Abstract
This present study aims to evaluate the insecticidal activity of a bioactive molecule, menthol, against a pest species, Rhyzopertha dominica (F. 1792) (Coleoptera: Bostrichidae). Effects were examined on mortality, enzymes of intermediary metabolism (ALP, GOT and GPT), nutritional reserves, digestive enzymes, and oxidative stress biomarker. Toxicological tests revealed the insecticide activity of this treatment with a dose–response relationship. In addition, ingestion (LC50 at 24 h: 0.69 µl/ml) is the most effective mode of application compared to fumigation (LC50 at 24 h: 302 µl/L air). The obtained results revealed an increase in the percent repellency as a function of concentrations. Moreover, the biochemical study shows that the treatment decreases the protein content and the energy reserves. In addition, it disrupts the activity of enzymes of intermediary metabolism (ALP, GOT and GPT) in R. dominica adult. Finally, menthol also disrupts the digestive enzymes activity in treated adults compared to controls. Indeed, the treatment reduces the specific activity of α-amylase, protease, chitinase, and lipase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical pesticides and fumigants (methyl bromide, phosphine) are crucial tools for controlling stored grain insect pests. However, these compounds led to possible risks to none-target organisms and had contributed to pest resistance development to pesticides (Venkatesan et al. 2016). These issues have prompted scientists to search for new classes of safer pest control agents that would be inexpensive, biodegradable, non-toxic to humans, and environmentally friendly.
Some studies have shown insecticidal and physiological effects of essential oils of aromatic plants and their constituents (Oftadeh et al. 2020; Dutra et al. 2020; Gong and Ren 2020). Insecticidal activity, antifeedant, repellency, and insect growth regulation are just a few of the many properties that pesticides of a botanical origin might have (Guettal et al. 2021a, b; Sayada et al. 2021a). Before using these substances to combat pests, it is crucial to have a thorough understanding of plant essential oils (EOs), or their active metabolites, and how they interact with the physiology of insect pests (Shahriari et al. 2019). The majority of the reports are based on the fumigant activity of essential oils rather than their constituents. Several studies have confirmed that these activities are due to their compounds (menthol, menthone, carvone, limonene, β-ocimene, and dihydrotagetone), which act on the nervous system of the insect by disrupting the functions GABAergic (Tong and Coats 2012) and aminergic (Enan 2005) systems and by inhibiting acetylcholinesterase (Abdelgaleil et al. 2009).
Plant essential oils are typically composed of complex mixtures of mono-and sesquiterpenoids, such as 1,8-cineole, eugenol, and menthol (El-Saadony et al. 2022). Menthol and its derivatives were reported to exhibit insecticidal (Samarasekera et al. 2008) and antifeedant activity (Rajkumar et al. 2019) against flies, beetles, mosquitoes, and mites and affect also their behavior (Abed-Vieillard et al. 2014; Himmel et al. 2019). The insect repellent activity of menthol and its derivatives against stored product pests has been reported (Aggarwal et al. 2001; Shimomura et al. 2020).
So, we have tested the toxicity, the repellent activity, and the biochemical effects of menthol with an emphasis on digestive enzymes such as α-amylase, lipase, and general protease of Rhyzopertha dominica (F. 1792) (Coleoptera: Bostrichidae).
Additionally, the effects of this bioactive molecule on the amounts of protein, carbohydrate, and lipid in the treated adult were also taken into consideration under laboratory conditions. Finally, enzymes of intermediary metabolism have been analyzed.
Materials and methods
Insects rearing
The mass rearing of R. dominica was carried out in the Laboratory of Water and Environment in Larbi Tebessi University (Tebessa, North east Algeria). The insect rearing carried out in cubic containers with 1 kg wheat was maintained at temperature of 27 ± 1 °C and relative humidity of 65 ± 5%. All experiments used adult insects aged 7 to 14 days.
Menthol
( ±)- Menthol (> 98%) were purchased from Sigma-Aldrich.
Fumigant bioassay
After preliminary screening, menthol dissolved in acetone (3% w/v) was applied at different concentrations: 83.33, 166.67, 333.33, and 666.67 μl/L air to disks of Whatman filter paper (2.5 cm in diameter), attached to the underside of plastic vial caps. This test is carried out in plastic vials (60 mL) ; in each of them 10 adults (both sexes) were released. Insects used as controls were maintained in the same conditions without treatment. Each concentration was replicated three times. The number of dead and alive insects was counted after 24, 48, and 72 h from the start of exposure.
After correction of observed mortality (Abbott 1925), lethal concentrations (LC25, LC50 and LC90) with their 95% confidence interval (CI) were determined.
Contact toxicity
After preliminary screening, 0.5 ml aliquot of the appropriate dilutions of menthol (0.5; 1; 2 and 4 mg/ml in acetone) was applied to 5 g of wheat grains in plastic bottles. The grains were shaken thoroughly to ensure uniform distribution product in grains. After complete evaporation of the solvent for 15 min, 10 adults of R. dominica without sexing are introduced into the bottle. The bioassay was carried out in four repetitions for each concentration. A control series is conducted in parallel, and the wheat grains receive only acetone solvent (0.5 ml). Insects used as controls were maintained in the same conditions with acetone. The mortalities recorded at 6, 12 and 24 h after treatment were corrected according to Abbott's (1925) correction formula in order to eliminate natural mortalities:
where Mt = mortality with treatment
Mc = mortality with control
A nonlinear regression was used to determine the lethal concentrations (LC25, LC50, and LC90) and their fiducial limits (95% FL).
