Abstract
Plant-based insecticides can play an important role in integrated insect pest management (IPM), especially in protecting stored grains. The aim of this study was to evaluate the bioactivity of derivatives (powder, ethanolic extract, and essential oil (EO)) from the leaves of Pimenta pseudocaryophyllus (Myrtaceae), a Brazilian native species, against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae), the main insect pest of stored corn. The powder and essential oil prepared from leaves showed a repellent effect. Moreover, the EO exhibited promising insecticidal activity through residual contact (LC50 = 1522 mg kg−1) and significantly decreased the F 1 progeny and the percentage of damaged grains. However, the essential oil obtained from P. pseudocaryophyllus leaves did not result in significant mortality of S. zeamais adults after 72 h of exposure by fumigation in concentrations up to 400 μL L−1 of air. Based on GC-MS analysis, 20 compounds were identified in the essential oil of P. pseudocaryophyllus leaves, being chavibetol (38.14%), methyl eugenol (11.35%), and terpinolene (9.17%) as the major constituents. Essential oil from P. pseudocaryophyllus leaves is an interesting source of compounds with grain-protectant properties and should be analyzed in future studies aiming to develop new bioinsecticides to use in the IPM of stored grains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The storage environment is characterized by a series of interactions among physical, chemical, and biological factors that may alter the quality of the stored products. One of the most important causes of qualitative and quantitative losses is the presence of insect pests and microorganisms (Neethirajan et al 2007). Many pest species are found in warehouses in tropical regions; for example, the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) is frequently found in all economically important stored cereals (Kehinde & Angela 2004). Infestations by this insect pest can substantially reduce grain weight and increase grain moisture, thus providing favorable conditions for the growth of decomposing fungi (Lazzari & Lazzari 2009) and leading to even higher levels of post-harvest loss.
Integrated pest management (IPM) in stored grains is currently challenged by the growing frequency of populations that are resistant to available insecticides. Thus, the development of new management tools and low-risk insecticidal compounds is necessary (Moreau & Isman 2012). Compounds produced by plant secondary metabolism are potential sources for developing low-risk insecticides, which differ from conventional synthetic insecticides in their low toxicity to mammals, rapid degradation, and local availability (Isman 2006, 2011, Regnault-Roger et al 2012).
The plant family Myrtaceae is one of the most important in the neotropics due to its high abundance (the highest within the order Myrtales) and species diversity (Paula et al 2010b). In addition, these plants secrete a large diversity of secondary metabolites, especially in the form of essential oils (EOs; mainly sesquiterpenes). Some of these metabolites exhibit important biological activities (Stefanello et al 2011), and the pharmacological and toxicological properties of various Myrtaceae species have received multidisciplinary interest. Recent studies have shown that many insect pest species are affected by essential oils from Myrtaceae plants. These essential oils can have several effects, including insecticidal action (knockdown effect), repellency, deterrence of feeding and oviposition, and growth inhibition (Ebadollahi 2013).
Pimenta pseudocaryophyllus is a native Brazilian species of the family Myrtaceae and is highly abundant in the Atlantic Forest and Cerrado biomes (Landrum & Kawasaki 1997). In addition to the known ethnopharmacological applications of various species of this genus (Paula et al 2008), previous studies have shown that compounds derived from P. pseudocaryophyllus have several biological effects, including potent antibacterial and antifungal activity (Lima et al 2006, Paula et al 2009, Custódio et al 2010). Furthermore, phytochemical studies revealed great chemical diversity in P. pseudocaryophyllus derivatives (Lima et al 2006, Paula et al 2008), indicating its potential as a source of a diversity of bioactive molecules, including those that could have insecticidal or insectistatic activity against insect pests such as insect pests of stored grains. Therefore, the primary objective of this study was to assess the bioactivity of derivatives (plant powder, ethanolic extract, and essential oil) obtained from P. pseudocaryophyllus leaves against the maize weevil. In addition, chemical analyses were performed to determine the composition of the active derivative(s).
Material and Methods
Collection of the plant material
Leaves of P. pseudocaryophyllus were collected on June 26, 2011 from specimens grown in the Caiçara de Pedrinhas neighborhood, Ilha Comprida municipality, state of São Paulo, Brazil (24°54ʹ09.2ʺS, 47°47ʹ10.8ʺW, 31 m asl). One voucher specimen, verified by Prof. Dr. Vicente Coffani Nunes (Universidade Estadual Paulista “Júlio de Mesquita Filho”, Registro Campus), was deposited in the ESA herbarium (http://splink.cria.org.br/manager/detail?resource=ESA&setlang=pt) of the Departamento de Ciências Biológicas, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo (ESALQ/USP), Piracicaba, SP, Brazil (record number 121119).
