Abstract
The survival, feeding response, and detoxification mechanism of Rhynchophorus ferrugineus Olivier, a key pest responsible for destruction of date palm, was examined with different extracts of Piper nigrum and its major constituent (piperine) identified by GC-MS. In the present study, toxicity of different extracts of black pepper was evaluated by incorporating diffferent doses of extracts into the artificial diet of red palm weevil larvae. All extracts showed dose-dependent insecticidal activity to the tested eighth-instar red palm weevil larvae. Among all the extracts, maximum larvicidal activity was exhibited by chloroform (LD50 = 342.62 mg/l), followed by dichloromethane (LD50 = 357.78 mg/l), acetone (LD50 = 372.57 mg/l), and ethanol (LD50 = 408.88 mg/l). However, piperine, a major constituent of all black pepper extracts identified by GC-MS in the present work, was found to be the most potent treatment exhibiting the least LD50 (219.88 mg/l). In addition, nutritional indices evaluated by calculating the efficiency of the conversion of ingested food (ECI) and digested food (ECD) at the same dose (219.88 mg/l) showed that there was maximum reduction in the ECI (49.90%) and ECD (62.21%) index of larvae fed diets incorporated with piperine. Larvae that were fed diets incorporated with different black pepper extracts experienced increases in the expression of detoxification genes (glutathione S-transferase and cytochrome P450), and this upregulation in detoxification genes (glutathione S-transferase, cytochrome P450 and esterase) was tremendously high in larvae fed diets incorporated with piperine. Results suggest that piperine is a promising bio-pesticide agent for the control of R. ferrugineus Olivier.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phoenix dactylifera L. is an important palm species that grows in arid, sub-tropical, and tropical regions (Khiyami and Alyamani 2008). This species not only tolerates harsh desert temperatures but is also resistant to drought and high levels of salinity. However, desert beauty (date palm) is not able to tolerate the invasion of the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Curculionidae), which results in serious threats to the date industry, especially in Gulf Cooperation Council (GCC) countries (Hussain et al 2013b).
Red palm weevil control is primarily based on the frequent application of synthetic pesticides (Hussain et al 2013a). However, environmental pollution, possible deleterious effects on non-target animals, applicator safety issues, concerns over human health, and the development of resistance among red palm weevils to insecticides have forced scientists to search for and develop new and sustainable methods of biological control (Al-Ayedh et al 2016). The development of plant-based red palm weevil control technology appears to be an attractive alternative.
The use of plant extracts is known to have a wide array of effects due to the presence of several active compounds. These plant extracts are comprised of secondary metabolites. These metabolites are concentrated in bark, leaves, fruit, and seeds (Isman 2006). The incorporation of plant extracts in pest management strategies aimed to kill or repel pests has a long history, as these extracts have been used throughout the world because of their ability to produce chemicals (Isman 2006). These chemicals function as defense mechanisms that reduce feeding injury caused by pests. Numerous modes of action of these chemicals have been described, including (1) cuticle disruption, (2) reduced fecundity, (3) molting inhibition, (4) growth reduction, (5) respiratory inhibition, (6) anti-feeding activity, (7) repellent , and (8) toxic effects (Isman 2000, Tsao et al 2002, Akhtar and Isman 2004, Maia and Moore 2011, Erdogan et al 2012, Deletre et al 2013). In addition, botanical pesticides, unlike synthetic pesticides, contain complex mixtures of several compounds (Isman 2006). These diverse characteristics are advantageous over synthetic pesticides because they delay the development of resistance among pest populations (Feng and Isman 1995, Joseph et al 2012). Species of Piper are a good source of naturally occurring insecticides (Scott et al 2007b). For instance, piperine is a compound derived from P. nigrum and is known to have insecticidal properties against pests (Su 1977, Scott et al 2007b).