Repellent activity
The Talukder and Howse (1994) method was used to examine menthol's repellency. Filter paper circles of 9 cm in diameter were cut into two halves. On one half of each of the filter-papers, 0.5 ml of each of the four menthol solutions (5, 10, and 20 l/ml) was uniformly applied. On the second halves that acted as the control, acetone was applied. The treated and untreated half-circles are dried until the total evaporation of the solvent; then, they were joined by adhesive tape and placed in the Petri dish. In the center of the dish, ten adults were liberated. Each treatment was replicated five times, and the percentages of insects present on treated (G) and control (P) areas were recorded after 15 min, 30 min, 1 h, 2 h, and 3 h. The repulsion percentage (RP) was calculated using the following formula:
where Nc is the percentage of beetles present in the control half.
The average values were calculated and assigned as ranked by Mc Donald et al. (1970) by a repulsive different class varying from 0 to V [Class 0 (RP < 0.1%), class I (RP = 0.1% −20.0%), class II (RP = 20.1–40.0%), class III (RP = 40.1–60.0%), class IV (RP = 60.1–80.0%), and class V (RP = 80.1–100.0%)].
Determination of biochemical profile
In order to investigate the sublethal effects of menthol on the biochemistry and physiology, insects were treated with LC25 and LC50. These two concentrations permit us to obtain a sufficient number of survivors following treatment to perform different experiments compared to LC90 (Kissoum et al. 2020). Samples were collected from control and treated series and subjected to extraction. The method described by Shibko et al. (1966) was used to extract the biochemical components (proteins, carbohydrates, and lipids). The whole body of the control and treated insects (3 replicates each containing ten individual) was weighed and then placed in 1 ml of 20% trichloroacetic acid. Using bovine serum albumin as a standard and Coomassie Brilliant Blue as the reagent, proteins were quantified following the Bradford (1976) technique. Using anthrone as the reagent and glucose as the standard, carbohydrates were determined using the Duchateau and Florkin (1959) method. The method of Goldsworthy et al. (1972) was used to measure lipids, with vanillin serving as a reagent and sunflower oil as the standard.
Digestive enzyme assay
Control and treated (LC25 and LC50) adults were sampled for digestive enzymes analysis. Dinitrosalicylic acid (DNS) was used as the reagent and 1% soluble starch as the substrate to measure the activity of α-amylase (Bernfeld 1955). According to Garcia-Carreno and Haard's (1993) method, protease activity was measured using casein (1%) as a substrate. The lipase assay was performed according to the method of Tsujita et al. (1989).
Enzymes of intermediary metabolism
A BIOSCAN commercial kit was used to assess the activity of alanine and aspartate aminotransferase. At 340 nm, the absorbance was measured. The alkaline phosphatase was quantified by BIOLABO commercial kit. At 405 nm, the absorbance was measured.
Statistical analysis
The mean ± SEM are used to present the data. Numbers of individuals and repetitions were also mentioned. Data were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post hoc test to compare treatment means using GraphPad Prism v.7.00 for Windows (GraphPad Software, Inc., http://www.graphPad.com).
Results
Insecticidal activity
Figure 1 shows the percent mortality in R. dominica treated with different concentrations of menthol by fumigation. These mortalities increase significantly according to the applied doses and the time after treatment in R. dominica treated by fumigation at 24 h (F3,8 = 85.78; p < 0.0001), 48 h (F3,8 = 96.53; p < 0.0001), and 72 h (F3,8 = 136; p < 0.0001). On the lethal times, the concentration of 0.5 μl/ml of menthol eliminated 50% of the population of R. dominica in 42.97 h and 90% during 251.90 h of treatment. When 4 μl/ml of menthol is applied, LT50% was 2.26 h, while the LT90% was 6.00 h (Table 2).
Otherwise, application of menthol by contact revealed an increase in mortality as a function of the applied doses and the time after treatment at 6 h (F3,12 = 109.3; p < 0.0001), 12 h (F3,12 = 136.2; p < 0.0001), and 24 h (F3,12 = 75.27; p < 0.0001) (Fig. 2). The results show that menthol applied by contact exerts an insecticidal activity with a dose–response relationship against R. dominica. We calculated LC25 and LC50 values of menthol along with their fudicial limits (Table 1). In addition, it is noted that menthol applied by contact is more toxic compared to fumigation.
On the lethal times, the concentration of 83.33 μl/L of menthol eliminated 50% of the population of R. dominica in the 169.10 h and 90% during 793.40 h of treatment. When 666.67 μl/L of menthol is applied, LT50% is 4.78 h, while the LT90% is 18.52 h (Table 2).
Repellent activity
The percentage of repellency shows an increase according to the applied concentrations. The high repulsion rates (93.33%) are observed at 30 min with the highest concentration (20 µl/ml). These percentages increase at 30 min after treatment and decrease thereafter with exposure time. In addition, we note that this treatment is classified in category V of repulsion (Table 3).
Effects on digestive enzyme activities
α-amylase activity decreases significantly (F5,12 = 18,49: p < 0,0001) in the treated adults with menthol without dose effect (LC25 Vs LC50: p > 0.05). The results of general protease activity showed a significant reduction in the treated series (LC25 and LC50) (F5.12 = 39.75: p < 0.0001) compared to the control with a dose effect at 48 h and 72 h after treatment (LC25 vs. LC50: p < 0.0001). Finally, the results of lipase activity revealed a significant decrease (F5,12 = 27.79: p < 0,0001) in the treated series with LC25 and LC50 as compared to control (Table 4).