After collection, the leaves were separated from the remaining structures. Some of the leaves were used fresh to extract the essential oil, and the remaining leaves were dried to obtain the plant powder.
Preparation of the plant derivatives
Plant powder.
Leaves were dehydrated in a convection drying oven at 40°C for 48 h. Afterwards, the leaves were ground in a knife mill, and the resulting plant powder was stored in hermetically sealed glass receptacles and protected from light until use.
Ethanolic extract.
The crude extract was prepared by cold maceration in ethanol (99.5%) as a solvent, using a proportion of 1:5 plant powder/solvent (w/v). The resulting solutions were stirred for 10 min and left to rest for 72 h, after which the material was filtered through filter paper. The extraction process was then repeated with the material remaining on the filter using the same plant powder/solvent ratio. This procedure was repeated three times, resulting in a total of four filtrations. The solvent was removed from the remaining sample in a rotary evaporator at 50°C and −600 mmHg. The efficiency of the extraction process was determined after the complete evaporation of the solvent in an air-flow chamber.
Essential oil.
Fresh leaves were separated into 100-g samples, washed in running water, and cut into small pieces to optimize the extraction process by increasing the contact surface. The samples were then subjected to hydrodistillation in a Clevenger-type apparatus for 2 h at 110°C. The resulting mixture of water and oil (hydrolate) was decanted to separate the fractions and dried by adding anhydrous sodium sulfate (Na2SO4). The extraction yield was calculated from the fresh weight of the plant material (g of oil/g of fresh leaves). The essential oil was stored in a domestic freezer (∼−10°C) until use.
Chemical analysis
The components of the essential oil were qualitatively and quantitatively analyzed in a 17A gas chromatograph (Shimadzu Corporation, Kyoto, Japan) coupled to a mass spectrometer (GC-MS) QP5000. The following conditions were used: J&W Scientific DB-5MS (5% phenyl, 95% methylpolysiloxane) fused silica capillary column with 30-m length × 0.25-mm inner diameter × 0.25-μm film thickness; 1.2 mL min−1 flow rate of helium as the carrier gas (99.99%); 0.5 μL injection volume; 1:20 split ratio; 250°C injector temperature; and 280°C detector temperature. The oven temperature was programmed to 50°C for 1.5 min, followed by a rise of 4°C min−1 up to 200°C, then a rise of 10°C min−1 up to 250°C, and finally, a 5-min isotherm at 250°C. The mass spectrometer operated at 70 eV with rapid scanning at 0.5 scan s−1 for masses between 45 and 500 Da. The amount of each component was estimated from the normalized area (%) calculated using the areas of the GC-MS peaks sorted by elution order.
The volatile components were identified by comparing their mass spectra with spectra available in the literature (Adams 2007) and in the equipment’s database (WILEY8, NIST05, NIST21, and NIST107) and by comparing the retention indices with those found in the literature. The retention indices (RI) were calculated from a homologous series of n-alkanes (C9H20–C20H42) injected under the same chromatographic conditions as the samples, using the equation proposed by Vand Den Dool & Kratz (1963).
Chavibetol was identified by mass spectrometry, retention index and confirmed by nuclear magnetic resonance (NMR1H and 13C) of the essential oil in comparison to data published by Santos et al (2009). The NMR spectra (400 MHz for the frequency of 1H and 100 MHz for 13C) were acquired on a Bruker Avance spectrometer III 9.4 T using tubing Norell 5 mm ID, using deuterated chloroform (CDCl3) as solvent.
Effect of the derivatives on the adult survival, F1 progeny, and damage caused by S. zeamais
All bioassays were carried out under controlled laboratory conditions (25 ± 2°C; 60 ± 10% RH; 14 h photophase) under a completely randomized experimental design.