Few studies have explored the potential of plant-based products as control agents for red palm weevils. The findings of Salama and Ismail have found that Ambrosia maritima, Calotropis procera, and Curcuma longa are highly toxic to red palm weevils (Salama and Ismail 2007). In another study, neem extract and insect growth regulator (flufenoxuron) were used to investigate their potential to control red palm weevils. Their results suggested that both neem and IGR at higher doses (500 mg/l) caused 22.2 and 14.3% adult mortality, respectively (El-Bokl et al 2010). Plant-based essential oils have also been used to investigate their potential to control red palm weevils (Shukla et al 2012). Their findings have shown that the essential oils from Eupatorium adenophorum flowers and Artemisia nilagirica aerial parts promote anti-feeding activity at a concentration of 1000 ppm. Recently, the insecticidal potential of phenylpropanoids, an important class of plant secondary metabolites against red palm weevils, was evaluated. Results showed that coumarin greatly disturbed the growth and detoxification mechanism of red palm weevils that ultimately leads to the death of R. ferrugineus Olivier larvae (AlJabr et al 2017). However, none of the study has investigated the insecticidal potential of P. nigrum extracts to control red palm weevils. Our study aimed to (1) compare the toxicity of P. nigrum extracts with different solvents to the main chemical component (piperine), (2) assess the impact of P. nigrum extracts and piperine on the larval growth and development of R. ferrugineus Olivier, and (3) evaluate the impact of extracts and piperine on the quantitative expression of detoxification genes from the gut of R. ferrugineus Olivier larvae. Determining how P. nigrum extracts and piperine affect the growth and detoxification mechanisms of R. ferrugineus Olivier will facilitate the development of inexpensive IPM strategies to control R. ferrugineus Olivier infestations in date palm plantations.
Material and Methods
Rearing of experimental insects
Rhynchophorus ferrugineus Olivier adults were collected in October–December 2014 from infested date palms (25°23′02.9″N 49°36′33.6″E). Mated females were shifted to pineapples for egg laying. Second instar larvae were reared in the laboratory with a 16-h light photoperiod at 30 ± 1°C, 75% ± 5% relative humidity (RH) on artificial diets as per the protocol standardized previously (Hussain et al 2015).
Extraction procedure
Piper nigrum seeds were ground to fine powder in an electric grinder and loaded into extraction thimbles. Piper nigrum powder was extracted using pure solvents (>99%) including acetone, chloroform, dichloromethane, or ethanol separately until the solvent ran clear in the soxhlet apparatus. Extracts were evaporated until they were dry under reduced pressure. The extracts were freeze dried until a powder was formed and were then stored at −20°C. The desired stock solution of each solvent P. nigrum extract was prepared with their respective solvent. Further dilutions of each extract were prepared with ddH2O.
Identification of active compounds
Qualitative analysis of P. nigrum extracts was performed with a GCMS-QP 2010 Plus (Shimadzu). The capillary column used was RTx®-1 (30 m × 0.32 mm × 0.25 μm) operated with the following program: the initial oven temperature (60°C) was maintained for 60 s following injection, and then the temperature was raised to 180°C at a rate of 10°C/min and held for 1 min. Next, the oven temperature was raised to 280°C at 20°C/min and held for 15 min. Carrier gas, helium (99.99% purity), was used. Electron ionization (EI) was induced at 70 eV, and mass spectra were repetitively scanned between 35 and 335 atomic mass unit (amu). Identification of major compound (e.g., piperine) was confirmed by comparing the retention time and mass spectra with pure standard purchased from Sigma-Aldrich.
Laboratory toxicological bioassays
Preliminary laboratory toxicity bioassays (data not shown) were performed to identify a range of each P. nigrum extract dose to be used for toxicological bioassays. Separate preparations of artificial diets were prepared using individual P. nigrum extract solutions. Based on preliminary laboratory toxicity bioassays, five doses (150, 300, 450, 600, and 750 mg/l) were used to evaluate the toxicity of acetone, chloroform, dichloromethane, and ethanol extract of P. nigrum to newly molted eighth-instar larvae. Different doses of piperine, the major constituent of each extract as depicted from GC-MS, were prepared by dissolving it in 1 ml of acetone with ddH2O to obtain 100, 200, 300, 400, and 500 mg/l of piperine. A control treatment diet was prepared using ddH2O along with similar volume (1 ml) of the respective solvent used to prepare the artificial diet for each extract. All of the treatments were incubated at 30 ± 1°C with 75% ± 5% relative humidity in an incubator (Sanyo). Each replicate consisted of 25 larvae. Five replicates for each treatment from separate generations were prepared on different dates. The study was repeated over time. Dose mortality response was recorded daily until 100% mortality was achieved. A larva was considered dead if no signs of movement were observed. Control mortality was adjusted using Abbott’s formula (Abbott 1925). Corrected percent mortality data were angularly transformed. Corrected angularly transformed cumulative percent mortality data were analyzed by repeated measures ANOVA with Fisher’s LSD test (Statistix 2003). Lethal dose to kill 50% (LD50) of red palm weevil larvae and lethal time to kill 50% (LT50) larvae were calculated using probit analysis using POLO software (Russell et al 1977).
