Abstract
In the present work, vortex-assisted liquid-phase microextraction (VA-LPME) and ultrasound-assisted liquid-phase microextraction (UA-LPME) techniques were used for the speciation of chromium (Cr3+/Cr6+) followed by their determination using flame atomic absorption spectrometry. 1-(2-Pyridylazo)-2-naphthol (PAN) was used as a chelating agent to form a hydrophobic complex with Cr3+; then, it was extracted by choline chloride–phenol mixture, as an extraction solvent. A solution of 1.0 mol L−1 ascorbic acid was used as a reducing agent to convert Cr6+ to Cr3+ (total chromium), and the concentration of Cr6+ was determined from the subtraction of Cr3+ form total chromium. The efficiency of two microextraction techniques was compared in terms of type and amount of extraction solvent, type and volume of extraction solvent, pH of solution, volume of tetrahydrofuran (THF), and extraction time. Based on the obtained results, the extraction recoveries of VA-LPME and UA-LPME techniques are 52% and 60%, respectively. Therefore, UA-LPME is superior technique than VA-LPME. By optimizing different parameters affecting the recovery percentage (RP) of Cr3+, the calibration curve was depicted in the concentration range of 1.5–375.0 ng mL−1 Cr3+ with a correlation coefficient of 0.9937. The limit of detection was 0.4 ng mL−1, and the relative standard deviation (RSD%) for seven replicate analyses of 50.0 µg L−1 of Cr3+ was 3.6%. Finally, the UA-LPME method was successfully applied for the determination of Cr species in different food and water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, leakage of heavy metals from different industries has caused serious damages to the environment [1, 2]. Chromium (Cr) is one of the most applicable heavy metals in different fields such as electroplating [3], batteries [4, 5], dyes and pigments [6], and catalyst [7,8,9,10]. In the environment, chromium mainly exists in two oxidation states including trivalent (Cr3+) and hexavalent chromium (Cr6+). These chemical forms have different solubility, mobility, bioavailability, and toxicity. In biologically views, Cr6+ is more toxic than Cr3+ because it could be permeated through the sulfate transport system and interact with protein and nucleic acid. Cr6+ compounds are known to have toxic, mutagenic, and carcinogenic effects on humans and animals. However, Cr(III) is less toxic and it is actually an essential human nutrient [11, 12]. Therefore, accurate determination of chromium species is highly required. Up to now, different preconcentration methods including solid-phase extraction (SPE) [13,14,15,16] and liquid-phase microextraction (LPME) [17,18,19,20,21,22,23] have been used for the speciation of chromium ions. LPME has significant advantages such as simplicity, highly rapidity, and high efficiency [24,25,26]. However, one of the critical parameters in LPME is the selection of extraction solvent which should be green (non-toxic) and has high availability and tendency to extract the analyte.
Deep eutectic solvents (DESs) have been recently introduced and used as extraction solvent in LPME techniques. DESs are generally composed of two or more green and inexpensive components that associate with each other with hydrogen bonds, to form an eutectic mixture with a melting point less than that of each individual component [27, 28]. DESs are usually obtained by the complexation of choline chloride salt as a quaternary ammonium salt (ChCl) with hydrogen bond donor (HBDs) compounds or metal oxides [27]. Due to the many advantages of DESs such as non-toxicity, biodegradability, easy to synthesize, and low costs, these solvents could be considered as green extraction solvents for LPME methods. Also, these DESs exhibit similar physico-chemical properties to the traditionally used ionic liquids (IL). As compared to ILs, DESs have unique advantages including (1) convenient synthesis, (2) very low price due to their accessible chemicals, and (3) their low toxicity, especially DESs derived from ChCl and renewable chemical [29].
Vortex-assisted liquid-phase microextraction (VA-LPME) and ultrasonic-assisted liquid-phase microextraction (UA-LPME) are the kinds of liquid-phase microextraction techniques, in which the extraction solvent could be dispersed in the whole of the sample solution using vortex stream and ultrasonic waves, respectively. Therefore, the surface-to-area ratio of the extraction solvents increases impressively and the extraction of analyte takes place in a short time [30,31,32].