Effect in enzymes of intermediary metabolism
Treatment of adults with two doses of menthol significantly increased the activity of alanine and aspartate aminotransferases at 12 and 24 h after treatment (ALT: F5,12 = 8.88: p = 0.0010 and AST: F5,12 = 44.17: p < 0.0001). However, the activity of alkaline phosphatases was increased significantly in all treatments compared to the control (F5,12 = 40.53: p < 0.0001) (Table 5).
Effect in nutritional reserves
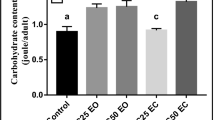
A significant reduction in the total protein content is noted after treatment with the two concentrations applied (F5.12 = 106.1; p < 0.0001). A dose effect was observed at 24 h (LC25 vs. LC50: p < 0.0001) and 48 h (LC25 vs. LC50: p = 0.0132) (Fig. 3A). The results mentioned in Fig. 3B showed a significant decrease in lipid content in the treated series at LC25 and LC50 (F5.12 = 537.6: p < 0.0001) with a dose effect at 24 h (LC25 vs LC50: p = 0.0132) and 48 h (LC25 vs. LC50: p < 0.0001). The carbohydrate content revealed a significant decrease in the treated series at LC25 and LC50 (F5,12 = 85.77: p < 0.0001) compared to the controls, with a dose effect at 48 h (LC25 vs LC50: p < 0.0001) (Fig. 3C).
Discussion
Insecticidal activity
In our study, menthol applied on R. dominica was evaluated. Mortality increases with the concentration and the exposure time. The fumigant activity of menthol and 1,8-cineole, on different stored product pests, was demonstrated (Erler 2005). They achieved less than 99% mortality against many insect species tested. Menthol was most effective as fumigant against Tribolium castaneum and Callosobruchus maculatus (Tripathi et al. 2001). The fumigant activity of six main monoterpenoids of EOs from aromatic plants grown in Turkey such as 1,8-cineole, γ-terpinene, carvacrol, menthol, thymol, and terpinene-4-ol was tested against Tribolium confusum adults and Ephestia kuehniella larvae. The results showed a variation in the fumigant activity of these molecules with the most active constituent being carvacrol (Erler 2005). In terms of LT50 values (h) at 46.2 mg/l air, the following monoterpenoids had the highest levels of activity against T. confusum adults: carvacrol, 3.9; thymol, 15.1; -terpinene, 19.1; terpinen-4-ol, 162.2; 1,8-cineole, and menthol, both of which were quite potent. However, Mentha piperita oil and menthol showed either less or no activity against Aedes aegypti (Samarasekera et al. 2008).
Effect in digestive enzymes
Currently, one of the most important aspects of pest control is the selective inhibition of digestive enzymes of many insect pests (Yazdani et al. 2013). In the midgut, digestive enzymes hydrolyze food macromolecules into smaller molecules, thus facilitating their absorption. Proteins, carbohydrates, and lipids constitute the majority of food macromolecules and are hydrolyzed by proteases, amylases, and lipases, respectively. The application of menthol disrupts the activity of digestive enzymes in adults of R. dominica compared to controls with a dose–response relationship. The activity of three enzymes studied was significantly reduced. These results are consistent with previous studies which were demonstrated the reduction of α-amylase, proteases, and lipases activities in pests such as R. dominica treated with Lavandula angustifolia EO (Sayada et al. 2021a), Trogoderma granarium treated with Eucalyptus globulus EO (Tine et al. 2021a), and R. dominica treated with Schinus molle EO (Tine et al. 2021b).α-amylase is a midgut and salivary enzyme involved in the metabolism of starch and other carbohydrates, and its level of activity is diet dependent (Shekari et al. 2008). Some plant extracts and plant-derived molecules inhibited α-amylase activity in vitro (Yazdani et al. 2013). The reduced activity of α-amylase by plant-based compounds could imply their cytotoxic effect on the midgut epithelial cells those synthesize insect α-amylase (Senthil-Nathan et al. 2006; Zibaee and Bandani 2010). This result is consistent with previous studies which were demonstrated the reduction of α-amylase activities in pests such as E. kuehniella (Shahriari et al. 2017), Ectomyelois ceratoniae (Ramzi et al. 2014), Glyphodes pyloalis (Yazdani et al. 2013), Pieris rapae (Hasheminia et al. 2011) after treatment with botanical toxins.
Proteases are a class of enzymes that catalyze the hydrolysis of peptide bonds in proteins to release their corresponding amino acids (Pascual-Ruiz et al. 2009). They can be altered by botanical insecticides which interfere with the production of certain types of proteases and prevent them from digesting ingested proteins (Senthil-Nathan et al. 2006). Similar results have been reported in Periplaneta americana (Paranagama et al. 2001) and G. pyloalis (Khosravi and Sendi 2013) treated with azadirachtin, in T. granarium larvae treated with E. globulus EO (Tine et al. 2021a), in R. dominica treated by S. molle EO (Tine et al. 2021b), and Lavandula angustifolia (Sayada et al. 2021a). Many insect species have reported a decrease in protease activity after exposure to botanical insecticides (Paranagama et al. 2001).