Feeding performance bioassays
To evaluate the impact of different extracts and their major constituent (piperine) on the growth and development of P. nigrum, nutritional analyses were performed. The experiment was conducted using eighth-instar (newly molted) larvae. In this experiment, 25 larvae per treatment per replicate were fed individually on artificial diet supplemented with either piperine, acetone, chloroform, dichloromethane, or ethanol extract of P. nigrum at the dose of 219.88 mg/l based on the LD50 value of the most potent treatment (piperine) as determined from experiments on eighth-instar red palm weevil larvae. Five replicates for each treatment from separate generations were prepared on different dates. A control treatment diet was prepared using ddH2O along with a similar volume of the respective solvent used to prepare the artificial diet for each extract. All of the experimental units were incubated at 30 ± 1°C with 75 ± 5% relative humidity in an incubator (Sanyo). The initial weight of the artificial diet offered to the larvae and the food remaining after 3 days were measured using an analytical balance. In addition, the frass produced over 3 days and the initial and final weights of the larvae were also measured. Insect, diet and frass weights were used to calculate nutritional indices. These indices were calculated by computing the efficacy of conversion of ingested food [ECI = 100 × dry weight gained by the larva/dry weight of food consumed by larva] and efficacy of conversion of digested food [ECD = weight gained by the larva/(food ingested by the larva − dry weight of frass excreted by larvae)] as described earlier by Hussain et al (2009). Another important nutritional index, approximate digestibility (AD), was calculated as (food ingested − frass weight)/food ingested × 100 (Hussain et al 2016). Significant differences between nutritional indices generated from larvae fed on different diets were determined using one-way ANOVA and Fisher’s LSD test (SAS Institute 2000).
Quantitative expression of detoxification genes of red palm weevils by qRT-PCR
For total RNA isolation, eighth-instar red palm weevil larvae were fed on artificial diet incorporated with piperine, acetone, chloroform, dichloromethane, or ethanol extract of P. nigrum at the dose of 219.88 mg/l based on the LD50 value of the most potent treatment (piperine) as determined from experiments on eighth-instar red palm weevil larvae. After 72 h, larvae were dissected in saline and then transferred to a mortar for fine grinding in liquid nitrogen. Total RNA from the mid-gut of red palm weevil larvae was extracted using RNeasy® Universal Mini Kit (cat no. 73404; Qiagen). Total RNA for each treatment was separately reverse-transcribed as per the protocol of PrimeScript First Strand cDNA Kit (cat no. 6110A; TaKaRa, Clontech). The synthesized first-strand cDNA was used as a template to quantify target gene expression sequenced from Macrogen sequencing facility (Macrogen, South Korea) (Table 1) with specific primers synthesized from Macrogen Korea in CFX96 Touch™ (Bio-Rad) as per the manufacturer’s protocol of the SYBR® Premix Ex Taq™ II kit (cat no. RR820W; TaKaRa Clontech). Three replicates were prepared using three larvae mid-guts. The results of each experimental unit were compared with those of the control by relative fold expression obtained by transforming the obtained results into absolute values using 2−ΔΔCt (Livak and Schmittgen 2001). The relative expression of each gene was set to 1 for the uninfected (control) treatment. Significant differences between gut samples extracted from different treatments were determined using one-way ANOVA and Fisher’s LSD test (SAS Institute 2000).
