fe) on the degradation of progesterone are shown in Fig. 2a, b. The results obtained indicate that an increase in ferric concentration resulted in an increased percentage conversion of progesterone. Increasing concentrations of ferric is usually beneficial in the decomposition of H2O2 and this enhanced progesterone degradation until excess ferric (above 75 mg/L), did not further improve the degradation process but started to act as hydroxyl radical scavenger. All further experiments were conducted at a ferric concentration of 30 mg/L. This trend is consistent with previous studies by Babuponnusami and Muthukumar (2012) and Li et al. (2012). They observed that the increase in iron concentration yielded greater degradation of contaminants. This could be as a result of the increased formation of Fe(III)–H2O2 complexes, resulting in increased ferrous ion and hydroxyl radical formation, consequently accelerating degradation. The total degradation of 2 mg/L of progesterone was achieved by the Fenton-like process at a maximum ferric concentration of 75 mg/L as Fe, while complete degradation was achieved for the photo-assisted reaction process at a maximum ferric concentration of 30 mg/L as Fe. The amount of ferric concentration required for complete degradation is reduced by more than 50 % with the photo-assisted reaction. This can be attributed to the increased production of hydroxyl radicals under photo-irradiation, caused by the continuous recycling of Fe(II), as its formation from the reduction of Fe(III) is enhanced by the irradiation process (Zepp et al. 1992). Additionally, in the photo-Fenton-like degradation process, H2O2 is dissociated under irradiation to produce hydroxyl radical, as the emission wavelength of the UV (254 nm) light used is within the range of the absorbance spectrum of H2O2.
Reaction time and kinetics
The efficiency of the photo-Fenton process has been reported to depend on the rate of formation and scavenging of hydroxyl radicals, which vary according to the type of organic substrate and the irradiation time (Elmolla and Chaudhuri 2009; Alalm et al. 2015). Figure 3 shows the effect of time on the degradation of progesterone at pH 3 and ferric concentration of 30 mg/L. It was observed that the degradation of progesterone took place in two stages: the first where it was rapidly degraded and a second where degradation rate slowed. This initial rapid removal could be as a result of ferrous ions reacting very quickly with H2O2 to generate large numbers of hydroxyl radicals, which then rapidly react with progesterone in the initial stage. The slower second stage reaction could be as a result of the slow regeneration of Fe(II) from Fe(III) with increases in reaction time. A similar result was obtained by Zhang and Wang (2011), who found the optimum reaction time of 30 min for the Fenton treatment with a batch reactor for treatment of landfill leachates. This result also compares favourably with a previous study conducted by Frontistis et al. (2011), who observed an 80 % removal of 17β-estradiol (E2) from wastewater from a WWTP in China after 20 min of irradiation time when compared to the Fenton oxidation, which achieved a 35 % removal of E2 after 20 min.
For an evaluation of the kinetics of both oxidation processes for the degradation of progesterone in wastewater, the pseudo-first-order and pseudo-second-order kinetics were used to fit the experimental data. The expressions guiding the reaction kinetics are those of Lagergren pseudo-first-order and pseudo-second-order kinetic models. The pseudo-first-order kinetic model is expressed in Eq. 3. The reaction rate constant was determined from the equation below:
where C 0 is the initial concentration of the progesterone, k the rate constant and t the time in minutes. Representing the equation in an integral form, it becomes
The pseudo-second-order kinetics model is expressed as shown below:
Representing the Equation in an integral form, it becomes
A graph of ln C/C 0 against different time intervals showing pseudo-first-order reaction and a graph of \(\frac{1}{C} - \frac{1}{{C_{0} }}\) against different time intervals depicting a pseudo-second-order reaction were plotted to determine the kinetic model which best suits the degradation of progesterone in Milli-Q water (Table 1). The values of the apparent rate constants were calculated by linear regression and the summaries are presented in Table 2 with their corresponding correlation coefficients.
Previous studies on the advanced oxidation of oestrogenic steroid hormones reported that the reaction followed second-order kinetics (Deborde et al. 2004; Ifelebuegu and Ezenwa 2011). In this work, both the Fenton-like and photo-Fenton-like oxidation of progesterone showed a better fit to the pseudo-first-order kinetic model compared to the second-order kinetic model by the correlation coefficient, suggesting that the degradation rate may be dependent on the initial concentrations of progesterone.