Lipases play a very important role in the storage and mobilization of lipids. These enzymes are also involved in several physiological processes such as reproduction, growth, and defense against pathogens (Lemaitre and Miguel-Aliaga 2013). Similar observations were noted in Chilo suppressalis treated with A. annua (Zibaee et al. 2008), in Cnaphalocrocis medinalis treated with azadirachtin (Senthil Nathan et al. 2006), in T. granarium larvae treated with E. globulus EO (Tine et al. 2021a) and R. dominica exposed to S. molle EO (Tine et al. 2021b). Decreased lipase activity by botanical insecticide could be due to disturbance of digestion and metabolism processes (Senthil- Nathan et al. 2006; Zibaee and Bandani 2010).
Exposure to lethal concentrations considerably affected the enzymatic activities of an organism, reflecting the biochemical disturbances (Kiran et al. 2015). Indeed, any disturbance in the activity of digestive enzymes reduces access to nutrients essential to the functioning of the body. Furthermore, this reduction in nutrient utilization capacity may be related to a conversion of the energy required for biomass production into induction enzymes activity involved in detoxification essential oils and their components (Senthil-Nathan et al. 2005). Inactivation of digestive enzymes leads to poor nutrient utilization, retarded development and death by starvation (Gatehouse and Gatehouse 1999).
Effect on intermediary-involved enzymes
In the body of insects, transamination is the most important physiological process, for formation of protein needed for various functions (Chapman et al. 2013). It also plays an important role in insect energy processes such as alanine to proline conversion (Hakkak et al. 2018; Sugeçti and Büyükgüzel 2018). Transaminase enzymes (GOT and GPT) are mitochondrial enzymes which involved in transamination and found in hemolymph and fat bodies of insects (Nation 2008). They are released in the hemolymph of insects only when the cells are damaged or destroyed (Abo El Makarem et al. 2015a; b; Pradel and Albert 2021). They are crucial for the Krebs cycle's transformation of aspartate and α-ketoglutarate into oxalate and glutamate, respectively, which enables insects to adapt to oxidative stress (Hakkak et al. 2018). Our results revealed an increase in GOT, GPT, and PAL in R. dominica treated with menthol. These results might suggest that menthol application enhanced transaminase enzyme activity due to toxic stress. The study of Abo El Makarem et al. (2015a; b) showed a significant increase in the GPT activity after treatment of S. granarius with sublethal concentrations of basil and clove while basil oil increased the activity of GOT indicating of increased synthesis of both enzymes. Significant differences were found among activities of ALT and AST in the hemolymph of Chrysodeixis chalcites larvae (Lepidoptera: Noctuidae) reared on lemon balm, corn, and dill, respectively (Mardani-Talaee et al. 2014). Increased transaminase activity might have been required by insects to metabolize amino acids to obtain energy under stress. In fact, the varying effect of plant extracts on GOT and GPT activities might be due to the effect on the synthesis or functional levels of these enzymes directly or indirectly by altering the cytomorphology of the cells (Nath 2000). Our results agreed with some other findings on the effect plant oils on GOT and GPT activates (Abdel-Latif and Al Moajel 2004; Arshad et al. 1999; Hassan 2002; Tabassum et al. 1994). However, decreased enzymatic activity of transaminases was reported in Helicoverpa armigera (Lepidoptera: Noctuidae) fed on a diet containing β-cytosterol with dose-dependent (Mishra et al. 2020). The studies of Khater and El-Shafiey (2015) showed a decrease in alanine and aspartate aminotransferase activity in Tribolium castaneum treated with Wedelia trilobata and Melissa officinalis EOs. Decreased alanine aminotransferase activity may be due to lack of energy supply through proline or the need for amino acids due to tissue damage caused by the compounds used (Goharrostami et al. 2022).
Alkaline phosphatase is hydrolytic enzyme that separate phosphate groups from various molecules such as nucleotides, proteins, and alkaloids in alkaline conditions (Nation 2016). The activity of this enzyme indicates the efficiency of digestion and absorption of nutrients in the stomach and their transfer to fat bodies (Goharrostami et al. 2022). The results of this study revealed an increase in PAL in R. dominica adults treated with menthol. Our results disagree with those found in G. pyloalis larvae treated with thyme EO, thymol, and carvacrol compounds (Goharrostami et al. 2022) and in E. kuehniella treated with Teucrium polium (Lamiaceae) EO and α-pinene (Shahriari et al. 2019). Significant differences were found among activities of alkaline (ALP) phosphatase in the hemolymph of Chrysodeixis chalcites larvae (Lepidoptera: Noctuidae) reared on lemon balm, corn, and dill, respectively (Mardani-Talaee et al. 2014). The lack of digestive function and decreased metabolism caused by a decrease in the release of phosphate groups for the production of energy can be indicated by a drop in the activity of this enzyme group (Selin-Rani et al. 2016; Senthil-Nathan 2006).
Effect on biochemical composition
Essential oils and their components interfere with several insect functions: metabolic, biochemical, physiological, and behavioral (Mann and Kaufman 2012). Several studies have shown fluctuation of biochemical composition of insect’s body treated with EOs and their components (Guettal et al. 2021a, b; Sayada et al. 2021b; Tine et al. 2021a, b). In the present investigation, the application of menthol to R. dominica adults resulted in a decrease in the biochemical parameters (carbohydrates, lipids and proteins content) at different periods after treatment. This decline could be due to the reduction in the diet of insects since menthol could have a deterrent effect.
The reduction of proteins is a frequent phenomenon in insects treated with toxic products (Nation et al. 2008); it can be attributed to one or more factors, such as the reduction in their synthesis or increase in their degradation to detoxify the active ingredients present in plant extracts or essential oils (Vijayaraghavan et al. 2010). The breakdown of proteins into amino acids is intended to facilitate their incorporation into the Krebs cycle as ketone acids to compensate for low energy levels caused by stress (Nath et al. 1997). Moreover, the reduction in protein reserve may also be due to the physiological adaptation of the insect to a state of stress caused by insecticides (Ribeiro et al. 2001).