Results
Insecticidal activities
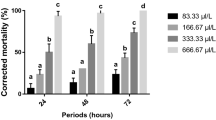
All of the extracts and the major constituent of black pepper (piperine) exhibited insecticidal activities against red palm weevil larvae. Overall, piperine-fed larvae had the lowest LD50 value (219.88 mg/l) as shown in Table 2. The mortality of red palm weevil larvae fed on piperine differed significantly after 3, 6, 9, and 12 days post-feeding time intervals (F 3, 64 = 1260.23, P < 0.0001), doses (F 4, 64 = 316.29, P < 0.0001), and their interaction (F 12, 64 = 38.71, P < 0.0001) (Fig 1). In addition, the most potent compound (piperine) showed 2.40 days to impart 50% larval mortality (LT50) at 500 mg/l as shown in Table 3. Red palm weevil larvae fed on diets incorporated with ethanol extract of black pepper showed the lowest response, resulting in the highest LD50 value (408.88 mg/l) (Table 2), along with the highest LT50 values (11.60 days) as shown in Table 3. However, ethanol extract-fed larvae differed significantly at all of the studied time intervals (F 3, 64 = 1697.90, P < 0.0001), doses (F 4, 64 = 313.17, P < 0.0001), and their interaction (F 12, 64 = 21.55, P < 0.0001) (Fig 2). Treatment of chloroform extract incorporated diet was also toxic to the tested larvae, resulting in significant differences in mortality at different time intervals (F 3,64 = 2019.51, P < 0.0001), doses (F 4,64 = 253.57, P < 0.0001), and their interaction (F 12,64 = 39.60, P < 0.0001) (Fig 3). Mortality percentages of red palm weevil larvae fed black pepper dichloromethane extract showed significant differences at all of the studied time intervals (F 3, 64 = 2024.50, P < 0.0001), doses (F 4, 64 = 270.11, P < 0.0001), and their interaction (F 12, 64 = 39.91, P < 0.0001) (Fig 4). Red palm weevil mortality was also highly significantly different at all of the studied time intervals (F 3, 64 = 1728.39, P < 0.0001), doses (F 4, 64 = 309.63, P < 0.0001), and their interaction (F 12, 64 = 25.75, P < 0.0001) among larvae fed diets incorporated with black pepper acetone extract (Fig 5).
Average cumulative corrected percent mortality of Rhynchophorus ferrugineus Olivier larvae fed artificial diets incorporated with piperine. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Average cumulative corrected percent mortality of Rhynchophorus ferrugineus Olivier larvae fed artificial diets incorporated with ethanol extract. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Average cumulative corrected percent mortality of Rhynchophorus ferrugineus Olivier larvae fed artificial diets incorporated with chloroform extract. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Average cumulative corrected percent mortality of Rhynchophorus ferrugineus Olivier larvae fed artificial diets incorporated with dichloromethane extract. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Average cumulative corrected percent mortality of Rhynchophorus ferrugineus Olivier larvae fed artificial diets incorporated with acetone extract. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Growth-retarding activities
Nutritional indices of tested compounds greatly differed against red palm weevil larvae at a dose of 219.88 mg/l. The larvae fed diets incorporated with piperine showed the greatest reduction (49.90%) in ECI and remained different compared to all other diets incorporated with black pepper extracts (F 5, 24 = 364, P < 0.0001). However, the lowest reduction (5.37%) was observed in larvae fed diets incorporated with black pepper ethanol extract (Table 4). Similarly, ECD also differed (F 5, 24 = 348, P < 0.0001) between larvae fed different diets. The most potent compound (piperine) greatly reduced (62.21%) ECD (Table 4). However, black pepper ethanol extract caused little reduction (8.56%) in ECD compared to control larvae. Larvae fed diets incorporated with black pepper chloroform, dichloromethane, and acetone extracts generated 48.36, 39.27, and 22.72% reductions in ECD compared to control larvae, respectively (Table 4).
The feeding of artificial diet incorporated with different extracts of black pepper and piperine resulted in significant increase in AD of red palm weevil larvae (F 5, 24 = 182, P < 0.0001). The most potent piperine tremendously enhanced the AD (24.57%) of red palm weevil larvae compared with control larvae (Table 4). Chloroform and dichloromethane extract of black pepper increases 16.78 and 17.77% AD compared with the control, respectively. The least potent extract (ethanol) of black pepper negligibility (3.34%) enhance AD of red palm weevil larvae compared to control larvae (Table 4).
Quantification of detoxification genes by qRT-PCR
The toxicity of different treatments induced different levels of detoxification genes such as cytochrome P450, glutathione S-transferase, and esterase. The expression of detoxification genes (F 2, 20 = 283.63, P < 0.0001), upon exposure to different treatments (F 4, 20 = 671.32, P < 0.0001) and their interaction (F 8, 20 = 53.58, P < 0.0001), showed significant differences. Cytochrome P450 was highly expressed compared to esterase (Fig 6). Overall, red palm weevil larvae fed on diets incorporated with piperine greatly induced the expression of cytochrome P450. However, the lowest fold expression of cytochrome P450, glutathione S-transferase, and esterase was induced by the least toxic treatment of black pepper ethanol extract (Fig 6).
