Abstract
The sonochemical degradation of 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) in water and wastewater was investigated at ultrasonic frequency of 850 kHz. The effects of pH, initial concentrations, temperature, power and dissolved organic carbon were examined. The results obtained indicated that the rate of ultrasonic degradation of E2 and EE2 in water and wastewater is influenced by the pH, power, air sparging and the dissolved organic content of the aqueous solutions. Mass degradation rates of E2 and EE2 per kW ranged from 1.7 to 4.0 mg kW−1 at varying process parameters. The degradation process followed the pseudo-second-order kinetic model with rate constant of 1.71 × 10−2 min−1 at 25 °C. The value for activation energy (E a = 15.21 kJ mol−1) obtained from Arrhenius-type plot, indicated that the ultrasonic degradation of steroid hormones is thermodynamically feasible, and does not progress only on radical reactions but other intermediate reaction processes. In wastewater, the higher dissolved organic carbon significantly reduced the effectiveness of degradation of the E2 and EE2 showing that ultrasound treatment will be more effective as a tertiary treatment option in wastewater applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The widespread occurrence of endocrine disrupting chemicals (EDCs) in treated wastewater particularly the steroidal hormones is well recognised, and there are concerns about the adverse effect that they may have on both the aquatic environment and humans (Diamanti-Kandarakis et al. 2009; Ifelebuegu 2011). The steroid hormones particularly 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) are among the most potent EDCs. Effluent from wastewater treatment plants (WWTPs) is a major source of E2 and EE2 across the globe at levels which significantly cause endocrine disruption, and conventional wastewater treatment is not effective in degrading these chemicals to levels below their potentially non-effective concentrations (Esperanza et al. 2007; Gomez et al. 2007; Kanda and Churchley 2008).
Recent studies have explored the development of non-biological methods for the removal of EDCs in water and wastewater with particular consideration to advanced oxidation processes (AOPs) (Klavarioti et al. 2009). A number of AOPs have been investigated for treatment of emerging contaminants in various water environments (Esplugas et al. 2007). Techniques that have been used include: TiO2 photocatalysis induced by UV-A (Rizzo et al. 2009), Fenton’s oxidation (Hsueh et al. 2005), artificial solar irradiation (Méndez-Arriaga et al. 2008), degradation by photo-Fenton oxidation based on sunlight (Shemer et al. 2006), ozonation (Vogna et al. 2004), chlorine dioxide oxidation (Deborde and von Gunten 2008) and Fenton-like oxidation (Ifelebuegu and Ezenwa 2011). Some of the AOPs have got drawbacks related to formation of by-products that could have harmful effects. In view of the increased demand for higher water quality standards without toxic by-product concerns, significant attention has been drawn to the use of ultrasound for treatment of pharmaceutical and personal care products in water and wastewater (Gogate and Pandit 2004; Naddeo et al. 2009; Chiha et al. 2011). This is because the generation of hydroxyl radicals through acoustic cavitations results in oxidative degradation of contaminants (Torres et al. 2008). The cavitations involve the formation, growth and consequent collapse of micro bubbles, which occur in very short periods and release large amount of energy over a very small area (Gogate and Pandit 2002).
Ultrasonic irradiation has the capacity to degrade a variety of organic compounds particularly hydroxylated and halogenated aromatics and hydrocarbons (Loan et al. 2007; Méndez-Arriaga et al. 2008; Hartmann et al. 2008). E2 and EE2 both have similar aromatic structures (Fig. 1a, b) and are amenable to sonochemical degradation. Sonochemical reactions result from the collapse of cavitation bubbles in a liquid which leads to thermal dissociation of water vapour into hydroxyl radicals. It can also result from the interfacial sheath between the bubble and the surrounding liquid or the solution bulk (Ince et al. 2001; Mason and Pétrier 2004). The hydroxyl radical generated is predominantly responsible for the oxidation of various organic compounds.
The purposes of this research which was conducted at the Sonochemistry Centre, Coventry University between 2009 and 2010 were to investigate the degradation of E2 and EE2 by ultrasonic irradiation and to determine the effects of process parameters as well as the thermodynamic properties. Much uncertainty still exists about the mechanics for effective degradation of EDCs in water and wastewater, hence the need for this research. The kinetic and thermodynamic data will find application in modelling of industrial scale application of ultrasound in water and wastewater treatment.