In the present work, we have used DESs for the preconcentration of trace levels of Cr3+ followed by its determination with flame atomic absorption spectrometry (FAAS). 1-(2-Pyridylazo)-2-naphthol (PAN) was used as a chelating agent to form a hydrophobic complex with Cr3+. By using choline chloride–phenol (as extraction solvent) and THF (aprotic solvent), vortex-assisted liquid-phase microextraction (VA-LPME) and ultrasonic-assisted liquid-phase microextraction (UA-LPME) techniques were used to investigate their efficiency for the extraction of Cr3+-PAN complex. Based on our knowledge, there is no report for the comparison of VA-LPME and UA-LPME as low cost, efficient, green (using biodegradable extraction solvents), and rapid microextraction techniques for speciation of chromium ions. The main parameters affecting the recovery percentage (RP) such as pH, amounts of PAN, volume of THF, and the extraction time were completely investigated, and optimum conditions were selected.
Experimental
Instruments
A Shimadzu AA-670 (Shimadzu, Japan) flame atomic absorption spectrometer equipped with a 100-mm burner head, deuterium background correction, and an air-acetylene flame was utilized. A chromium hollow-cathode lamp (Hamamatsu Photonics, Shizuoka, Japan) at 4 mA and 0.3 nm slit width at a wavelength of 257.9 nm was used as a radiation source. The pH values were measured with a pH meter (Metrohm 827 pH lab, Switzerland). An ultrasonic bath (Sonica 2200 ETH, Italy) was used for sonication of sample solution, and a vortex Gilson mixer (Villiers Le Bel, France) at 2800 rpm was used for mixing of the sample solution. Phase separation was assisted using a Centurion Scientific Centrifuge (Model Andreas Hettich D72, Tuttlingen, Germany). An electronic balance (Libror, Shimadzu, Japan) was used for weighing of samples. Conical centrifuged tubes (15 mL) were used throughout the analysis and washed with deionized water, followed by drying at 100 °C before uses.
Reagents
Deionized water was used throughout the analysis. Stock solutions of 1000.0 mg L−1 Cr3+ and Cr6+ were prepared by dissolving appropriate amounts of Cr(NO3)3.9H2O and K2Cr2O7 in deionized water, respectively. 1-(2-Pyridylazo)-2-naphthol (PAN) was used as a chelating agent and purchased from Merck (Darmstadt, Germany). Choline chloride, urea, glycerol, ethylene glycol, and phenol were purchased from Merck (Darmstadt, Germany). HCl (Merck, 35.5%) was used for sample digestion. L-Ascorbic acid (99.0%, Sigma-Aldrich, USA) was used as a reducing agent to convert Cr6+ to Cr3+. Solutions of 0.1 mol L−1 NaOH and HCl were used for adjusting pH values.
Experimental work
Vortex-assisted and ultrasonic-assisted liquid-phase microextraction
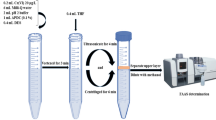
Ten milliliters of sample solution containing 50.0 ng mL−1 Cr3+ and optimum amounts of PAN as a complexing agent were transferred into the conical centrifuge tube followed by adjusting the pH value at 6.5. Then, 350 µL of choline chloride–phenol (as an extraction solvent) was added to the sample solution. The resulting mixture was vortexed for 2 min at 2800 rpm (VA-LPME) or sonicated for 90 s in an ultrasonic bath (UA-LPME) to completely disperse the extraction solvent into the sample solution. By centrifuging the resulting solution at 5000 rpm for 4 min followed by addition of 400 µL THF to the mixture, the fine droplets of extraction solvent were floating on the top of sample solution. The extraction solvent containing Cr3+-PAN complex was collected by a Hamilton syringe (200 ± 10 µL) and injected into the FAAS to measure the absorbance of Cr.