Effect of black pepper extracts and piperine on the expression of detoxifying genes of red palm weevils. Newly molted eighth-instar larvae were fed for 72 h on artificial diets incorporated with piperine, chloroform, dichloromethane, ethanol, or acetone extract of black pepper. Bars (means ± SE) followed by different letter(s) are significantly different (Fisher’s LSD test, α = 0.05).
Piperine percentage in different extracts of black pepper
The GC-MS analysis of the crude extracts of black pepper revealed marked differences in the proportion of piperine. Overall, the highest proportion of piperine was detected from the chloroform extract of black pepper (74.15%). On the other hand, the lowest piperine proportion (14.07%) was detected from the least toxic ethanol extract of black pepper (Fig 7).
Discussion
Laboratory dietary bioassays of black pepper extracts and its major constituent (piperine) had various biological effects on the red palm weevil, R. ferrugineus Olivier. All of the tested treatments showed insecticidal activities against red palm weevil larvae from moderate to high degrees. The difference in toxicity among all of the extracts probably stemmed from the piperine content. The toxicity of the treatments was measured by their ability to disrupt larval growth and development, induce larval weight loss, enhance detoxification gene expression levels, and increase mortality.
Higher mortality rates were observed when R. ferrugineus Olivier larvae were fed artificial diets incorporated with black pepper chloroform extract. These exposed larvae could not tolerate this extract and failed to grow. In addition, these larvae were exceptionally sluggish and all of the larvae died within 6 days at 500 mg/l. The present black pepper chloroform extract shows promise in its ability to control R. ferrugineus Olivier larvae. The efficacy of this extract is equivalent to the efficacy observed by Scott et al (2007a), who examined the effect of this extract on common home and garden insect pests. They found that P. nigrum extract showed the best control efficacy against the eastern tent caterpillar, Malacosoma americanum Fabricius (Lasiocampidae: Lepidoptera). Khani et al (2012) reported acute toxicity (LD50 = 12.52 μl/ml) of P. nigrum extract against the rice moth, Corcyra cephalonica Stainton (Pyralidae: Lepidoptera). In addition, previous research has found that crude hexane extract of P. nigrum performed better among other black pepper extracts, having a 1.806 mg/g LD50 against Spodoptera litura Fabricius (Noctuidae: Lepidoptera) (Fan et al 2011). However, the acute toxicity levels of crude hexane extract of P. nigrum against S. litura Fabricius appear to be much higher compared to the P. nigrum extracts evaluated in the current study. Even the least potent ethanol extract of P. nigrum incorporated into the diet in the current study exhibited a much lower LD50 (408.88 mg/l) compared to the results of Fan et al (2011). Current toxicological studies of red palm weevils suggest that all of the tested extracts of P. nigrum are toxic. However, their toxicity to the weevils varies with the solvent. The difference in toxicity may stem from the chemical composition of extracts. GC-MS analyses of crude extract revealed marked differences in the proportion of piperine (Fig 7). P. nigrum extract with the least toxicity showed a relatively low percentage of piperine and vice versa. Toxicological bioassays, in which piperine was incorporated into the artificial diet, showed acute toxicity, resulting in the lowest LD50 (219.88 mg/l) against red palm weevil larvae. Therefore, our findings suggest that high larvicidal activity of P. nigrum extracts are apparently attributed to higher percentages of the main constituent piperine. In the past, the insecticidal activity of piperine has been well documented in other insect pests (Scott et al 2003). However, the present work, for the first time, highlights the potential of piperine to be used as a botanical insecticide against red palm weevils.