Materials and methods
Chemicals
The model solutes used for this research (E2 and EE2) were purchased from Sigma Aldrich (Gillingham, UK). All reagents used were all of HPLC grade and were purchased from Fischer Scientific (Loughborough, UK). The stock solutions of E2 and EE2 were prepared by dissolving the required amounts in HPLC grade water. The aqueous solutions used for the sonication were made from the stock using deionised water (DI), treated wastewater final effluent (FE) and settled wastewater (SW). pH adjustments were carried out by using sodium hydroxide and hydrochloric acid.
Wastewater
The wastewater used for the research was obtained from a local sewage treatment plant located in the West Midlands, UK. The treated final effluent (FE) and the settled wastewater (SW) from the primary sedimentation tank were sampled. The characteristics of the wastewater used are presented in Table 1.
Apparatus
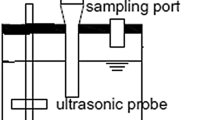
Sonochemical treatment was carried out using a cylindrical water-jacketed ultrasonic baths operating at a frequency of 850 kHz (Meinhardt Ultraschalltechnik K80-5). A cooling system JULABO Labortechnik GmbH, 230 V/50 Hz, 115 V/60 Hz, temperature range from −20 °C to +40 °C and temperature stability of ±0.5 °C, was used to keep temperature constant at 10, 20, 25 and 30 °C for thermodynamic studies.
Procedures
Experiments were conducted using 200 mL of 1 mg L−1 aqueous solutions of E2 and EE2 in deionised and wastewater with the temperature maintained between 10 and 30 °C. Although the steroid hormones occur in ng L−1 levels in surface and wastewater, higher concentrations were used for the study of the effects of operating parameters and the thermodynamic properties, which is concentration independent (Annamalai and Puri 2002).
Sonication was carried out for 60 min, and the temperature and pH were monitored with a thermocouple immersed in the solution. Blank samples were also sonicated for quality control purposes. Aliquots for HPLC analysis were withdrawn at different intervals between 0 and 60 min. Experiments were conducted at fixed frequencies of 850 kHz with varying powers and intensities. The experiments were conducted at varying pH, varying initial concentrations, varying temperatures, varying dissolved organic (DOC) contents for the wastewater, and varying power and with and without air sparging.The calorimetric method was used for the calculation of applied power using Eq. 1:
where C p = heat capacity of water (4.198 J g−1 K−1), M = mass of water (kg). dT/dt is the temperature rise per second.
4-Nitrophenol dosimetry
Para-nitrophenol (PNP) dosimetry was used to assess the rate of hydroxyl radical formation by monitoring the oxidation of PNP to 4-nitrocatechol (4-NC) photometrically. Aqueous PNP has an ultraviolet maximum at 401 nm and the hydroxylated product 4-NC at 512 nm. The number of hydroxyl radicals formed during sonication was quantified by observing the amount of product formed. 4-Nitrophenol (0.278 g) was added to 2 L of distilled water and raised to pH 5. Two hundred millilitres of this solution was analysed on the 850 kHz ultrasonic bath, operated at a power of 27.82 W, for 90 min. 1.5 mL Aliquots were withdrawn into the cuvettes at 5, 15, 30, 45, 60 and 90 min for analysis on the UV–VIS spectrophotometer. Prior to analysis, equal amounts (1.5 mL) of 0.2 M NaOH were added to the sonicated PNP, resulting in a final concentration of 0.1 M NaOH. Absorbance measurements at 401 nm and 512 nm were taken, indicating the decrease of PNP and the subsequent formation of 4-nitrocatechol (4-NC), respectively. A plot of concentration (μM) against time (mins) gave a linear graph, and the rate of formation of hydroxyl radical was calculated. PNP and 4-NC were quantified by the measurement of their absorbencies in 0.1 M NaOH at 401 nm (extinction coefficient = 19,200 M−1 cm−1 for PNP) and 512 nm (extinction coefficient = 12,300 M−1 cm−1 for 4-NC), respectively.
Analyses
Analysis of EDCs
EDCs were analysed on the Hewlett Packard 1050-series HPLC, with the fluorescence detector wavelengths of 280 nm (excitation) and 310 nm (emission). Details of the analytical methods have been previously described in Ifelebuegu et al. (2010). The limit of detection (LOD) and the Instruments Quantization Limit (IQD) based on signal to noise were evaluated as described in Sarkar and Panchagnula (2006). The LOD values were 0.11 and 0.031 μg L−1 and IQL values were 0.34 and 0.093 μg L−1 for E2 and EE2, respectively.