The insecticidal activity of the plant derivatives (plant powder, ethanolic extract, and essential oil) was assessed using corn samples (10 g) placed in Petri dishes (6.1 cm in diameter and 2.1 cm in height) and separately treated with each derivative. The dishes were infested with 20 unsexed, 10–20-day-old adult weevils. The weevils originated from a population kept in the laboratory for approximately 20 generations with wheat grains used as breeding substrate. Adult survival was assessed on the 10th day of infestation. Individuals whose extremities were fully extended and that did not react to contact with a brush after 1 min of observation were considered dead. Ten replicates were used for each treatment (n = 200).
The same sampling units used in the previous test were used to assess the effects of the plant derivatives on the F 1 progeny and on the damage sustained by the treated samples. For this purpose, the grains were treated with the respective derivatives and infested as before. After 10 days of infestation, the adults were removed and the sampling units were kept under the controlled conditions. The number of emerged adults was counted in each Petri dish 60 days after the initial infestation. The damage caused by S. zeamais feeding was also estimated at that time by counting the number of damaged or perforated grains in each sample based on a visual inspection of each grain. The grain weight loss (%) was then estimated using the equation proposed by Adams & Schulten (1976):
where Wl = weight loss (%), Ndg = number of damaged grains, tNg = total number of grains, and C = 0.125 if the corn is stored as loose grain or in cobs without husks and 0.222 if the corn is stored in cobs with husks.
The concentrations of each derivative in grams per kilogram (g of plant powder per kg of corn) were selected based on previous studies, as specified below for each derivative.
Plant powder.
To assess the bioactivity of the plant powder, corn samples (10 g) were treated with plant powder in concentrations of 10, 20, 40, and 80 g kg−1. The control treatment did not receive any plant powder.
Ethanolic extract.
To assess the bioactivity of the ethanolic extract, corn samples (10 g) were sprayed with the extract at concentrations of 0.25, 0.5, 1, and 2 g kg−1 using a microatomizer coupled to a pneumatic pump, which was adjusted to supply a pressure of 0.5 kgf cm−2 through a spray volume of 30 L t−1. These conditions were selected based on previous studies (Ribeiro et al 2013, 2014). The control samples were treated only with the solvent used to resuspend the extract [acetone/methanol (1:1, v/v)].
Essential oil.
To evaluate the insecticidal activity of the essential oil, corn samples (10 g) were treated with different concentrations of the essential oil (0.25, 0.5, 1, and 2 g kg−1) solubilized in acetone using the same equipment and conditions earlier described. Similarly, the control samples were treated only with the solvent used to dissolve the essential oil (acetone).
Effects of the derivatives on the behavior of S. zeamais adults
The effects of the derivatives on the attractiveness of the corn to S. zeamais adults were assessed in two arenas composed of five Petri dishes (6.1 cm in diameter and 2.1 cm in height) mounted on a plastic base (30 × 30 cm). In each arena, one of the dishes was fixed to the center of the base and connected to the other dishes by plastic tubes of equal length.
Corn samples (10 g each) were treated with the respective derivatives in the same concentrations and using the same application procedures described above. The samples were placed in two Petri dishes located symmetrically on opposite sides of the arena, and grains treated only with the solvents used to solubilize the derivatives were placed in the other two Petri dishes (control). Afterwards, 50 unsexed, 10–20-day-old adults of S. zeamais were released in the central container. The number of insects found in each Petri dish was counted after 24 h. Each treatment level consisted of 10 replicates (n = 500).
The different treatments were compared using a repellency index adapted from Lin et al (1990):
where RI = repellency index, G = percent of insects on the grain treated with the derivative being tested, and P = percent of insects on the control grains. The RI and standard deviation were used to determine the classification interval (ClassI) for the treatment means using the following formula:
where t = tabulated t value ( n-1; α:0.05), SD = standard deviation, and n = number of replicates. The derivative were considered neutral when the RI interval was within the ClassI being evaluated, repellent when the RI was below the lowest value obtained for the ClassI, and attractive when the RI was above the highest calculated ClassI value. In turn, the mean percentage repellency of each treatment was calculated from the following equation (Obeng-Ofori 1995):
where PR = mean percentage repellency; NC = total number of insects on the control grain, and NT = total number of insects on the grains treated with the derivative being tested.