Piperine caused significant reductions in the growth and development of R. ferrugineus Olivier eighth-instar larvae, when it incorporated into the diet at a dose of 219.88 mg/l. Eighth-instar larvae fed on piperine-containing diets showed the lowest efficacy of conversion of digested and ingested diet, which meant that less food was available for growth because most of the energy was metabolized for the degradation of toxin. This finding was confirmed in the present work by comparing ECI and ECD, which were higher for larvae fed on diets incorporated with black pepper ethanol extract. Silva et al (2009) observed similar nutritional indices in Anagasta kuehniella Zeller larvae when they studied the insecticidal potential of Croton urucurana extracts by incorporating them in their artificial diet. The chloroform extract (LD50 = 342.62 mg/l), dichloromethane extract (LD50 = 357.78 mg/l), and acetone extract (LD50 = 372.57 mg/l) of P. nigrum incorporated into the artificial diet also interfered with nutritional indices resulting in 37.98, 26.17, and 14.82% reductions in ECI and 48.36, 39.27, and 22.72% reductions in ECD indices compared to control larvae, respectively. Similar growth inhibition patterns of ECI and ECD in R. ferrugineus Olivier larvae were observed upon exposure to conidial suspension (1 × 107 conidia/ml) of Beauveria bassiana (Hussain et al 2015). The decrease in ECI and ECD values compared to the control larvae reported before, and confirmed in the present work, revealed that most of the energy from ingested food is being used to perform physiological activities to combat the toxin, which means that less food is being utilized for larval growth.
The most potent compound, piperine, incorporated into the artificial diet of red palm weevil larvae tremendously increased the AD values compared to control treatment. The increase in AD values in piperine-fed larvae might reveal the fact that these larvae demand more energy for host defense. This energy shortfall could only be acquired through the use of intrinsic abilities to increase the AD of the limited foodstuff. In the past, similar enhanced AD response to fungal stress (Hussain et al 2015, 2016) and labramin (Martinez et al 2012) was obtained against R. ferrugineus Olivier and A. kuehniella, respectively.
Concerning the physiological impact, black pepper extract caused a tremendous increase in the expression of detoxification genes. This increase was even more pronounced in larvae fed diets incorporated with piperine. Enhanced expression of detoxification genes, including glutathione S-transferase, cytochrome P450, and esterase, is well documented as a defense mechanism against deleterious plant metabolites. The present laboratory experiments show that among the detoxification genes, cytochrome P450 was greatly expressed in the mid-gut as a detoxification defense. We have noted approximately 35-fold higher expression of cytochrome P450 from the eighth-instar red palm weevil larvae fed on diets incorporated with piperine. Similar enhanced activity (45-fold) of cytochrome P450 was reported in variegated cut-worm fed peppermint leaves (Yu et al 1979). Furthermore, each extract of black pepper induced different levels of cytochrome P450 expression. However, all of the extracts could only induce up to <12-fold expression in red palm weevil larvae. Our results are consistent with the findings of Rashid et al (2013), who demonstrated that sub-lethal concentrations of cantharidin in the artificial diet of Helicoverpa armigera Hübner enhanced cytochrome P450 gene expression.
In the present study, all of the black pepper extracts were not able to induce esterase expression of eighth-instar red palm weevil larvae. These results are consistent with those reported by Liu et al (2008), who reported that fraxinellone-treated food was not able to induce the activity of esterase in Ostrinia furnacalis Guenee. In contrast, the most potent piperine-fed artificial diet greatly enhanced the expression of esterase, resulting in approximately a 15-fold increase in expression that coincides with the findings of Khosravi et al (2011), who reported that the crude extract of Artemisia annua Linnaeus significantly enhanced esterase activity of Glyphodes pyloalis Walker larvae.
Glutathione S-transferase is a gene with several functions but is primarily involved in detoxification. Data presented in the current study demonstrate that different extracts of P. nigrum significantly enhanced GST expression, and this expression was more prominent in piperine-fed larvae of red palm weevils. The findings of Zibaee and Bandani (2010) also showed high GST levels in Eurygaster integriceps Puton treated with A. annua Linnaeus. However, high levels of energy consumption in Cydia pomonella Linnaeus and G. pyloalis Walker were observed during detoxification that lead to a reduction in growth (Boivin et al 2001, Khosravi et al 2011). Similarly, our results do not reveal that there was any inhibition in the expression of detoxification genes, suggesting that they were involved in the detoxification mechanism of R. ferrugineus Olivier larvae.