Effect of natural organic matter on reaction kinetics
The effect of dissolved organics on the degradation of progesterone was also investigated by using effluent wastewater from a local WWTP (Table 1). The experiments were conducted at temperature of 25 ± 2 °C and pH 3. It can be seen from Table 3 that there was no significant changes in the first and second-order rate constants for the dark Fenton-like process in Milli-Q water (k = 0.015 min−1, k 2 = 21 M−1s−l) compared to wastewater (k = 0.012 min−1, k 2 = 25 M−1s−l), while the rate constants for the photo-assisted process increased at a more significant rate (k = 0.044 min−1, k 2 = 22 M−1s−l for Milli-Q water and k = 0.098 min−1, k 2 = 119 M−1s−l for wastewater). This suggests that the presence of dissolved organics enhanced the photo-assisted degradation of progesterone. This can be attributed to the presence of humic acid (HA) in the wastewater effluent. Previous studies have reported significant enhancement of the degradation of phenolic moieties in the presence of HA (He et al. 2010; Jiang et al. 2010). Leech et al. (2009) also reported an enhanced degradation of oestrogens during irradiation due to the organic radical formation from the interaction of humic acid, sunlight, and oxygen. We postulate that the improved photo-Fenton degradation of progesterone in the presence of humic acid is due to Fe(III)-HA complex formation, which under irradiation encourages the formation of Fe(II) by ligand-to-metal charge transfer (Ou et al. 2009).
Effects of temperature on reaction kinetics
The effects of temperature on the degradation rates of progesterone by Fenton-like and photo-Fenton-like degradation were investigated at varying temperatures (20, 25, 30 and 35 °C), pH 3 and ferric concentration of 30 mg/L. This is represented in Fig. 4. The observed increase in degradation rate with temperature can be attributed to the fact that at higher temperatures, the generation of hydroxyl radicals are enhanced (Khamaruddin et al. 2011; Aygun et al. 2012), increasing the degradation rate constant and hence accelerating progesterone degradation.
The significant activation energies of the degradation of progesterone were determined from the Arrhenius equation expressed as:
where k is the rate constant for the reaction, A 0 is the Arrhenius factor, E a is the activation energy for the reaction, T is the solution temperature, and R is the gas constant (8.314 J/(mol K)). A plot of ln k against 1/T was used to evaluate the activation energy (E a).
Fenton–like oxidation
E a and A 0 for the reaction were evaluated from the slope and intercept of the Arrhenius plot of ln(k) against 1/T, which had a good linear relationship (R 2 = 0.9593). The value of the activation energy, E a, obtained from the Arrhenius plot shown in Fig. 5a, b was found to be 103.6 kJ/mol and A 0 = 4.27 × 1016 min−1, which indicates the rate of collision of molecules.
The value of the activation energy E a obtained from the Arrhenius plot shows that the reaction rate of Fenton oxidation of progesterone in wastewater is very sensitive to temperature change and also that it is hard for the reaction to occur (degradation of progesterone), as the activation energy value was observed to be high. This is in support of the findings of Chen and Zhu (2007), who stated that the values of activation energy of thermal reactions are usually between 60 and 250 kJ/mol. Also, the high activation energy can be attributed to the presence of the carbon–carbon double bond that exists in progesterone molecules (Cendejas-Santana et al. 2002), as progesterone is known to belong to the ketone and oxygenated functional with a carbon–carbon bond possessing energies around 376.81 kJ/mol (Nicotra 2009). So, for degradation of progesterone to occur, these bonds have to be broken, resulting in high activation energy.
Photo-Fenton-like oxidation
In the photo-Fenton-like oxidation process, an activation energy E a value of 42.01 kJ/mol (R 2 = 0.9436) was obtained from the slope of the plot and a pre-exponential factor value of 2.41 × 109 min−1 from the y-intercept of the plot. The low activation energy obtained in the photo-Fenton process (a 60 % reduction compared to the activation energy of the dark Fenton-like process) could be as a result of the presence of the recycled Fe2+ catalyst and the photo-catalytic effects of the UV irradiation, which speed up the reaction by lowering the transition state. Lower activation energy will result in larger rate constants as presented in Table 3, because the rate of a reaction is directly complementary to the rate constant k.