Fumigant insecticidal activity of the essential oil
The fumigant insecticidal activity of the P. pseudocaryophyllus essential oil was assessed in 250-mL plastic containers used as fumigation chambers. Each chamber received 20 g of corn grains and was then infested with 30 adult weevils of undetermined sex and between 10 and 20 days of age. The essential oil was administered on rectangular patches of filter paper (5 × 2 cm), which were fixed in place on the lower part of each container’s lid and isolated by a thin fabric (voile) to avoid direct contact of the weevils with the oil. Doses of 50, 100, 200, and 400 μL L−1 of air were tested. The control treatment contained no essential oil. The number of dead insects in each chamber was counted after 72 h of exposure. Six replicates of each concentration were used, with 30 weevils per replicate (n = 180).
Response-concentration curves of the active derivatives
The results of the bioassays carried out with each derivative in the different methods of exposure were used to estimate the LC50 and LC90 (the concentrations required to kill 50% and 90% of the weevil population, respectively) for the active treatment. For this purpose, preliminary tests were performed with each derivative to determine the base concentrations that produced mortality rates of approximately 95% of the weevils and the concentrations that produced mortality rates similar to that observed in the control, according to the method described by Finney (1971). These tests were used to select the concentrations to be studied [six concentrations (range = 0–4000 mg kg−1)]. Subsequently, the same bioassay procedures and experimental conditions earlier described were used.
Data analysis
Generalized linear models (Nelder & Wedderburn 1972) of the quasi-binomial type were used to analyze the proportions of mortality and damaged grains, whereas the quasi-Poisson model was used to analyze the number of emerged insects. The goodness-of-fit was assessed with a half-normal probability plot with a simulation envelope (Hinde & Demétrio 1998). When there were significant differences between treatments, multiple comparisons (Tukey’s test, p < 0.05) were carried out using the glht function in the multicomp package with adjusted p values for the treatments with qualitative levels, whereas nonlinear regressions were used for the treatments with quantitative levels. The possible relationships between the study variables were assessed using Spearman’s non-parametric correlation analysis (p < 0.05). All analyses were performed in the statistical software “R” version 2.15.1 (R Development Core Team 2012).
The lethal concentrations (LC50 and LC90) were estimated using a binomial model with a complementary log-log link function (gompit model) via the PROBIT procedure in SAS software version 9.2 (SAS Institute 2011).
Results
None of the concentrations of the plant powder or ethanolic extract from P. pseudocaryophyllus leaves had a significant effect (p > 0.05) on adult survival, F 1 progeny, or damage by S. zeamais (Table 1). However, the essential oil extracted from P. pseudocaryophyllus leaves caused significant adult mortality through residual contact in a concentration-dependent manner (Table 1). In addition to acute toxicity [LC50 = 1.52 g kg−1 (95% CI = 1.41–1.61), χ2 = 5.68, df = 4, n = 1.200], the essential oil significantly reduced the F1 progeny and therefore the damage caused by S. zeamais in the treated samples (Table 1). Adult mortality was negatively correlated with the F 1 progeny (r s = −0.8438; p < 0.0001) and with the percentage of damaged grains (r s = −0.9052; p < 0.0001), possibly indicating that acute toxicity is the main effect of the essential oil on S. zeamais; this effect was reflected in the other variables studied. In all cases, a second-degree polynomial model was fitted to the data to describe the effect of concentration on the studied variables (Fig 1). However, the essential oil obtained from P. pseudocaryophyllus leaves did not result in significant mortality of S. zeamais adults after 72 h of exposure by fumigation in concentrations up to 400 μL L−1 of air (Table 2).
Bioactivity of different concentrations of the essential oil extracted from Pimenta pseudocaryophyllus leaves against Sitophilus zeamais. a Adult mortality by day 10. b Number of emerged insects (F 1 progeny). and c Damage sustained by the corn samples after 60 days of infestation, estimated as the proportion of damaged grains.
The powder and essential oil extracted from P. pseudocaryophyllus leaves had a repellent effect on S. zeamais adults (Table 3), with larger concentrations of each derivative resulting in greater repellency.
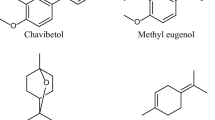
The extraction yield of the essential oil using the hydrodistillation was 0.81% (g of oil/g of fresh leaves). The GC-MS identified 20 constituents in the essential oil obtained from P. pseudocaryophyllus leaves. The major compounds were the phenylpropanoids chavibetol (38.14%) and methyl eugenol (11.35%) and the terpenoid terpinolene (9.17%) (Table 4, Fig 2). However, two other components of essential oil (0.89% of their relative composition) were not identified by means of this technique.