Conclusion
In summary, incorporation of piperine in R. ferrugineus Olivier larval diet significantly inhibited the feeding performance signifying its utility as eco-friendly bio-pesticide against red palm weevils. The alterations in the expression of genes provide important insights for the first time about the detoxification mechanism of red palm weevils. Overall, detailed toxicological, growth inhibitory, and physiological responses at the molecular level in the current study demonstrate the efficacy of using environmentally friendly commercial development of piperine to control red palm weevil infestations.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. doi:10.1093/jee/18.2.265a
Akhtar Y, Isman MB (2004) Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. J Appl Entomol 128:32–38. doi:10.1046/j.1439-0418.2003.00806.x
Al-Ayedh H, Hussain A, Rizwan-ul-Haq M, Al-Jabr AM (2016) Status of insecticide resistance in field-collected populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae). Int J Agric Biol 18:103–110. doi:10.17957/IJAB/15.0070
AlJabr AM, Hussain A, Rizwan-ul-Haq M, Al-Ayedh H (2017) Toxicity of plant secondary metabolites modulating detoxification genes expression for natural red palm weevil pesticide development. Molecules 22:169. doi:10.3390/molecules22010169
Boivin T, D’Hieres CC, Bouvier JC, Beslay D, Sauphanor B (2001) Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella. Entomol Exp Appl 99:381–386. doi:10.1046/j.1570-7458.2001.00838.x
Deletre E, Martin T, Campagne P, Bourguet D, Cadin A, Menut C, Bonafos R, Chandre F (2013) Repellent, irritant and toxic effects of 20 plant extracts on adults of the malaria vector Anopheles gambiae mosquito. PLoS One 8:e82103. doi:10.1371/journal.pone.0082103
El-Bokl MM, Baker RF, El-Gammal HL, Mahmoud MZ (2010) Biological and histopathological effects of some insecticidal agents against red palm weevil Rhynchophorus ferrugineus. Egypt Acad J Biol Sci Histol Histochem 1:7–22
Erdogan P, Yildirim A, Sever B (2012) Investigations on the effects of five different plant extracts on the two-spotted mite Tetranychus urticae Koch (Arachnida: Tetranychidae). Psyche A J Entomol 2012:1–5. doi:10.1155/2012/125284
Fan LS, Rita M, Dzolkhifli O, Mawardi R (2011) Insecticidal properties of Piper nigrum fruit extracts and essential oils against Spodoptera litura. Int J Agric Biol 13:517–522
Feng R, Isman M (1995) Selection for resistance to azadirachtin in the green peach aphid, Myzus persicae. Experientia 51:831–833. doi:10.1007/BF01922438
Hussain A, Tian MY, He YR, Ahmed S (2009) Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci 16:511–517. doi:10.1111/j.1744-7917.2009.01272.x
Hussain A, Rizwan-ul-haq M, Al-Jabr AM (2013a) Red palm weevil: understanding the fungal disease mechanism and host defense. In: Microbial pathogens and strategies for combating them: science. Technology and Education. Formatex Research Center, Badajoz, Spain, pp 1278–1286
Hussain A, Rizwan-ul-Haq M, Al-Jabr AM, Al-Ayied HY (2013b) Managing invasive populations of red palm weevil: a worldwide perspective. J Food, Agric Environ 11:456–463
Hussain A, Rizwan-ul-Haq M, Al-Ayedh H, Ahmed S, Al-Jabr AM (2015) Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 60:849–859. doi:10.1007/s10526-015-9682-3
Hussain A, Rizwan-ul-Haq M, Al-Ayedh H, AlJabr A (2016) Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int J Mol Sci 17:1518. doi:10.3390/ijms17091518
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608. doi:10.1016/S0261-2194(00)00079-X
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66. doi:10.1146/annurev.ento.51.110104.151146
Joseph B, Sowmya, Sujatha S (2012) Insight of botanical based biopesticides against economically important pest. Int J Pharm Life Sci 3:2138–2148
Khani M, Awang RM, Omar D, Rahmani M (2012) Bioactivity effect of Piper nigrum L. and Jatropha curcas L. extracts against Corcyra cephalonica [Stainton]. Agrotechnology 2:1–6. doi:10.4172/2168-9881.1000105
Khiyami M, Alyamani E (2008) Aerobic and facultative anaerobic bacteria from gut of red palm weevil (Rhynchophorus ferrugineus). African Jorunal Biotechnol 7:1432–1437