Mineralisation and degradation by-products
Figure 6 shows the change in the concentration of progesterone during the photo-Fenton degradation process and the corresponding changes in DOC. The values demonstrate the presence of degradation by-products of progesterone oxidation. The double bonds in the progesterone molecule between C4 and C5, the hydroxyl group on C12 and double bond oxygen on C3 (Fig. 1a, b), are the likely points of hydroxyl radical attack. Hydroxyl radicals are known to transform olefinic moieties by electrophilic attack at these double bonds (Lee and von Gunten 2010; Yang et al. 2012). The proposed pathway is, therefore, a 2 + 2 addition to the olefinic bonds by the organometallic complex formed (Yang et al. 2012). There is also the possibility of hydroxyl radical addition to the olefinic bond forming cyclic esters (Yang et al. 2012). The increased BOD/COD ratios also suggest increased biodegradability of the post-photo-Fenton-like oxidation effluent, suggesting the transformation of the by-products through ring rupturing reactions into simpler molecules like carboxylic acids and acetaldehydes (Zhao et al. 2008). Tables 4 and 5 indicate the identified predominant by-products. It can be concluded that there was a multi-step degradation of the progesterone involving several intermediate transformation by-products that went through further degradation and ring rupture to form simpler organic molecules, carbon dioxide, and water. This is supported in Fig. 6 by the gradual drop in the DOC after 30 min of degradation time. Figure 7, therefore, proposes a possible pathway for the UV photo-assisted degradation of progesterone.
Conclusion
The mechanism and reaction pathway of UV photo-assisted Fenton-like degradation of progesterone in water and wastewater were investigated in this study. There was a 60 % reduction in the ferric requirement by the photo-assisted Fenton-like process compared to the dark Fenton process. The kinetic data obtained fitted the pseudo-first-order model for both processes. The activation energy for the Fenton-like degradation of 104 kJ/mol was significantly reduced to 42 kJ/mol with the UV photo-assisted process due to the photocatalytic effect. The presence of humic acid in wastewater effluent enhanced the UV photo-assisted degradation process through the formation of Fe(III)-HA complexes, which produced Fe(II) by ligand-to-metal charge transfer. The overall degradation of progesterone was a step change with initial oestrogen-like by-products and eventual ring rupture, resulting in a complete transformation to simpler organics, carbon dioxide, and water.
References
Alalm MG, Tawfik A, Ookawara S (2015) Degradation of four pharmaceuticals by solar photo-Fenton process: kinetics and costs estimation. J Environ Chem Eng 3:46–51
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA, Washington, DC
Aygun A, Yilmaz T, Nas B, Berktay A (2012) Effect of temperature on Fenton oxidation of young landfill leachate: kinetic assessment and sludge properties. Glob NEST J 14:487–495
Babuponnusami A, Muthukumar K (2012) Advanced oxidation of phenol: a comparison between Fenton, electro- Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 183:1–9
Balabanič D, Hermosilla D, Merayo N, Klemenčič AK, Blanco Á (2012) Comparison of different wastewater treatments for removal of selected endocrine -disruptors from paper mill wastewater. J Environ Sci Heal A 47:1350–1363
Bartelt-Hunt SL, Snow DD, Kranz WL, Mader TL, Shapiro CA, Donk SJV, Zhang TC (2012) Effect of growth promotants on the occurrences of endogenous and synthetic steroid hormones on feedlot soils and in run-off from beef cattle feeding operations. Environ Sci Technol 46:1352–1360
Basile T, Petrella A, Petrella M, Boghetich G, Petruzzelli V, Colasuonno S, Petruzzelli D (2011) Review of endocrine disrupting compounds removal technologies in water and wastewater treatment plants: an EU perspective. Ind Eng Chem Res 50:8389–8401
Cendejas-Santana G, Hinojosa-Torres J, Castaño VM (2002) Progesterone crystallization from a solvent: a new procedure. Mater Res Innov 6:252–255