Results and discussion
Effects of pH
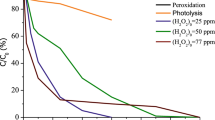
In water and wastewater treatment, pH is a crucial parameter. The effects of pH change for the removal of E2 and EE2 were investigated by varying the pH under constant process parameters. pH adjustments were done with dilute sodium hydroxide and sulphuric acid solutions. Figure 2 shows the results of the effects of pH on the degradation of EE2. Similar result was obtained for E2 (not included). It can be observed from the results in Fig. 2 that the degradation of the steroid hormones was faster at acidic and neutral pH and much slower at alkaline conditions. The difference in degradation rates between pHs 3, 5 and 7 is relatively low consistent with the findings of Gultekin and Ince (2008) during the sonochemical degradation of another EDC bisphenol-A. It however got increasingly very significant at alkaline pH. This is consistent with reports of pH having significant effect in certain organics at alkaline pH ranges (Méndez-Arriaga et al. 2008; Torres et al. 2008).
The structures of E2 and EE2 are shown in Fig. 1a, b. They both have a phenolic moiety, and it has been reported by others that the sonochemical degradation of phenolic compounds is favoured at lower pH ranges (Ku et al. 1997; Chiha et al. 2011). Also, the pKa values of E2 and EE2 are 10.4 and 10.7, respectively (Clara et al. 2004), and hence, at lower and neutral pH (3, 5 and 7), both E2 and EE2 exist in non-ionic molecular form and can easily diffuse into the cavity–liquid interface, whereas at pH 9 they are partially ionic due to the deprotonation of the phenolic group and hence more hydrophilic, and less likely to approach the cavity–liquid interface where the concentration of hydroxide radical is predominant.
Effects of initial EDCs concentration
The effects of EDCs concentrations were investigated, and the sonication experiments were carried out at 1, 2 and 3 mg L−1 of individual aqueous solutions of E2 and EE2 at ultrasound frequency of 850 kHz, 50 W in DI Water. It can be seen in Fig. 3a that the percentage removal efficiency with time relative to the initial concentration is inversely related to the initial concentrations of the EDCs. This is similar to the findings of Méndez-Arriaga et al. (2008), Gultekin and Ince (2008) and Chiha et al. (2011). This behaviour is due to the fact that the relative number of hydroxyl radical available to react with the substrate becomes less in comparison with the EDCs molecules. The mass of the EDCs degraded per unit power was investigated. Mass degraded per unit power was calculated using Eq. 2 below:
where C 0 and C t are the initial and final EDCs concentrations in mg L−1, V is volume of solution in the reactor in litres and P is the ultrasonic power in watts.
Figure 3b represents the mass of the EDCs degraded per unit power. It can be seen that the mass degraded per unit power with increasing initial concentrations of E2 and EE2 remained relatively the same. This shows that the mass of EDCs degraded per unit power is independent of the initial concentrations of the EDCs.
Effect of air sparging
Sonochemical degradation of organic pollutants in aqueous solutions is affected by air or gas bubbling (Pétrier et al. 2007; Chiha et al. 2011). To investigate the effects of this on the sonochemical degradation of E2 and EE2, continuous injection of air at equivalent flow rates was monitored during the sonication process. The result of this study indicates that with air sparging, there was increased mass degradation rate per unit power of the EDCs. As illustrated in Fig. 4, higher degradation of 3.4, 3.8 and 4.0 mg kW−1 was obtained with air sparging compared to 1.7, 1.9 and 2.4 mg kW−1 obtained without air sparging at 15, 20 and 30 min sonication time, respectively. This increase in the mass degraded per kilowatt with the introduction of air is due to the fact that the components of the air such as nitrogen and oxygen act as nucleation sites for cavitations. These findings are consistent with those of Méndez-Arriaga et al. (2008) which showed that the introduction of air promoted the formation of hydroxyl radical, thus contributing to the degradation of ibruprofen. Moreover, previous studies by Pétrier et al. (2007), Torres et al. (2008) and Chiha et al. (2011) have also noted the importance of gas saturation on the sonochemical treatment of pollutants in water. In this experiment, the reaction medium became slightly acidic as the pH changed from 6 ± 0.3 to 4 ± 0.3, resulting to the generation of nitrate, nitrite and hydroxyl radicals as shown in Eqs. 3–10 (Gultekin and Ince 2008). These equations show that higher degradation of E2 and EE2 with air bubbling is as a result of the generation of nitric acid which enhances the reaction by reducing the pH and by the formation of excess hydroxyl, nitrite and nitrate radicals which are more reactive with aromatic compounds and improve the sonochemical degradation process.