Khosravi R, Sendi JJ, Ghadamyari M, Yezdani E (2011) Effect of sweet wormwood Artemisia annua crude leaf extracts on some biological and physiological characteristics of the lesser mulberry pyralid, Glyphodes pyloalis. J Insect Sci. doi:10.1673/031.011.15601
Liu ZL, Ho SH, Goh SH (2008) Effect of fraxinellone on growth and digestive physiology of Asian corn borer, Ostrinia furnacalis Guenee. Pestic Biochem Physiol 91:122–127. doi:10.1016/j.pestbp.2008.03.003
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Maia M, Moore SJ (2011) Plant-based insect repellents: a review of their efficacy, development and testing. Malar J 10:S11. doi:10.1186/1475-2875-10-S1-S11
Martinez DST, Freire M das GM, Mazzafera P, Araujo-Júnior RT, Bueno RD, MLR M (2012) Insecticidal effect of labramin, a lectin-like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J Insect Sci 12:62. doi:10.1673/031.012.6201
Rashid M, Khan RA, Zhang YL (2013) Over-expression of cytochrome P450s in Helicoverpa armigera in response to bioinsecticide, cantharidin. Int J Agric Biol 15:993–997
Russell RM, Robertson JL, Savin NE (1977) POLO: a new computer program for Probit analysis. Bull Entomol Soc Am 23:209–213. doi:10.1093/besa/23.3.209
Salama H, Ismail I (2007) Potential of certain natural extracts for the control of the red palm weevil, Rhynchophorus ferrugineus (Oliver). Arch Phytopathol Plant Prot 40:233–236. doi:10.1080/03235400500383669
SAS Institute (2000) SAS user’s guide: statistics. SAS Institute, Cary
Scott IM, Jensen H, Scott JG, Isman MB, Arnason JT, Philogène BJ (2003) Botanical insecticides for controlling agricultural pests: Piperamides and the Colorado potato beetle Leptinotarsa decemlineata say (Coleoptera: Chrysomelidae). Arch Insect Biochem Physiol 54:212–225. doi:10.1002/arch.10118
Scott IM, Helson BV, Strunz GM, Finlay H, Sánchez-Vindas PE, Poveda L, Lyons DB, Philogène BJR, Arnason JT (2007a) Efficacy of Piper nigrum (Piperaceae) extract for control of insect defoliators of forest and ornamental trees. Can Entomol 139:513–522. doi:10.4039/n06-040
Scott IM, Jensen HR, Philogène BJR, Arnason JT (2007b) A review of piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem Rev 7:65–75. doi:10.1007/s11101-006-9058-5
Shukla P, Vidyasagar PSPV, Aldosari SA, Abdel-Azim M (2012) Antifeedant activity of three essential oils against the red palm weevil, Rhynchophorus ferrugineus. Bull Insectology 65:71–76
Silva LB, Silva W, Macedo MLR, Peres MTLP (2009) Effects of Croton urucurana extracts and crude resin on Anagasta kuehniella (Lepidoptera: Pyralidae). Brazilian Arch Biol Technol 52:653–664. doi:10.1590/S1516-89132009000300018
Statistix (2003) Statistix 8.1 Tallahassee, FL: Analytical Software.
Su HCF (1977) Insecticidal properties of black pepper to rice weevils and cowpea weevils. J Econ Entomol 70:18–21. doi:10.1093/jee/70.1.18
Tsao R, Peterson CJ, Coats JR (2002) Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol 2:5. doi:10.1186/1472-6785-2-5
Yu S, Berry R, Terriere L (1979) Host plant stimulation of detoxifying enzymes in a phytophagous insect. Pestic Biochem Physiol 12:280–284. doi:10.1016/0048-3575(79)90113-5
Zibaee A, Bandani AR (2010) A study on the toxicity of a medicinal plant, Artemisia annua L. (Asteracea) extracts to the Sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). J Plant Prot Res 50:79–85. doi:10.2478/v10045-010-0014-4
Acknowledgments
Deanship of King Faisal University (150083) of the Kingdom of Saudi Arabia provided the funds to carry out the whole experimentation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Moisés J Zotti - UFPel
Rights and permissions
About this article
Cite this article
Hussain, A., Rizwan-ul-Haq, M., Al-Ayedh, H. et al. Toxicity and Detoxification Mechanism of Black Pepper and Its Major Constituent in Controlling Rhynchophorus ferrugineus Olivier (Curculionidae: Coleoptera). Neotrop Entomol 46, 685–693 (2017). https://doi.org/10.1007/s13744-017-0501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0501-7