Discussion
This study reports for the first time the biological activity of P. pseudocaryophyllus against an insect pest species of stored grains. Our results show that the activity of derivatives from this species against S. zeamais may vary with the extraction method (and the consequent modifications of the chemical profile of the constituents found in the derivative), the type of exposure (contamination) of the target insect to the product, and the concentration used.
The variables analyzed in our bioassays enabled us to assess the potential activity of the essential oil extracted from P. pseudocaryophyllus leaves as a grain protector. The phenylpropanoid chavibetol, which is the major constituent of the oil (38.14% of its total composition) is reported as the major component of the essential oil from P. pseudocaryophyllus leaves (Santos et al 2009, Marques et al 2010, Barata et al 2011). However, edaphoclimatic and seasonal differences among the regions where the specimens used in the different studies were cultivated may explain the differences in the proportions of the major constituents and in the presence or absence of minor constituents (Paula et al 2010a, Barata et al 2011). Still, the occurrence of intraspecific chemical variation (chemotypes) has been reported in this Myrtaceae species (Paula et al 2011) and should be considered and analyzed in future studies aiming to develop new bioinsecticides from this species.
In general, terpenes are neurotoxic to insects by affecting the acetylcholinesterase activity or the octopamine receptors (Isman 2000). Based on a Periplaneta americana Linnaeus cell culture and Drosophila melanogaster Meigen brains, Enan (2001) showed that eugenol [C10H12O2 (isomer of chavibetol)] acts on insects by mimicking the activity of octopamine and increasing the levels of intracellular calcium. Terpenic compounds may reach their target sites by entering the organism (insect) through its airways, thus rapidly interfering with the insect physiology (Rajendran & Sriranjini 2008), although we did not observe it in our fumigation bioassay. Although some of the compounds found in this essential oil have been shown to have fumigant insecticidal activity against various pest species of stored grains (Mondal & Khalequzzaman 2010, Coitinho et al 2011, Liu et al 2013), the low concentrations of the active constituents in the crude essential oil or possible changes in the physical-chemical properties (especially the volatility) of the fumigant insecticidal compounds when used in blends may explain these results. Similarly, Cox et al (2001) demonstrated that the non-oxygenated terpenes found in the essential oil of Melaleuca alternifolia (Myrtaceae) reduce the antimicrobial activity of terpinen-4-ol (the main active component of this oil) by reducing its aqueous solubility, possibly through an increase in the carbon-chain length of the total composition.
In addition to the insecticidal and repellent activity of the essential oil, the P. pseudocaryophyllus leaf powder also had a significant repellent effect on adult S. zeamais. This effect was most likely due to the presence of more polar and less volatile terpenic compounds in the plant powder, which were not lost when the leaves were dried due to the exposure to a slow drying in a low-temperature process. Although the action of chavibetol has not been studied yet, the effects of other components of the essential oil of P. pseudocaryophyllus on the behavior of various pest species of stored grains have been previously reported (Obeng-Ofori & Reichmuth 1997, Ogendo et al 2008, Zapata & Smagghe 2010, Ukeh & Urnoetok 2011, Suthisut et al 2011). In these studies, the compounds were tested in isolation or as major constituents of the essential oils of different aromatic species, and the attractiveness level varied with the concentrations and proportions of the oil constituents. In light of these findings, layers of P. pseudocaryophyllus leaves might be used for grain protection using the technique of grain enveloping, especially in the storage units of smallholders in the Vale do Ribeira (southern of the state São Paulo, Brazil), where P. pseudocaryophyllus is abundant. This grain protection technique is already used with inert powders and involves placing layers of plant material below and above the grain mass, thus preventing insect pests from entering the grain through the effect of the plant material on host-plant selection behavior (repellency).
