Abstract
In this study, a switchable solvent was used to preconcentrate trace amounts of Cd from aqueous solution for its determination by flame atomic absorption spectrometry (FAAS). Protonation of N,N-dimethylbenzylamine by dry ice (solid CO2) made it water soluble, and addition of sodium hydroxide converted it back to its original nonionic state for phase separation and subsequent extraction of Cd. A slotted quartz tube (SQT) was attached to the flame burner head to increase the residence time of Cd atoms in the light path. Under the optimum conditions, limits of detection and quantification were determined as 0.7 and 2.6 μg L−1, respectively. Low relative standard deviations calculated from seven replicate measurements of the lowest concentration indicated high precision. Accuracy of the developed method was checked by using a standard reference material (SRM 1633c). Spiked recovery tests were also performed on lake water and wastewater samples at different concentrations to check the applicability of the developed method, and the results obtained (90–103%) established high recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are several anthropogenic activities such as mining, improper waste disposal, industrial discharge, accidental chemical spillage, and fossil fuel consumption that have led to contaminated environmental resources, and these in turn enter the human body to cause health disorders (Hughes et al. 2015; Akkaya et al. 2017). Cd is an example of a toxic metal which has the potential to cause cancers of the lungs, liver, and kidneys (Jancic and Stosic 2014). Even at trace levels, it has the potential to accumulate in some organs over time and its adverse effects manifest upon reaching a threshold limit (Newbigging et al. 2015). The International Agency for Research on Cancer has classified Cd as a group 1 carcinogen (IARC 1993). Drinking water is one of the common modes through which humans are exposed to contaminants, and the World Health Organization (WHO) and Environmental Protection Agency (EPA) have therefore set a maximum allowable Cd limit in drinking water at 3.0 and 5.0 μg L−1, respectively (Fırat et al. 2017). This therefore calls for sensitive and accurate analytical methods to quantify Cd at trace levels.
The conventional flame atomic absorption spectrometer has been widely used for the determination of many metals due to its robustness and durability. It has relatively low operational cost and does not require high level of expertise to be operated (Miller-Ihli and Baker 2017). The down side of the FAAS is low sensitivity for most metals due to poor nebulization efficiency of sample to the flame (Wu et al. 2009). Other atomic absorption techniques such as hydride generation (HGAAS), graphite furnace (GFAAS), thermospray flame furnace (TS-FF-AAS), and photochemical vapor generation (PVG-AAS) provide higher sensitivities, but they are relatively expensive and need high level of expertise (Corazza and Tarley 2016; Zhong et al. 2016; Büyükpınar et al. 2017; Čundeva et al. 2003){Stafilov, 2001 #25}. Inductively coupled plasma techniques with mass spectrometer (ICP-MS) and optical emission spectrometry (ICP-OES) offer simultaneous determination of multi-elements, and ICP-MS has an added advantage of high sensitive for a wide range of elements (Søndergaard et al. 2015; Amorello et al. 2016).
In place of buying sensitive but expensive instrument(s), preconcentration methods and basic modifications are coupled to FAAS to lower the detection limits (Büyükpınar et al. 2017). Microextraction methods such as nondispersive ionic liquid microextraction (NDILME), solid-phase microextraction (SPME), single drop microextraction (SDME), dispersive liquid-liquid microextraction (DLLME), solidified floating organic drop microextraction (SFODME), and cloud point extraction (CPE) have been used to preconcentrate Cd and other organics/inorganics to get lower detection limits (Moreda-Piñeiro and Moreda-Piñeiro 2015; Al-Saidi and Emara 2014; Heydari 2014; Borkowska-Burnecka et al. 2010; Naeemullah et al. 2014). Recent studies have reported the use of peculiar solvents, whose physical properties (polarity) can be changed to switch the solvent from water-immiscible to water-miscible state for the extraction of analytes in aqueous medium (Memon et al. 2017). This switching phenomenon is achieved with the addition of dry ice (solid CO2) to an immiscible mixture of water and low polarity amines to obtain a single phase upon protonation of amine into a hydrophilic bicarbonate form. After extraction, the addition of an acid or base, or inert gases with heating causes the removal of CO2 and a subsequent reversion back to the non-polar amine and separation of phases (Soylak et al. 2016).
Despite the buildup of analyte amounts by extraction methods, the rapid exit of atoms from the flame causes less interaction with source radiation and as such, maximum absorbance is not obtained. A basic component that can be attached to the burner head to increase atom dwell time in the light path is a slotted quartz tube (SQT) (Akkaya et al. 2017). The hollow tube confines the flame within its walls and aspirated sample aerosol directly entering the tube is atomized in the confined space. Depending on the type of metal, absorbance signal could be increased between 2 and 5 times using this simple apparatus (Bakirdere et al. 2011). SQTs can also be used to trap atoms for a certain period of time, and the trapped atoms released all at once to obtain a sharp analytical signal (Titretir et al. 2008; Bakirdere et al. 2011).
This study was aimed at developing a sensitive and accurate analytical method for trace determination of Cd using slotted quartz tube to increase the residence time of Cd atoms in the FAAS system after preconcentration by switchable liquid-liquid microextraction.