However, an increase in protein levels has been reported in R. dominica treated with S. molle (Tine et al. 2021a), in T. granarium treated with E. globulus (Tine et al. 2021b), in R. dominica (Tine et al. 2017) and Sitophilus granarius treated with azadirachtin (Guettal et al. 2021a, b).
Carbohydrate plays a major metabolic role in the development cycle (Steele 1981) and constitutes an essential source of energy. Our results show a significant reduction in carbohydrate levels in adult of R. dominica treated with menthol. Similar results were reported in S. granarius treated with citrus oil and azadirachtin (Guettal 2021a), in T. castaneum treated with the of Agastache foeniculum EO (Ebadollahi 2013), in S. granarius treated with C. limonum EO (Guettal et al. 2020), in T. granarium treated with S. molle (Tine et al. 2021b), and in R. dominica treated with E. globulus (Tine et al. 2021a). Glucose depletion may be due to the stress conditions imposed on these insects which require more energy to cover energy expenditure via induction by neuropeptides (Mojarab-Mahboubkar et al. 2015). It may also be due to an acceleration of glycogenolysis in the fat body, the transport of glycogen from the fat body to the hemolymph in response to energy depletion when individuals are exposed to toxins (Zibaee 2011).
Lipids are the main source of energy in insects (Beenakers et al. 1985). Our results showed that treatment of adult R. dominica with menthol induced a significant decrease in lipid content. The same observations were made in S. granarius treated with Citrus oil (Guettal et al. 2020), azadirachtin (Guettal et al. 2021b) and combination Aza-EO (Guettal et al. 2021a), in R. dominica treated with E. globulus EO (Tine et al. 2021a), and in T. granarium treated with S. molle EO (Tine et al. 2021b). The depletion of this biochemical component after treatment is due to the stress induced following exposure to an insecticide (Sancho et al. 1998) which results in an alteration of their synthesis (Klowden 2007), to hormonal dysfunction that controls lipid metabolism (Steele 1981), to the use of this metabolic reserve (Sak et al. 2006), to the formation of lipoproteins, to the repair of cellular damage, and to the increase lipolysis to provide energy (Lohar and Wright 1993; Steele 1985).
Reducing the amount of energy resources, the proteins, carbohydrates, and lipids considered one of the main strategies in the insect pest management because of their important roles in insect biochemical pathways, growth, metamorphosis, reproduction, and diapause (Arrese and Soulages 2010; Senthil-Nathan 2013).
Conclusions
The menthol exhibited contact and fumigant toxicity against lesser grain borer adults confirming its potential use as a natural alternative to synthetic insecticides against stored-product insect pests. In addition, the strong repellent activity evidence suggests a possible application to flush out insect infestation from empty stores before fresh grain is introduced. Moreover, the perturbation of digestive enzymes affects growth and reproductive events via depressive effects on energy reserves. This study gives additional information on plant derivative products and their use in integrated pest management in the storage sites.
References
Abbott WB (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abdelgaleil S, Mohamed M, Badawy M, El-arami S (2009) Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J Chem Ecol 35:518–525. https://doi.org/10.1007/s10886-009-9635-3
Abdel-Latif AM, Moajel NH (2004) Some biochemical effects of natural mint oil on some species of stored grain pests. Mansoura J Agr Sci 9(9).
Abed-Vieillard D, Cortot J, Everaerts C, Ferveur JF (2014) Choice alters Drosophila oviposition site preference on menthol. Biol Open 3:22–28
Abo El Makarem HE, El Kholy S, Abdel-Latif AI, Seif A (2015a) Physiological and biochemical effects of some essential oils on the granary weevil, Sitophilus granarius (L) (Coleoptera: Curculionidae). Egypt Soc Exp Biol 11(2):117–123
Aggarwal KK, Tripathi AK, Ahmad A, Prajapati V, Verma N, Kumar S (2001) Toxicity of l-menthol and its derivatives against four storage insects. Int J Trop Insect Sci 21:229–235
Arrese EL, Soulages JL (2010) Insect fat body: Energy, metabolism, and regulation. Ann Rev Entomol 55:207–225
Arshad M, Hasan SN, Kham MF, Akhtar K, Khan FX (1999) Comparative toxicological studies of RBa (neem extract) and (permethrin + bioallethrin) against Sitophilus oryzae with reference to their effects on oxygen consumption and GOT, GPT activity. Turk J Zool 22:307–310
Beenakers AM, Van der Horst DJ, Van Marrewijk WJ (1985) Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res 24(1):19–67
Bernfeld P (1955) Amylases, alpha and beta. Methods Enzymol I:149–158
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Bio Chem 72(1–2):248–254
Chapman RF, Stephen J, Simpson A, Douglas E (2013) The insects structure and function. Press, Cambridge, Cambridge Uni
Duchateau G, Florkin M (1959) Sur la tréhalosémie des insectes et sa signification. Arch Inter Physiol Bioch 67(2):306–314
Dutra K, Wanderley-Teixeira V, Guedes C, Cruz G, Navarro D, Monteiro A, Agra A, Neto CL, Teixeira A (2020) Toxicity of essential oils of leaves of plants from the genus Piper with influence on the nutritional parameters of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae). J Essent Oil Bear Plants 23:213–229. https://doi.org/10.1080/0972060X.2020.1756423
Ebadollahi A (2013) Essential oils isolated from Myrtaceae family as natural insecticides. Ann Res Rev Biol 148–175.