Based on these results, we conclude that P. pseudocaryophyllus is an interesting source of active compounds with insecticide activity against stored-product pest. However, more studies are required for the development of a botanical insecticide based on P. pseudocaryophyllus essential oil, especially devoted to the isolation and identification of the main active compound(s) and to the understanding of the role of each constituent in their overall biological activity. Moreover, complementary studies are also necessary to determine the activity-structure relationships (configurations and functional groups) of these terpenic compounds and to modulate their biological properties to facilitate the development of more efficient and inexpensive artificial blends to use in the IPM of stored grains.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectroscopy, 4th edn. Allured, Carol Stream, p 804
Adams JM, Schulten GGM (1976) Losses caused by insects, mites and microorganisms. In: Harris KL, Lindblad CJ (eds) Post harvest grain loss assessment methods. Slough, England, pp 83–93
Barata LES, Santos BCB, Marques FA, Baroni ACM, Oliveira PR, Einloft P, Ribeiro JCL, Guerrero PG Jr (2011) Seasonal variation of the volatile constituents from leaves of Pimenta pseudocaryophyllus (Gomes). J Essent Oil Res 23:54–57
Coitinho RLBC, Oliveira JV, Gondim Junior MGC, Câmara CAG (2011) Toxicidade por fumigação, contato e ingestão de óleos essenciais para Sitophilus zeamais Motschulsky, 1885 (Coleoptera: Curculionidae). Ciênc Agrotecnol 35:172–178
Cox SD, Mann CM, Markham JL (2001) Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol 91:492–497
Custódio DL, Burgo RP, Moriel B, Barbosa AM, Rezende MI, Daniel JFS, Pinto JP, Bianchini E, Faria TJ (2010) Antimicrobial activity of essential oils from Pimenta pseudocaryophyllus and Tynanthus micranthus. Braz Arch Biol Technol 53:1363–1369
Ebadollahi E (2013) Essential oils isolated from Myrtaceae family as natural insecticides. Ann Rev Res Biol 3:148–175
Enan E (2001) Insecticidal activity of essential oils: octopaminergic site of action. Comp Biochem Physiol 130:325–327
Finney DJ (1971) Probit analysis. Cambridge University Press, Cambridge, p 31
Hinde J, Demétrio CGB (1998) Overdispersion: models and estimation. Comput Stat Data Anal 27:151–170
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Isman MB (2011) Botanical insecticides: for richer, for poorer. Pest Manag Sci 64:8–11
Kehinde K, Angela OJ (2004) Comparative biological activity of Synzygium aromaticum (L.) and Xylopia ethiopica on Rhizopertha dominica F. (Coleoptera: Bostrychidae) and Sitophilus zeamais Motsch. (Coleoptera: Cuculionidae) in maize grains. J Asia Pac Entomol 7:339–342
Landrum LR, Kawasaki ML (1997) The genera of Myrtaceae in Brazil: an illustrated synoptic treatment and identification keys. Brittonia 49:36–58
Lazzari SMN, Lazzari FA (2009) Insetos-praga de grãos armazenados. In: Panizzi AR, Parra JRP (eds) Bioecologia e nutrição de insetos. Embrapa Informação Tecnológica, Brasília, pp 667–732
Lima MEL, Cordeiro I, Young MCM, Sobra MEG, Moreno PRH (2006) Antimicrobial activity of the essential oil from two specimens of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae) native from São Paulo State Brazil. Pharmacol Online 3:589–593
Lin H, Kogan M, Fischer D (1990) Induced resistance in soybean to the Mexican bean beetle (Coleoptera: Coccinellidae): comparisons of inducing factors. Environ Entomol 19:1852–1857
Liu ZL, Zhou LG, Liu ZL, Du SS (2013) Identification of insecticidal constituents of the essential oil of Acorus calamus rhyzomes against Liposcelis bostrychophila Badonnel. Molecules 18:5684–5696
Marques FA, Wendler EP, Baroni ACM, Oliveira PR, Sasaki BS, Guerrero PG Jr (2010) Leaf essential oil composition of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum native from Brazil. J Essent Oil Res 22:150–152
Mondal M, Khalequzzaman M (2010) Toxicity of naturally occurring compounds of plant essential oil against Tribolium castaneum (Herbst). J Biol Sci 10:10–17
Moreau TL, Isman MB (2012) Combining reduced-risk products, trap crops and yellow sticky traps for greenhouse whitefly (Trialeurodes vaporariorum) management on sweet peppers (Capsicum annum). Crop Prot 34:42–46
Neethirajan S, Karunakaran C, Jayas DS, White NDG (2007) Detection techniques for stored-product insects in grain. J Food Control 18:157–162