Degradation kinetics
Some studies have suggested that degradation of organic compounds by sonication does not obey first-order kinetics (Torres et al. 2008). To investigate the rate of E2 and EE2 removal by sonochemical degradation, the data generated were evaluated using the pseudo-first-order and pseudo-second-order kinetic models. The EDCs degradation can be represented by the following nth-order reaction kinetics in Eq. 11:
where C represents the EDCs concentration, n the order of the reaction, k the reaction rate coefficient and t the time. The integrated form of Eq. 11 for a second-order reaction is given by equation 12:
where C 0 is the initial EDCs concentration and K 2 is the second-order rate constant. A plot of 1/C − 1/C 0 against t gave a straight-line graph with the rate constants evaluated from the slope of the graphs (Fig. 5). The pseudo-second-order rate constant k 2 and the corresponding linear regression correlation coefficient values R 2 are given in Table 3, and it can be concluded that the ultrasonic degradation of the estrogens can be evaluated by second-order kinetic models. Although the first-order evaluation (not included) showed a good fit, the second-order model gave a better fit. This suggests that the sonochemical degradation of EDCs is a complex reaction that can be described by both kinetic models at different stages of the reaction. Various authors have reported both first- and second-order kinetics for similar organic compounds (Koda et al. 2003; Pétrier et al. 2007; Hameed et al. 2007; Torres et al. 2008).
Effect of applied power
The effect of power on sonochemical degradation of EE2 was investigated by varying the US power, and maintaining the frequency of the 850 kHz bath. The mass degradation profile obtained at 850 kHz frequency for US powers of 50.00, 27.82, 16.17 and 6.76 W is shown in Fig. 6. The results show that the degradation rates increased linearly with increase in power. These findings are consistent with those of Méndez-Arriaga et al. (2008), Madhavan et al. (2010) and Chiha et al. (2011), which suggest that increased bubbles, pressure and temperature in the bubbles are directly related to acoustic power and enhance the degradation of contaminants. The applied power had a linear relationship with power intensity as shown in Table 2. With increasing applied power intensity, there was higher degradation of the EDCs (Fig. 6); this is due to the increased cavitational activity at higher levels of power as confirmed from the 4-nitrophenol dosimetry. Also, increased power leads to increased acoustic amplitude and causes a more violent collapse of the cavitation bubble (Hamdaoui and Naffrechoux 2008) and hence enhances the rate of degradation of the EDCs. The increased rate of degradation with applied power showed a direct relationship with the rate of hydroxyl radical generation as shown in Fig. 7. These findings are consistent with other studies which showed a linear correlation between reaction rates and applied power for the degradation of other pharmaceuticals and personal care products (PPCPs) such as ibuprofen (Méndez-Arriaga et al. 2008), chlorophenols (Madhavan et al. 2010) and diclofenac (Naddeo et al. 2010).
Effects of organic matter
The sonochemical degradation of organic compounds is a function of their physicochemical properties. To understand the impact of natural organic matter present in wastewater on the ultrasonic degradation, the mass degradation rates in deionised water were compared (DOC = 0.9 mg L−1): final effluent sample from wastewater treatment plant (DOC = 9.78 mg L−1), a 50 % dilution of settled wastewater with deionised water (DOC = 40.3 mg L−1) and settled wastewater (DOC = 80.6 mg L−1). The results of the mass degradation rates of the EDCs at 850 kHz ultrasonic frequency and applied power of 50 W are shown in Fig. 8. It can be seen that the relative mass degradation rates of E2 and EE2 reduced with increasing concentrations of dissolved organic carbon. The mass degradation rates of 1.80, 2.08, 2.80 and 3.10 mg kW−1 for E2, and 1.6, 2.51, 3.45 and 3.74 mg kW−1 for EE2 were observed at DOC concentrations of 80.6, 40.3, 9.78 and 0.9 mg L−1, respectively. This can be attributed to the fact that higher DOC in treated final effluent and settled wastewater acts as a scavenger for hydroxyl radicals and hence inhibits the hydroxide radical attack of the compounds (Torres et al. 2007; Lindsey and Tarr 2000a, b). This also confirms the role of free radicals in the degradation rates of E2 and EE2. It can be concluded that in wastewater treatment applications, ultrasonic treatment will only be effective at the tertiary treatment stage when most of the dissolved organics have been removed by biological means.