El-Saadony MT, Saad AM, Elakkad HA, El-Tahan AM, Alshahrani OA, Alshilawi MS, El-Sayed H, Amin SA, Ahmed AI (2022) Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4 °C through controlling chemical and microbial fluctuations. Saudi J Biol Sci 29:346–354
Enan EE (2005) Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch Insect Biochem Physiol 59:161–171. https://doi.org/10.1002/arch.20076
Erler F (2005) Fumigant activity of six monoterpenoids from aromatic plants in Turkey against the two stored-product pests confused flour beetle, Tribolium confusum, and Mediterranean flour moth. Ephestia Kuehniella J Plant Dis Prot 112(6):602–611
Garcia-Carreno FL, Haard NF (1993) Characterization of protease classes in langostilla (Pleuroncodes planipes) and crayfish (Pacifastacus astacus) extracts. J Food Biochem 17(2):97–113
Gatehouse JA, Gatehouse AMR (1999) Genetic engineering of plants for insect resistance. In: Rechcigl JE, Rechcigl NA (eds) Biological and biotechnological control of insect pests. CRC Press, Boca Raton, FL, pp 211–241
Goharrostami M, Sendi JJ, Hosseini R, Mahmoodi NA (2022) Chemical composition, toxicity and physiological effects of thyme oil and its two components on mulberry pyralid moth. Res Square. https://doi.org/10.21203/rs.3.rs-1613448/v1.
Goldsworthy GJ, Mordue W, Guthkelch J (1972) Studies on insect adipokinetic hormones. Gen Comp Endocrinol 18(3):545–551
Gong X, Ren Y (2020) Larvicidal and ovicidal activity of Carvacrol, p-cymene, and γ-terpinene from Origanum vulgare essential oil against the cotton bollworm, Helicoverpa armigera (Hübner). Environ Sci Pollut Res 27:18708–18716. https://doi.org/10.1007/s11356-020-08391-2
Guettal S, Tine S, Tine-Djebbar F, Soltani N (2020) Effect of Citrus limonum essential oil against granary weevil, Sitophilus granarius and its chemical composition, biological activities and energy reserves. Inter J Trop Insect Sci 41:1531–1541
Guettal S, Tine S, Kaouther H, Tine-Djebbar F, Soltani N (2021a) Combined effects of Azadirachtin and Citrus limonum essential oil against Sitophilus granarius: Toxicity and biological activities. Pest Res J 33(1):78–86
Guettal S, Tine S, Tine-Djebbar F, Soltani N (2021b) Repellency and toxicity of azadirachtin against granary weevil Sitophilus granarius L. (Coleoptera: Curculionidae). https://hal.archives-ouvertes.fr/hal-03169471.
Hakkak R, Gauss C, Bell A, Korourian S (2018) Short-term soy protein isolate feeding prevents liver teatosis and reduces serum alt and ast levels in obese female zucker rats. Biomed 6:55–66
Hasheminia SM, Sendi JJ, Jahromi KT, Moharramipour S (2011) The effects of Artemisia annua L and Achillea millefolium L crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pest Biochem Physiol 99(3):244–249
Hassan HA (2002) Biological and biochemical studies on the effects of some botanical extracts on cotton leaf worm Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Dissertation, University of Ain Shams.
Himmel NJ, Letcher JM, Sakurai A, Gray TR, Benson MN, Cox DN (2019) Drosophila menthol sensitivity and the Precambrian origins of transient receptor potential-dependent chemosensation. Phil Trans r Soc B 374:20190369. https://doi.org/10.1098/rstb.2019.0369
Khater KS, El-Shafiey SN (2015) Insecticidal effect of essential oils from two aromatic plants against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Egypt J Biol Pest Control 25:129–134
Khosravi R, Sendi JJ (2013) Effect of neem pesticide (Achook) on midgut enzymatic activities and selected biochemical compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). J Plant Prot Res 53(3).
Kiran S, Prakash B (2015) Assessment of toxicity, antifeedant activity, and biochemical responses in stored-grain insects exposed to lethal and sublethal doses of Gaultheria procumbens L. essential oil. J Agric Food Chem 48:10518–10524
Kissoum N, Bensafi-Gheraibia H, Hamida ZC, Soltani N (2020) Evaluation of the pesticide Oberon on a model organism Drosophila melanogaster via topical toxicity test on biochemical and reproductive parameters. Comp Biochem Physiol, Part C 228:108666. https://doi.org/10.1016/j.cbpc.2019.108666
Klowden MJ (2007) Physiological systems in insects. Amsterdam. Elsevier, 688 p. Academic Press.
Lemaitre B, Miguel-Aliaga I (2013) The digestive tract of Drosophila melanogaster. Annu Rev Genet 47:377–404
Lohar MK, Wright DJ (1993) Changes in the lipid content in haemolymph, fat body and oocytes of malathion treated Tenebrio molitor L. Adult Females Pak J Zool 25:57–57
Mann R, Kaufman P (2012) Natural product pesticides: their development, delivery and use against insect vectors. Mini-Rev Org Chem 9(2):185–202
Mardani-Talaee M, Rahimi V, Zibaee A (2014) Effects of host plants on digestive enzymatic activities and some components involved in intermediary metabolism of Chrysodeixis chalcites (Lepidoptera: Noctuidae). J Entomol Acarol Res 46:3224
Mc Donald LL, Guy RH, Speirs RD (1970) Preliminary evaluation of new candidate materials as toxicants, repellents, and attractants against stored-product insects. Washignton D.C, Agricultural Research Service.