Chen J, Zhu L (2007) Heterogeneous UV—Fenton catalytic degradation of dyestuff in water with hydroxyl—Fe pillared bentonite. Catal Today 126:463–470
Deborde M, Rabouan S, Gallard H, Legube B (2004) Aqueous chlorination kinetics of some endocrine disruptors. Environ Sci Technol 38(21):5577–5583
Elmolla E, Chaudhuri M (2009) Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solutions. J Hazard Mater 170:666–672
Fan X, Hao H, Wang Y, Chen F, Zhang J (2012) Fenton-like degradation of nalidixic acid with Fe3+/H2O2. Environ Sci Poll Res 20:3649–3656
Fayad PB, Zamyadi A, Broseus R, Prévost M, Sauvé S (2013) Degradation of progestagens by oxidation with potassium permanganate in wastewater effluents. Chem Cent J 7:84
Frontistis Z, Xekoukoulotakis NP, Hapeshi E, Venieri D, Fatta-Kassinos D, Mantzavinos D (2011) Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem Eng J 178:175–182
Gadd JB, Tremblay LA, Northcott GL (2010) Steroid estrogens conjugated estrogens and estrogenic activity in farm dairy shed effluents. Environ Poll 158:730–736
He D, Guan X, Ma J, Yang X, Cui C (2010) Influence of humic acids of different origins on oxidation of phenol and chlorophenols by permanganate. J Hazard Mater 182:681–688
Ifelebuegu AO (2011) The fate and behavior of selected endocrine disrupting chemicals in full-scale wastewater and sludge treatment unit processes. Int J Environ Sci Technol 8:245–254
Ifelebuegu AO, Ezenwa CP (2011) Removal of endocrine chemicals in wastewater treatment by Fenton-like oxidation. Water Air Soil Poll 217:213–220
Ifelebuegu AO, Onwugbuta NE (2016) An evaluation of the removal of progesterone in wastewater by adsorption onto waste tea leaves. Proceedings of the Fourth International Conference on Advances in Applied Science and Environmental Technology, Bangkok, Thailand, 7–8 May 2016. doi: 10.15224/978-1-63248-097-2-43
Ifelebuegu AO, Theophilus SC, Bateman MJ (2010) Mechanistic evaluation of the sorption properties of endocrine disrupting chemicals in sewage sludge biomass. Int J Environ Sci Technol 7:617–622
Ifelebuegu AO, Onubogu J, Joyce E, Mason T (2014) Sonochemical degradation of endocrine disrupting chemicals 17β-estradiol and 17α-ethinylestradiol in water and wastewater. Int J Environ Sci Technol 11(1):1–8
Jiang J, Pang SY, Ma J (2010) Role of ligands in permanganate oxidation of organics. Environ Sci Technol 44:4270–4275
Kanda R, Churchley J (2008) Removal of endocrine disrupting chemicals during conventional wastewater treatment. Environ Technol 29:315–323
Kavitha V, Palanivelu K (2004) The role of ferrous ion in Fenton and photo-Fenton processes for the degradation of phenol. Chemosphere 55:1235–1243
Khamaruddin PF, Bustam MA, Omar AA (2011) Using Fenton’s reagent for the degradation of diisopropanolamine: effect of temperature and pH. International Conference on Environment and Industrial Innovation (IPCBEE)
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35:402–417
Ko EJ, Kim KW, Kang SY, Kim SD, Bang SB, Hamm SY, Kim DW (2007) Monitoring of environmental phenolic endocrine disrupting compounds in treatment effluents and river waters, Korea. Talanta 73:674–683
Kraigher B, Kosjek T, Heath E, Kompare B, Mandic-Mulec I (2008) Influence of pharmaceutical pesticides residues on the structure of activated sludge bacterial communities in wastewater treatment bioreactors. Water Res 42:4578–4588
Kueh CSW, Lam JYC (2008) Monitoring of toxic substances in the Hong Kong marine environment. Mar Pollut Bull 56:744–757
Lee Y, von Gunten U (2010) Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res 44:555–566
Leech D, Snyder MT, Wetzel RG (2009) Natural organic matter and sunlight accelerate the degradation of 17ß-estradiol in water. Sci Total Environ 407:2087–2092
Li Y, Zhang A (2014) Removal of steroid estrogens from waste activated sludge using Fenton oxidation: influencing factors and degradation intermediates. Chemosphere 105:24–30
Li W, Nanaboina V, Zhou Q, Korshin GV (2012) Effect of Fenton treatment on the properties of effluent organic matter and their relationships with the degradation of pharmaceuticals and personal care products. Water Res 46:403–412