Effects of temperature
Temperature has a significant effect on the reaction kinetics as shown in Table 3 and Fig. 5. The rate constants for EE2 increased from 1.25 × 10−2 to 1.91 × 10−2 min−1, respectively, when the temperature of the reaction increased from 10 to 30 °C. The increase in rate constant with higher temperature is due to the easier cavitation as a result of higher vapour pressure of solution (Mason 1999). Increase in temperature increases the number of cavitation bubbles and hence number of hydroxyl radicals consequently an increased degradation rates. Although an increase in temperature can result to inward motion of bubbles during collapse due to increased vapour pressure. However, in this work within the temperature range of 10 to 30 °C considered, an increase resulted to higher degradation rates for E2 and EE2.
The second-order rate constants were correlated by an Arrhenius-type expression given by Eq. 13:
Given K = the rate constant for the reaction, A = proportionality constant, e = the base of natural logarithms, E a = activation energy, R = ideal gas constant = 8.314 J mol K−1 and T = temperature in Kelvin. The Arrhenius equation was used to calculate the activation energy, and the pseudo-second-order rate constant is expressed as a function of temperature (Eq. 14)
A graph of lnk2 against 1/T (min−1) resulted in a straight-line graph with R 2 = 0.9726 (Fig. 9). From the slope (−E a/R), the activation energy (E a) was calculated as 15.21 kJ mol−1.
The low activation energy (E a = 15.21 kJ mol−1) demonstrates that the ultrasonic degradation of EDCs is thermodynamically feasible. It also suggests that the ultrasonic degradation does not involve only radical reactions but also intermediate reactions steps involving radical–molecule or ion–molecule reactions and a diffusion-controlled reaction (Mason 1999; Kim et al. 2001). Factors such as the structural difference of the target compounds, the concentration, the ultrasonic frequency, and power of the equipment affect the rate of degradation of the steroid hormones.
Conclusion
The main conclusions drawn from this study include:
-
The degradation of E2 and EE2 by sonication in water and wastewater is affected by pH, especially in the alkaline pH range. The rate of degradation is favoured in the acidic pH ranges and affected by ultrasonic power, air sparging and sonication temperatures.
-
Mass of E2 and EE2 degraded per kilowatt was 4.0 mg kW−1 with air sparging and 2.4 mg kW−1 without air sparging after 30 min of sonication time.
-
The degradation mechanism does not progress solely on hydroxyl radical generation, but appears to involve intermediate reaction steps involving radical–molecule reactions, ion–molecule reactions or pyrolysis.
-
Temperature affected the kinetics of the reaction, with higher percentage removal as temperature increased from 10 to 30 °C. The rate constants for EE2 increased from 1.25 × 10−2 to 1.91 × 10−2 min−1 with the increased temperature.
-
The sonolytic degradation followed the pseudo-second-order kinetic model for EE2, with R 2 values of 0.99 obtained at temperatures between 10 and 30 °C.
-
The low activation energy (E a = 15.21 kJ mol−1) obtained suggests that the ultrasonic degradation of EDCs is thermodynamically feasible, and the degradation does not involve only radical reactions.
-
Ultrasonic application for treatment of EDCs in wastewater will be more effective as a tertiary treatment stage.