Speciation of Cr3+ and Cr6+ in sample solution
To determination of total chromium, an aliquot of 1 mL of 1.0 mol L−1 ascorbic acid was added to 10 mL of sample solution containing Cr3+/Cr6+ to reduce of Cr6+ to Cr3+, followed by their analysis, according to the procedure explained above (total chromium). Then, the concentration of Cr6+ was determined from the subtraction of these results. Equation 1 shows chemical reaction for the reduction of Cr6+ by ascorbic acid.
Analysis of real sample
Tap and river water
In order to determine the chromium species in tap (Sabzevar, Iran) and river water (Kashaf Rood, Mashhad, Iran), 10 mL of each sample solution was adjusted at the pH value of 6.5 and analyzed according to the microextraction procedure.
Mushroom and soybean
Mushroom and soybean samples were purchased from Mashhad, Iran. 2.0 g of samples were weighted followed by addition of 10 mL concentrated HCl to them. Then, each sample was heated for 30 min at 60–70 °C, filtered by Whatman NO. 32 filter paper, and diluted to 50 mL with deionized water. Finally, 5 mL of each sample was adjusted at pH 6.5, and after dilution to 10 mL with deionized water, it was analyzed for determination of chromium content according to the microextraction procedure.
Analysis of CRM-TMDW
To the 10 mL of CRM-TMDW water sample, aliquot of 1 mL of 1 mol L−1 ascorbic acid was added to convert Cr6+ to Cr3+. Then, after adjusting the pH value at 6, it was analyzed according to the microextraction procedure.
Optimization of critical parameters
The main parameters affecting the extraction efficiency such as type of extraction solvent, volume of extraction solvent, pH, volume of THF, amounts of complexing agent (PAN), and extraction time were investigated to select the optimum conditions.
Effect of type of extraction solvent
The selection of extraction solvent is governed by its selectivity and hydrophobicity to extract of hydrophobic Cr3+-PAN complex. In this study, four types of DESs as extraction solvents including urea–choline chloride (Ur-ChCl), glycerol–choline chloride (Gly-ChCl), ethylene glycol–choline chloride (EthyGly-ChCl), and phenol–choline chloride (Phe-ChCl) were analyzed. Based on the obtained results, ChCl-Phe provides the maximum RP for preconcentration of Cr3+-PAN complex. Therefore, it was selected as the optimum extraction solvent (Fig. 1).
Also, in order to find the optimum mole ratio of ChCl-Phe components, different mixtures of ChCl:Phe including 1:1, 1:2: 1:3, and 1:4 were prepared and used as extraction solvents. Based on the obtained results, the mole ratio of 1:2 (ChCl:Phe) provides maximum RP for determination of Cr3+ ions.
Effect of volume of extraction solvent
In order to optimize the volume of extraction solvent (ChCl:Phe) in VA-LPME and UA-LPME methods, different volumes of extraction solvent in the range of 300–500 µL were tested. The results show that for both microextraction methods, 350 µL of ChCl-Phe provides maximum RP of Cr3+-PAN complex (Fig. 2). The increase of RP by increasing the volume of extraction solvent could be related to the higher surface-to-volume ratio of the extraction solvent in the aqueous solution. Therefore, 350 µL of extraction solvent was considered as the optimum value.
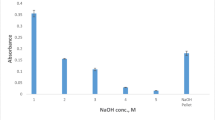
Effect of pH
pH of sample solution is one of the most important parameters affecting the stability of the metal-complexing agent complex; therefore, it has an important effect on the RP of analyte. The effect of pH on the RP of Cr3+-PAN complex was investigated in the range of 5.5–9.5. The results showed that (Fig. 3) pH values higher than 5.5 (in both microextraction methods) have similar effect on the RP of Cr3+-PAN complex. However, at pH values lower than 5.5, the RP decreases, which could be due to the instability of Cr3+-PAN complex in acidic media. Therefore, pH value of 6.5 was selected as the optimum value for both microextraction methods
Volume of THF
THF was used as an aprotic solvent to reduce the solubility of ChCl:Phe extraction solvent in the sample solution. The effect of THF on the RP of Cr3+-PAN complex was investigated by addition of different volumes of THF in the range of 200–600 µL. Based on the obtained results presented in Fig. 4, by increasing the volume of THF, the RP of Cr3+ increases which could be related to the complete aggregation of the extraction solvent until 400 µL THF that reaches to the maximum value for both microextraction methods. Therefore, 400 µL THF was selected as the optimum value.