Nelder JA, Wedderburn RWM (1972) Generalized linear models. J R Stat Soc 135:370–384
Obeng-Ofori D (1995) Plant oils as grain protectants against infestations of Cryptolestes pusillus and Rhyzopertha dominica in stored grain. Entomol Exp Appl 77:133–139
Obeng-Ofori D, Reichmuth C (1997) Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild) against four species of stored-product Coleoptera. Int J Pest Manag 43:89–94
Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, Omolo EO, Kariukim ST, Shaava E (2008) Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res 44:328–334
Paula JAM, Paula JR, Bara MTF, Rezende MH, Ferreira HD (2008) Estudo farmacológico das folhas de Pimenta pseudocaryophyllus (Gomes) L.R. Landrum – Myrtaceae. Rev Bras Farmacogn 18:265–278
Paula JAM, Paula JR, Pimenta FC, Rezende MH, Bara MTF (2009) Antimicrobial activity of the crude ethanol extract from Pimenta pseudocaryophyllus. Pharm Biol 47:987–993
Paula JAM, Paula JR, Bara MTF, Ferri PH, Santos SC, Silva LHS (2010a) Chemical differences in the essential oil of Pimenta pseudocaryophyllus (Gomes) L. R. Landrum leaves from Brazil. J Essent Oil Res 22:555–557
Paula JAM, Reis JB, Ferreira LHM, Menezes ACS, Paula JR (2010b) Gênero Pimenta: aspectos botânicos, composição química e potencial farmacológico. Rev Bras Plant Med 12:363–379
Paula JAM, Ferri PH, Bara MTF, Tresvenzol LMF, Sá FAZ, Paula JR (2011) Infraespecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae). Biochem Syst Ecol 39:643–650
R Development Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajendran S, Sriranjini (2008) Plant products as fumigants for stored-product insect control. J Stored Prod Res 44:126–135
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Ribeiro LP, Vendramim JD, Bicalho KU, Andrade MS, Fernandes JB, Moral RA, Demétrio CGB (2013) Annona mucosa Jacq. (Annonaceae): a promising source of bioactive compounds against Sitophilus zeamais Mots. (Coleoptera: Curculionidae). J Stored Prod Res 55:6–14
Ribeiro LP, Vendramim JD, Andrade MS, Bicalho KU, Silva MFGF, Vieira PC, Fernandes JB (2014) Tropical plant extracts as sources of grain-protectant compounds against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Neotrop Entomol 43:470–482
Santos BC, Silva JC, Guerrero PG Jr, Leitão GG, Barata LE (2009) Isolation of chavibetol from essential oil of Pimenta pseudocaryophyllus leaf by high-speed conter-current chromatography. J Chromatogr A 1216:4303–4306
SAS Institute (2011) Statistical analysis system: getting started with the SAS learning. Version 9.2. SAS Institute, NC
Stefanello MEA, Pascoal ACRF, Salvador MJ (2011) Essential oils from Neotropical Myrtaceae: chemical diversity and biological properties. Chem Biodivers 8:73–94
Suthisut D, Fields PG, Chandrapatva A (2011) Contact toxicity, feeding reduction, and repellency of essential oils from three plants from the Ginger family (Zingiberaceae) and their major components against Sitophilus zeamais and Tribolium castaneum. J Econ Entomol 104:1445–1454
Ukeh DA, Urnoetok SBA (2011) Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbest) and Rhyzopertha dominica (F.) in Calabar, Nigeria. Crop Prot 30:1351–1355
Vand Den Dool H, Kratz DJA (1963) Generalization of the retention index system including liner temperature programmed gas–liquid partition chromatography. J Chromatogr 11:463–467
Zapata N, Smagghe G (2010) Repellency and toxicity of essential oils from the leaves and bark of Laurelia sempervirens and Drimys winteri against Tribolium castaneum. Ind Crop Prod 32:405–410
Acknowledgments
The authors thank Odimar Zanuzo Zanardi, Gabriel Padoan Gonçalves, Marcos Conceschi, and Gláucia Pavarini for technical assistance. The authors also thank the São Paulo Research Foundation (FAPESP, grant 2010/52638-0) and the National Institute of Science and Technology for the Biorational Control of Insect Pests (INCT-CBIP, grant 573742/2008-1) for their financial support. This manuscript was translated and edited by American Journal Experts (Durham, NC, USA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Raul N Guedes – UFV
Rights and permissions
About this article
Cite this article
Ribeiro, L.P., Ansante, T.F., Niculau, E.S. et al. Pimenta pseudocaryophyllus Derivatives: Extraction Methods and Bioactivity Against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Neotrop Entomol 44, 634–642 (2015). https://doi.org/10.1007/s13744-015-0321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-015-0321-6