References
Annamalai K, Puri KI (2002) Advanced thermodynamics engineering. CRC Press, Boca Raton, FL
Chiha M, Hamdaoui O, Baup S, Gondrexon N (2011) Sonolytic degradation of endocrine disrupting chemical 4-cumylphenol in water. Ultrason Sonochem 18(5):943–950. doi:10.1016/j.ultsonch.2010.12.014
Clara M, Strenn B, Saracevic E, Kreuzinger N (2004) Adsorption of bisphenol-A, 17β-estradiol and 17α-ethinylestradiol to sewage sludge. Chemosphere 56(9):843–851. doi:10.1016/j.chemosphere.2004.04.048
Deborde M, von Gunten U (2008) Reactions of chlorine with inorganic and organic compounds during water treatment-kinetic and mechanisms: a critical review. Water Res 42(1–2):13–51. doi:10.1016/j.watres.2007.07.025
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev 30(4):293–342. http://edrv.endojournals.org/content/30/4/293.short
Esperanza M, Suidan MT, Marfil-Vega R, Gonzalez C, Sorial GA, McCauley P, Brenner R (2007) Fate of sex hormones in two pilot-scale municipal wastewater treatment plants: conventional treatment. Chemosphere 66(8):1535–1544. http://www.ncbi.nlm.nih.gov/pubmed/17083962. doi:10.1016/j.chemosphere.2006.08.020
Esplugas S, Bila DM, Krause LGT, Dezotti M (2007) Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater 149(3):631–642. doi:10.1016/j.jhazmat.2007.07.073
Gogate PR, Pandit AB (2002) Cavitation: an auxiliary technique in wastewater treatment schemes. Adv Environ Res 6(3):335–358. doi:10.1016/S1093-0191(01)00067-3
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment 2: hybrid methods. Adv Environ Res 8(3–4):553–597. doi:10.1016/S1093-0191(03)00032-7
Gomez MJ, Martinez Bueno MJ, Lacorte S, Fernandez-Alba AR, Aguera A (2007) Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 66(6):993–1002. http://www.ncbi.nlm.nih.gov/pubmed/16962638. doi:10.1016/j.chemosphere.2006.07.051
Gultekin I, Ince N (2008) Ultrasonic destruction of bisphenol-A: the operating parameters. Ultrason Sonochem 15(4):524–529. doi:10.1016/j.ultsonch.2007.05.005
Hamdaoui O, Naffrechoux E (2008) Sonochemical and photosonochemical degradation of 4-chlorophenol in aqueous media. Ultrason Sonochem 15(6):981–987. doi:10.1016/j.ultsonch.2008.03.011
Hameed BH, Ahmad A, Aziz N (2007) Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem Eng J 133(1–3):195–203. doi:10.1016/j.cej.2007.01.032
Hartmann J, Bartles P, Mau U, Witter M, Von Tuempling W, Hofmann J, Nietzschmann E (2008) Degradation of the drug diclofenac in water by sonolysis in presence of catalysts. Chemosphere 70(3):453–461. doi:10.1016/j.chemosphere.2007.06.063
Hsueh CL, Huang YH, Wang CC, Chen Y (2005) Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 58(10):1409–1414. doi:10.1016/j.chemosphere.2004.09.091
Ifelebuegu MO (2011) The fate and behaviour of selected endocrine disrupting chemicals in full scale wastewater and sludge treatment unit processes. Int J Environ Sci Technol 8(2):245–254
Ifelebuegu AO, Ezenwa CP (2011) Removal of endocrine disrupting chemicals in wastewater treatment by Fenton-like oxidation. Water Air Soil Poll 217(1–4):213–220. doi:1007/s11270-010-0580-010
Ifelebuegu AO, Theophilus SC, Bateman MJ (2010) Mechanistic evaluation of the sorption properties of endocrine disrupting chemicals in sewage sludge biomass. Int J Environ Sci Technol 7(4):617–622
Ince NH, Tezcanli G, Belen RK, Apikyan IG (2001) Ultrasound as a catalyzer of aqueous reaction systems: the state of the art and environmental applications. Appl Catal B Environ 29(3):167–176. doi:10.1016/S0926-3373(00)00224-1
Kanda R, Churchley J (2008) Removal of endocrine disrupting chemicals during conventional wastewater treatment. Environ Technol 29(3):315–323. http://www.informaworld.com/smpp/content~db=all~content=a792949752. http://pdfserve.informaworld.com/599460__792949752.pdf. doi:10.1080/09593330802099874
Kim JK, Huang CP, Chiu PC (2001) Sonochemical decomposition of dibenzothiophene in aqueous solution. Water Res 35(18):4370–4378. doi:10.1016/S0043-1354(01)00176-2