Amounts of complexing agent
The amounts of PAN, as a complexing agent, are one of the main parameters affecting the RP of Cr3+-PAN complex. By considering the fix amounts of Cr3+, different molar ratios of PAN/Cr3+ in the range of 350, 400, 500, 600, and 700 were examined. The results in Fig. 5 show that, by increasing the molar ratio of PAN/Cr3+, the RP improves and reaches to the maximum value at the molar ratio 500 PAN/Cr3+ for both microextraction methods. However, further increases in PAN/Cr3+ molar ratio have no significant effect on the RP of Cr3+. Therefore, the molar ratio of 500 PAN/Cr3+ was selected as optimum value.
Extraction time
Extraction time is also one of the significant parameters affecting the RP of analytes. It should be optimized critically to ensure that the equilibrium conditions between acceptor (extraction solvent) and donor (aqueous) phases being established. In VA-LPME, the vortex stream of solution besides its vibrating effect causes to accelerate of the extraction of Cr3+-PAN complex into the fine droplets of DESs. However, in UA-LPME method, the ultrasonic radiation causes to disperse of DES solvent into the nanosized droplets. Therefore, it could be expected that the extraction recovery of Cr3+-PAN complex in both microextraction techniques reaches to the maximum value in a short period of time. Figure 6 shows the RP of Cr3+-PAN complex at different extraction times for both microextraction methods. As it can be seen, 1.5 min extraction time is adequate to obtain maximum RP of Cr3+ for both methods. Therefore, 1.5 min extraction time was selected as the optimum value.
Effect of ionic strength
Ionic strength of the sample solution has two effects on the RP of the analyte. (1) salting out effect which related to the increasing of the analyte activity due to the engagement of water molecules around salt ions, therefore it causes to the increasing of RP and (2) salting in effect which causes to the decreasing of dispersion of the extraction solvent in the aqueous solution, therefore it causes to the decreasing of RP. The effect of ionic strength was examined in the concentration range of 0–1% (g mL−1) KCl. Based on the obtained results (data not shown), no significant changes were observed in the RP of Cr3+-PAN complex in this concentration range.
Effect of interfering ion
In order to test the effect of other ions on the RP of Cr3+, different cations and anions were added to the sample solution containing fix amounts of Cr3+ and subjected to the microextraction procedure. The results are presented in Table 1. As it can be seen, the proposed method has acceptable tolerance limit (ratio of the concentration of interfering ion to the analyte) for determination of Cr3+ in the presence of different cations and anions.
Analytical figures of merit
Under the optimum conditions, the analytical figures of merit were calculated. The calibration curve was linear in the range of 1.5–375.0 ng mL−1 Cr3+ with a correlation coefficient of 0.9937. The equation of the calibration curve was A = 0.0011CCr3+ + 0.0055, where CCr3+ is the concentration of Cr3+ in µg L−1 and A is the absorbance of sample solution. The limit of detection (LOD) based on three times of the standard deviation of the blank (n = 7) divided to the slope of the calibration curve after preconcentration step was 0.4 ng mL−1 Cr3+. Also, the relative standard deviation (RSD) for seven replicate analyses of 50.0 µg L−1 Cr3+ is 3.6%. For both microextraction methods, the preconcentration factor (PF) which could be calculated by the volume of donor phase (10 mL) to the acceptor phase (0.2 mL) is equal to 50. However, the enhancement factor (EF) which could be calculated by the ratio of the slope of the calibration curve after preconcentration step to that before preconcentration step is equal to 21 and 24 for VA-LPME and UA-LPME techniques, respectively. Also, the extraction recovery which calculated by dividing of EF to PF (EF/PF) for VA-LPME and UA-LPME techniques is 52% and 60%, respectively. Therefore, UA-LPME technique was used for speciation of chromium in different real samples.