Mishra M, Sharma A, Dagar VS, Kumar S (2020) Effects of β-sitosterol on growth, development and midgut enzymes of Helicoverpa armigera Hubner. Arch Biol Sci 72:271–278. https://doi.org/10.2298/ABS200308021M
Mojarab-Mahboubkar M, Sendi JJ, Aliakbar A (2015) Effect of Artemisia annua L essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). J Plant Prot Res 55(4):371–377
Nath BS (2000) Changes in carbohydrate metabolism in hemolymph and fat body of the silkworm, Bombyx mori L., exposed to organophosphorus insecticides. Pest Biochem Physiol 68(3):127–137
Nath BS, Suresh A, Varma BM, Kumar RS (1997) Changes in protein metabolism in hemolymph and fat body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to organophosphorus insecticides toxicity. Ecotox Envir Saf 36(2):169–173
Nation JL (2008) Insect physiology and biochemistry, 2nd edn. CRC Press, UK, London
Nation JL (2016) Insect physiology and biochemistry. CRC Press, London
Oftadeh M, Jalali-Sendi J, Ebadollahib A (2020) Toxicity and deleterious effects of Artemisia annua essential oil extracts on mulberry pyralid (Glyphodes pyloalis). Pestic Biochem Physiol 170:104702. https://doi.org/10.1016/j.pestbp.2020.104702
Paranagama PA, Kodikara KABCH, Nishantha HMI, Mubarak AM (2001) Effect of azadirachtin on growth and the activity of the midgut enzymes of cockroach Periplaneta americana. J Nat Sci Found Sri Lanka 29(1–2).
Pascual-Ruiz S, Carrillo L, Alvarez-Alfageme F, Ruiz M, Castanera P, Ortego F (2009) The effects of different preyregimes on the proteolytic digestion of nymphs of the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). Bull Entomol Res 99(5):487–491
Pradel SF, Albert MS (2021) Evaluation of the in vivo energy potency of ethanolic extracts and combinations of extracts from the leaves of Gnetum africanum Welv. and Gnetum buchholzianum Engl. (Gnetaceae): two plants with hepatic potential and antioxidant protection. Sci, Technol Dev 23:14–18 ((in Frensh with English abstract))
Rajkumar V, Gunasekaran C, Christy IK, Dharmaraj J, Chinnaraj P, Paul CA (2019) Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pestic Biochem Physiol 156:138–144
Ramzi S, Sahragard A, Zibaee A (2014) Effects of Citrullus colocynthis agglutinin on intermediary metabolism of Ectomyelois ceratoniae Zeller (Lepidoptera: Pyralidae). J Asia Pacific Entomol 17:273–279
Ribeiro PF, Johnson BK, Crow ML, Arsoy A, Liu Y (2001) Energy storage systems for advanced power applications. Proc IEEE 89(12):1744–1756
Sak O, Uçkan F, Ergin E (2006) Effects of cypermethrin on total body weight, glycogen, protein, and lipid contents of Pimpla turionellae (L.) (Hymenoptera: Ichneumonidae). Belg J Zool 136(1):53–58
Samarasekera R, Weerasinghe IS, Hemalal KDP (2008) Insecticidal activity of menthol derivatives against mosquitoes. Pest Manag Sci 4:290–295
Sancho E, Ferrando MD, Fernandez C, Andreu E (1998) Liver energy metabolism of Anguilla anguilla after exposure to fenitrothion. Ecotox Envir Saf 41(2):168–175
Sayada N, Tine S, Soltani N (2021a) Evaluation of a botanical insecticide, lavender (Lavandula angustifolia (M.)) essential oil as toxicant, repellent and antifeedant against lesser grain borer (Rhyzopertha dominica (F.)). Appl Ecol Envir Res 20(2):1301–1324
Sayada N, Tine S, Soltani N (2021b) Toxicity and physiological effects of essential oil from Lavandula angustifolia (M.) against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) adults. J Entomol Res 45(suppl):929–936
Selin-Rani S, Senthil-Nathan S, Revathi K, Chandrasekaran R, Thanigaivel A, Vasantha-Srinivasan P, Ponsankar A, Edwin ES, Pradeepa V (2016) Toxicity of Alangium salvifolium wang chemical constituents against the tobacco cutworm Spodoptera litura Fab. Pest Biochem Physiol 126:92–101
Senthil-Nathan S (2013) Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front Physiol 4:359
Senthil-Nathan SS, Kalaivani K, Murugan K, Chung PG (2005) The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guenée) the rice leaf folder. Pest Biochem Physiol 81(2):113–122
Senthil-Nathan S, Chunga PG, Muruganb K (2006) Combined effect of biopesticides on the digestive enzymatic profiles of Cnaphalocrocis medinalis (Guenee) (the rice leaf folder) (Insecta: Lepidoptera: Pyralidae). Ecotox Envir Saf 64:382–389
Shahriari M, Sahebzadeh N, Zibaee A (2017) Effect of Teucrium polium (Lamiaceae) essential oil on digestive enzyme activities and energy reserves of Ephestia kuehniella (Lepidoptera: Pyralidae). Invert Surv J 14:182–189
Shahriari M, Sahebzadeh N, Zibaee A (2019) Effects of Teucrium polium L. (Lamiaceae) essential oil and α-pinene on the detoxifying and intermediary engaged enzymes of Ephestia kuehniella Zeller, 1879 (Lep.: Pyralidae). Acta Agric Slov 113(2):251–261
Shekari M, Jalali Sendi J, Etebari K, Zibaee A (2008) Shadparvar A (2008) Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle, Xanthogaleruca luteola Mull (Coleoptera: Chrysomellidae). Pest Bioch Physiol 91:66–74
Shibko S, Koivistoinen P, Tratnyek CA, Newhall AR, Friedman L (1966) A method for sequential quantitative separation and determination of protein, RNA, DNA, Lipid, and glycogen from a single rat liver homogenate or from a subcellular fraction. Analyt Biochem 19:514–528
Shimomura K, Oikawa H, Hasobe M, Suzuki N, Yajima S, Tomizawa M (2020) Contact repellency by L-menthol is mediated by TRPM channels in the red flour beetle Tribolium castaneum. Pest Manag Sci. https://doi.org/10.1002/ps.616010.1002/ps.6160
Steele JE (1981) The role of carbohydrate metabolism in physiological function. Energ Metab Insects 101- 133.