Klavarioti M, Mantzavinos D, Kassinos D (2009) Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ Int 35(2):402–417. doi:10.1016/j.envint.2008.07.009
Koda SKT, Kondo T, Mitome H (2003) A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason Sonochem 10(3):149–156. doi:10.1016/S1350-4177(03)00084-1
Ku Y, Chen K, Lee K (1997) Ultrasonic destruction of 2-chlorophenol in aqueous solution. Water Res 31(4):929–935. doi:10.1016/S0043-1354(96)00372-7
Lindsey ME, Tarr MA (2000a) Inhibition of hydroxide radical reaction with aromatics by dissolved natural organic matter. Environ Sci Technol 34(3):444–449. doi:10.1021/es990457c
Lindsey ME, Tarr MA (2000b) Quantitation of hydroxyl radical during Fenton oxidation following a single addition of iron and peroxide. Chemosphere 41(3):409–417. doi:10.1016/S0045-6535(99)00296-9
Loan I, Wilson S, Lundanes E, Neculai A (2007) Comparison of Fenton and sono-Fenton bisphenol A degradation. J Hazard Mater 142(1–2):559–563. doi:10.1016/j.jhazmat.2006.08.015
Madhavan J, Grieser F, Ashokkumar M (2010) Combined advanced oxidation processes for the synergistic degradation of ibuprofen in aqueous environments. J Hazard Mater 178(1–3):202–208. doi:10.1016/j.jhazmat.2010.01.064
Mason TJ (1999) Sonochemistry. Oxford University Press, New York
Mason TJ, Pétrier C (2004) Advanced oxidation processes for water and wastewater treatment. In: Parson S (ed) Ultrasound processes. IWA Publishing, London
Méndez-Arriaga F, Esplugas S, Giménez J (2008) Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res 42(3):585–594. doi:10.1016/j.watres.2007.08.002
Naddeo V, Landi M, Belgiorno V, Napoli R (2009) Wastewater disinfection by combination of ultrasound and ultraviolet irradiation. J Hazard Mater 168(2–3):925–929. doi:10.1016/j.jhazmat.2009.02.128
Naddeo V, Belgiorno V, Kassinos D, Mantzavinos D, Meric S (2010) Ultrasonic degradation, mineralization and detoxification of diclofenac in water: optimization of operating parameters. Ultrason Sonochem 17(1):179–185. doi:10.1016/j.ultsonch.2009.04.003
Pétrier C, Combet E, Mason T (2007) Oxygen-induced concurrent ultrasonic degradation of volatile and non-volatile aromatic compounds. Ultrason Sonochem 14(2):117–121. doi:10.1016/j.ultsonch.2006.04.007
Rizzo L, Meric S, Kassinos D, Guida M, Russo F, Belgiorno V (2009) Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res 43(4):979–988. doi:10.1016/j.watres.2008.11.040
Sarkar MKS, Panchagnula R (2006) Development and validation of RP-HPLC and ultraviolet spectrophotometric methods of analysis for the quantitative estimation of antiretroviral drugs in pharmaceutical dosage forms. J Chromatogr B 830(2):349–354. doi:10.1016/j.jchromb.2005.11.014
Shemer H, Kunukcu YK, Linden GK (2006) Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 63(2):269–276. doi:10.1016/j.chemosphere.2005.07.029
Torres R, Abdelmalek F, Combet E, Petrier C, Pulgarin C (2007) A comparative study of ultrasonic cavitation and Fenton’s reagent for bisphenol A in deionised and natural waters. J Hazard Mater 146(3):546–551. doi:10.1016/j.jhazmat.2007.04.056
Torres R, Petrier C, Combet E, Carrier M, Pulgarin C (2008) Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason Sonochem 15(4):605–611. doi:10.1016/j.ultsonch.2007.07.003
Vogna D, Marotta R, Andreozzi R, Napolitano A, d’Ischia M (2004) Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res 38(2):414–422. doi:10.1016/j.watres.2003.09.028
Acknowledgments
The authors wish to express their gratitude to Department of Geography, Environment and Disaster Management and the Sonochemistry Centre, Coventry University for material and equipment support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ifelebuegu, A.O., Onubogu, J., Joyce, E. et al. Sonochemical degradation of endocrine disrupting chemicals 17β-estradiol and 17α-ethinylestradiol in water and wastewater. Int. J. Environ. Sci. Technol. 11, 1–8 (2014). https://doi.org/10.1007/s13762-013-0365-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0365-2