Lim SJ, Seo CK, Kim TH, Myung SW (2013) Occurrences and ecological hazard assessment of selected veterinary medicines in livestock wastewater treatment plants. J Environ Sci Heal B 48:658–670
Liu ZH, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Matilainen A, Sillanpaa M (2010) Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 80:351–365
Mert BK, Yonar T, Kiliç MY, Kestioğlu K (2010) Pre-treatment studies on olive oil mill effluent using physicochemical, Fenton and Fenton-like oxidations processes. J Hazard Mater 174:122–128
Mohapatra DP, Brar SK, Tyagi RD, Surampalli RY (2011) Concomitant degradation of bisphenol A during ultrasonication and Fenton oxidation and production of bio-fertilizer from wastewater sludge. Ultrasonic Sonochem 18:1018–1027
Mohapatra D, Brar S, Tyagi R, Picard P, Surampalli R (2013) A comparative study of ultrasonication, Fenton's oxidation and ferro-sonication treatment for degradation of carbamazepine from wastewater and toxicity test by yeast estrogen screen (YES) assay. Sci Total Environ 447:280–285
Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H (2006) Pharmaceuticals chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res 40:3297–3303
Nakrst J, Bistan M, Tišler T, Jana ZK, Zgajnar-Gotvajn A (2010) Feasibility of Fenton’s oxidation for removal of estrogens from aqueous solutions. Acta Chim Slov 57:90–99
Navarro S, Fenoll J, Vela N, Ruiz E, Navarro G (2011) Removal of ten pesticides from leaching water at pilot plant scale by photo-Fenton treatment. Chem Eng J 167:42–49
Nicotra F (2009) Organic and bio-molecular chemistry. Eolss Publishers Company Limited
Ou X, Chen S, Quan X, Zhao H (2009) Photochemical activity and characterization of the complex of humic acids with iron (III). J Geochem Explor 102:49–55
Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MÁ, Prados-Joya G, Ocampo-Pérez R (2013) Pharmaceuticals as emerging contaminants and their removal from water: a review. Chemosphere 93:1268–1287
Sin JC, Lam SM, Mohamed AR, Lee KT (2011) Degrading endocrine disrupting chemicals from wastewater by TiO2 photocatalysis: a review. Int J Photoenergy 2012:23
Tamimi M, Qourzal S, Barka N, Assabbane A, Ait-Ichou Y (2008) Methonyl degradation in aqueous solutions by Fenton’s reagent and photo-Fenton system. Sep Purif Technol 61:103–108
Vilar VJ, Moreira FC, Ferreira AC, Sousa MA, Gonçalves C, Alpendurada MF, Boaventura RA (2012) Biodegradability enhancement of a pesticide- containing bio-treated wastewater using a solar photo-Fenton treatment step followed by a biological oxidation process. Water Res 46:4599–4613
Xu EG, Liu S, Ying GG, Zheng GJ, Lee JH, Leung KM (2014) The occurrence and ecological risks of endocrine disrupting chemicals in sewage effluents from three different sewage treatment plants and in natural seawater from a marine reserve of Hong Kong. Mar Pollut Bull 85:352–362
Yang B, Ying GG, Zhao JL, Liu S, Zhou LJ, Chen F (2012) Removal of selected endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) during ferrate (VI) treatment of secondary wastewater effluents. Water Res 46:2194–2204
Yaping Z, Jiangyong H (2008) Photo-Fenton degradation of 17β-estradiol in presence of α-FeOOHR and H2O2. Appl Catal B-Environ 78:250–258
Zepp RG, Faust BC, Hoigne J (1992) Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron (II) with hydrogen peroxide: the photo-Fenton reaction. Environ Sci Technol 26:313–319
Zhang CJ, Wang YX (2011) Effect of dissolved organic matter in landfill leachate on photodegradation of environmental endocrine disruptors. Int J Environ Pollut 45:69–80
Zhao Y, Hu J, Jin W (2008) Transformation of oxidation products and reduction of estrogenic activity of 17beta-estradiol by a heterogenous photo-Fenton reaction. Environ Sci Technol 42(14):5277–5284
Acknowledgments
The authors acknowledge the support from Severn Trent Water, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ifelebuegu, A.O., Ukpebor, J. & Nzeribe-Nwedo, B. Mechanistic evaluation and reaction pathway of UV photo-assisted Fenton-like degradation of progesterone in water and wastewater. Int. J. Environ. Sci. Technol. 13, 2757–2766 (2016). https://doi.org/10.1007/s13762-016-1103-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1103-3