Steele JE (1985) Hormonal modulation of carbohydrate and lipid metabolism in fat body. Insect Biol future, Academic Press 253–271.
Sugeçti S, Büyükgüzel K (2018) Effects of oxfendazole on metabolic enzymes in hemolymph of Galleria mellonella L. (Lepidoptera: Pyralidae) larvae reared on artificial diet. Karaelmas Sci Engin J 8(2).
Tabassum R, Jahan M, Naqvi SNH (1994) Determination of toxicity of Sisthion and RB–a formulation (neem extract) against Tribolium castaneum (Herbst) adults and their effect on transaminases. Neem Newslett (india) 11(1):7–9
Talukder FA, Howse PE (1994) Repellent, toxic and food protectant effects of pithraj, Aphanamixis polystachya (Meliaceae) against the pulse beetle, Callosobruchus chinensis, in storage. J Chem Ecol 20:899–908
Tine S, Halaimia A, Chechoui J, Tine-Djebbar F (2017) Fumigant toxicity and repellent effect of azadirachtin against the lesser grain beetle, Rhyzopertha dominica (F.) (Col.: Bostrichidae). In: Recent advances in environmental science from the Euro-Mediterranean and surrounding regions, A Kallel, M Ksibi, H Ben Dhia and N. Khelifi (Eds), Springer, Cham, pp 399–401.
Tine S, Brahmi A, Yousfi R (2021a) Lutte contre les ravageurs des stocks. Noor Publishing. ISBN: 978-620-3-85842-6.
Tine S, Soltani M, Abess N (2021b) Utilisation des huiles essentielles dans la lutte contre les insectes des denrées stockées. Editions Universitaires Européennes. ISBN: 978-620-3-42165-1.
Tong F, Coats J (2012) Quantitative structure-activity relationships of monoterpenoid binding activities to the housefly GABA receptor. Pest Manag Sci 68(8):1122–1129
Tripathi AK, Prajapati V, Agvawal KK, Khanuja SPS, Kumar S (2001) Repellency and toxicity of oil from Artemisia annua to ceratin stored product beetles. J Econ Entomol 93:43–47
Tsujita T, Ninomiya H, Okuda H (1989) p-nitrophenyl butyrate hydrolyzing activity of hormone-sensitive lipase from bovine adipose tissue. J Lipid Res 30(7):997–620
Venkatesan T, Jalali SK, Ramya SL, Prathibha M (2016) Insecticide resistance and its management in mealybugs. In: Mani M, Shivaraju C (eds) Mealybugs and their management in agricultural and horticultural crops. Springer, India, pp 223–229
Vijayaraghavan C, Sivakumar C, Kavitha Z, Sivasubramanian P (2010) Effect of plant extracts on biochemical components of cabbage leaf webber, Crocidolomia binotalis Zeller. J Biopest 3(1):275–277
Yazdani E, Sendi JJ, Aliakbar A, Senthil-Nathan S (2013) Effect of Lavandula angustifolia essential oil against lesser mulberry pyralid Glyphodes pyloalis Walker (Lep: Pyralidae) and identification of its major derivatives. Pest Biochem Physiol 107(2):250–257
Zibaee A, Bandani AR (2010) Effects of Artemisia annua L. (Asteracea) on the digestive enzymatic profiles and the cellular immune reactions of the Sunnpest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauveria bassiana. Bull Entomol Res 100(2):185–196
Zibaee A, Bandani AR, Ramzi S (2008) Lipase and invertase activities in midgut and salivary glands of Chilo suppressalis (Walker) (Lepidoptera, Pyralidae), rice striped stem borer. Invert Surv J 5(2):180–189
Zibaee A, Bandani AR, Talaei-Hassanlouei R, Malagoli D (2011) Cellular immune reactions of the sunn pest, Eurygaster integriceps, to the entomopathogenic fungus, Beauveria bassiana and its secondary metabolites. J Insect Sci 11(1):1–16
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
TAO and TM involved in data curation, writing, methodology, investigation, formal analysis. F.T.-D., S.T., and N.S. took part in methodology, investigation, writing—review & editing, conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tine-Djebbar, F., Trad, M., Tine, A.O. et al. Effects of menthol on nutritional physiology and enzyme activities of the lesser grain borer, Rhyzopertha dominica (F. 1792) (Coleoptera: Bostrichidae). J Plant Dis Prot 130, 509–518 (2023). https://doi.org/10.1007/s41348-023-00727-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-023-00727-7