Analysis of real sample
The proposed UA-LPME technique was used for determination of Cr species in different real samples. In order to check the validity of the obtained results, spike tests were also performed on the samples. Also, a certified reference material CRM-TMDW water sample containing 20 µg L−1 Cr was examined for its chromium content and based on the obtained results was 19.5 ± 0.4 µg L−1 (Student’s t test, df = 4, 95% confident limit). As it can be seen in Tables 2 and 3, the proposed method could successfully determine the chromium species in water and food samples.
Comparison to other techniques
The proposed LPME method was compared to the other microextraction techniques, and the results are presented in Table 4. Based on the results, the main advantages of the proposed UA-LPME method are high rapidity (1.5 min extraction time), green approach, low cost, wide linear range, low RSD (3.4%), and low LOD value. Therefore, it could be considered as an efficient microextraction method for determination of low amounts of chromium in real samples.
Conclusion
In this paper, the efficiency of two microextraction procedures, vortex-assisted liquid-phase microextraction (VA-LPME) and ultrasound-assisted liquid-phase microextraction (UA-LPME), was examined for the chromium speciation and determination using FAAS. 1-(2-Pyridylazo)-2-naphthol (PAN) as a chelating agent forms the hydrophobic complex with Cr3+, and choline chloride–phenol was used as a green extraction solvent. After optimization of the critical parameters affecting the extraction recovery of analyte, including pH of sample solution, amounts of complexing agent, volume of extraction solvent, volume of THF, and extraction time, the obtained results show that both microextraction methods have short analysis time and high efficiency for determination of Cr3+. However, UA-LPME method provides better extraction recovery (ER = 60%). Also, the proposed UA-LPME has advantages such as wide linear range, low detection limit, high efficiency, short analysis time, easy to operate, and green aspect (biodegradable extraction solvent) which makes it a superior analytical method for speciation and determination of chromium in real samples.
References
Q. Song, J. Li, Waste Manag. 34, 2587 (2014)
K. Rehman, F. Fatima, I. Waheed, M.S.H. Akash, J. Cell. Biochem. 119, 157 (2018)
V.S. Protsenko, F.I. Danilov, Clean Technol. Environ. Policy 16, 1201 (2014)
J. Ma, S. Ni, J. Zhang, X. Yang, L. Zhang, Electrochim. Acta 176, 1420 (2015)
A.N. Filippin, M. Rawlence, A. Wäckerlin, T. Feurer, T. Zund, K. Kravchyk, M.V. Kovalenko, Y.E. Romanyuk, A.N. Tiwari, S. Buecheler, RSC Adv. 7, 26960 (2017)
L. Verger, O. Dargaud, M. Chasse, N. Trcera, G. Rousse, L. Cormier, J. Cult. Herit. 30, 26 (2018)
L.A. MacAdams, G.P. Buffone, C.D. Incarvito, A.L. Rheingold, K.H. Theopold, J. Am. Chem. Soc. 127, 1082 (2005)
M.P. Mc Daniel, Adv. Catal. 53, 123 (2010)
X. Liang, Y. Zhong, H. He, P. Yuan, J. Zhu, S. Zhu, Z. Jiang, Chem. Eng. J. 191, 177 (2012)
M. Zhu, I.E. Wachs, Catal. Today 311, 2 (2018)
D. Bagchi, S.J. Stohs, B.W. Downs, M. Bagchi, H.G. Preuss, Toxicology 180, 5 (2002)
A. Akhtar, A.I. Afridi, T.G. Kazi, F.N. Talpur, S.S. Arain, J.H. Baig, N. Khan, M. Khan, M. Bilal, Biol. Trace Elem. Res. 175, 312 (2017)
M. Tuzen, M. Soylak, J. Hazard. Mater. 129, 266 (2006)
I. Narin, A. Kars, M. Soylak, J. Hazard. Mater. 150, 453 (2008)
D.M. Adriá-Cerezo, M. Llobat-Estellés, A.R. Maurı́-Aucejo, Talanta 51, 531 (2000)
J. Chwastowska, W. Skwara, E. Sterlińska, L. Pszonicki, Talanta 66, 1345 (2005)
C. Zeng, Y. Lin, N. Zhou, J. Zheng, W. Zhang, J. Hazard. Mater. 237–238, 365 (2012)
P. Hemmatkhah, A. Bidari, S. Jafarvand, M.R. Milani Hosseini, Y. Assadi, Microchim. Acta 166, 69 (2009)
S.M. Yousefi, F. Shemirani, J. Hazard. Mater. 254–255, 134 (2013)
S. Wen, J. Wu, X. Zhu, J. Mol. Liq. 180, 59 (2013)
B. Majidi, F. Shemirani, Microchim. Acta 176, 143 (2012)
W. Ahmad, A. Al-Sibaai, A.S. Bashammakh, H. Alwael, M.S. El-Shahawi, RSC Adv. 6, 69492 (2016)
W. Ahmad, A.S. Bashammakh, A.A. Al-Sibaai, H. Alwael, M.S. El-Shahawi, J. Mol. Liq. 224, 1242 (2016)
S. Talpur, T.G. Kazi, H.I. Afridi, F.N. Talpur, S. Nizamani, A. Lashari, A. Akhtar, M. Khan, J. AOAC Int. 101, 883 (2018)
A. Akhtar, T.G. Kazi, H.I. Afridi, M. Khan, M. Bilal, N. Khan, J. Ind. Eng. Chem. 59, 320 (2018)
M. Khan, T.G. Kazi, H.I. Afridi, M. Bilal, A. Akhtar, N. Ullah, S. Khan, S. Talpur, Ultrason. Sonochem. 39, 313 (2017)
A. Shishov, A. Bulatov, M. Locatelli, S. Carradori, V. Andruch, Microchem. J. 135, 33 (2017)
C. Florindo, A.J.S. McIntosh, T. Welton, L.C. Branco, I.M. Marrucho, Phys. Chem. Chem. Phys. 20, 206 (2018)
Q. Zhang, K. De Oliveira Vigier, S. Royer, F. Jérôme, Chem. Soc. Rev. 41, 7108 (2012)
M. Chamsaz, A. Atarodi, M. Eftekhari, S. Asadpour, M. Adibi, J. Adv. Res. 4, 35 (2013)
M. Eftekhari, M. Chamsaz, M.H. Arbab-Zavar, A. Eftekhari, Environ. Monit. Assess. 187, 4129 (2015)
M. Chamsaz, M. Eftekhari, A. Eftekhari, A. Yekkebashi, Environ. Monit. Assess. 185, 9067 (2013)
A. Akhtar, T.G. Kazi, H.I. Afridi, S.G. Musharraf, F.N. Talpur, N. Khan, M. Bilal, M. Khan, Environ. Sci. Pollut. Res. 23, 25288 (2016)
N. Altunay, E. Yıldırım, R. Gürkan, Food Chem. 245, 586 (2018)
L. Boutorabi, M. Rajabi, M. Barzegar, A. Asghari, Microchem. J. 132, 378 (2017)
E. Kazemi, A.M. Haji Shabani, S. Dadfarnia, F. Izadi, Int. J. Environ. Anal. Chem. 97, 1080 (2017)
H.R. Sobhi, E. Azadikhah, M. Behbahani, A. Esrafili, M. Ghambarian, Spectrochim. Acta Part A Mol. Biomol. Spect. 202, 36 (2018)
S. Sadeghi, A.Z. Moghaddam, RSC Adv. 5, 60621 (2015)
E. Yilmaz, M. Soylak, Talanta 160, 680 (2016)
C. Zeng, Y. Lin, N. Zhou, J. Zheng, W. Zhang, J. Hazad. Mater. 237–238, 365 (2012)
Acknowledgements
All sources of funding for this research including the data collection, analysis, and interpretation, as well as manuscript writing, were supported by all authors. The authors declared that there is no conflict of interest and wish to thank Semnan University for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fasihi, M., Rajabi, M., Barfi, B. et al. Deep eutectic-based vortex-assisted/ultrasound-assisted liquid-phase microextractions of chromium species. J IRAN CHEM SOC 17, 1705–1713 (2020). https://doi.org/10.1007/s13738-020-01890-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01890-6