Materials and methods
Apparatus
A hollow quartz tube (16 mm i.d, 18 mm o.d.) was custom cut with two slots to fit the burner head of a flame atomic absorption spectrometer (Analytik Jena NovAA 300). The entrance slot was cut at a length of 5.5 cm to ensure that flame from the burner head with a 5.0 cm length fully entered the slotted quartz tube, while the exit slot was cut at a length of 3.0 cm. An air/acetylene mixture was used to produce the flame for atomization. A Cd hollow cathode lamp operating at 2.0 mA and spectral bandpass of 0.50 nm was used to determine Cd absorbances at the analytical line of 228.8 nm. A deuterium lamp (D2) was utilized for background correction.
Chemicals and reagents
Analytical grade reagents were used throughout the study. Aliquots from a 1000 mg L−1 Cd standard stock solution (High Purity Standards) were diluted to prepare working standard solutions. Deionized water for dilutions, sample preparations, and cleaning purposes was obtained from a Milli Q Reference Ultrapure Water Purification System. Buffer solutions were prepared by using dilute hydrochloric acid and sodium hydroxide to adjust primary salts (potassium hydrogen phthalate, potassium dihydrogen phosphate, disodium hydrogen phosphate) all obtained from Merck (Germany). Stock solution of diphenylcarbazone (DPC) (Riedel-de Haen) was prepared in methanol and used for the formation of Cd complexes. N,N-Dimethylbenzylamine and nitric acid were also obtained from Merck, and dry ice (solid CO2) was purchased from an ice cream and pastry café (Mado) in İstanbul, Turkey. A coal fly ash standard reference material (SRM 1633c) was obtained from the National Institute of Science and Technology (NIST).
Samples
Wastewater was taken into polypropylene plastic bottles from an Advanced Biological Wastewater Treatment plant in İstanbul. The wastewater was stored at 4.0 °C and filtered through 125 mm filter paper and 0.45 μm regenerative cellulose syringe filter. Water was sampled from Hazar Lake (Anatolian region of Turkey) by washing the polypropylene plastic bottle a few times with the lake water before filling it to the brim.
Switchable solvent synthesis
The switchable solvent was prepared using water and N,N-dimethylbenzylamine in a 1:1 v/v ratio (125 mL of each into an Erlenmeyer flask) for which two separate phases were initially observed. Under constant stirring, 18–20 g of dry ice was added intermittently to the two-phase system resulting in the formation of a cloudy upper phase solution. The cloudiness signifies protonation of N,N-dimethylbenzylamine. The addition of small amounts of dry ice was continued until a clear and distinct single-phase system was observed. Stirring was continued for an extra 30 min after observing the single phase to allow complete protonation.
Switchable liquid-liquid microextraction process
Cd-DPC complex was formed by successively adding 0.50 mL of pH 10 buffer solution and 1.0 mL ligand solution (0.05% w/v) to 8.0 mL aqueous sample/standard. The resulting solution was slightly shaken by hand to obtain an evenly distributed reddish-orange complex solution. Switchable solvent (1.0 mL) was injected into the complex solution, after which 2.0 mL of 1.0 M NaOH was added. Phase separation was facilitated by 2.0-min centrifugation at 3461g. The upper organic phase containing extracted Cd was transferred into a clean tube, and 150 μL conc. nitric acid (65%) was used to dissolve the Cd complex before aspirating to the FAAS system for absorbance measurements.
Results and discussion
The optimization process was started with 300 μg L−1 aqueous Cd standard solutions, but it was later reduced to 100 μg L−1 as the absorbance signals increased. One experimental parameter was optimized by maintaining a fixed variable for the other parameters. Optimum variables were selected according to the highest average absorbance obtained from triplicate measurements. Optimum conditions for the complexation of Cd with diphenylcarbazone was taken from the study performed by our research group and applied in this study (Fırat et al. 2017).
Switchable solvent volume
The switchable solvent was prepared in bulk for the entire optimization process and stored in the fridge at 4.0 °C when not in use. The optimum volume of switchable solvent required to efficiently extract the Cd-DPC complex was determined by injecting 1.0, 2.0, 3.0, and 3.5 mL into aqueous standard solutions. Prior to each study, it was ensured that the switchable solvent was in its single-phase state. Sodium hydroxide (3.0 M) was added to the extracted solutions for the reversible conversion of amine and phase separation. The absorbance signals recorded decreased significantly from 1.0 to 2.0 mL (≈ 70% difference), but the decrease became marginal at 3.0 and 3.5 mL. An increase in the final volume of upper amine phase with increasing extraction solvent volume suggested dilution of analyte after separation. Extraction volume of 1.0 mL had the lowest upper phase volume and a corresponding high absorbance value. For this reason, 0.50 mL extraction solvent volume was also tested for its extraction efficiency, but the upper amine phase resulting from this test was not easily distinguished from the aqueous solution. Collection of this low volume upper phase was not achieved and as such, further optimizations were performed using 1.0 mL switchable solvent.
Sodium hydroxide concentration
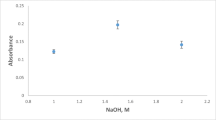
The separation of phases after the extraction process was carried out using sodium hydroxide. The hydroxyl group of sodium hydroxide attacks the protonated sites of the amine to convert it back into the immiscible hydrophobic compound. Thus, the Cd complex is extracted from the aqueous solution and concentrated into the low-volume upper amine phase. The effect of sodium hydroxide concentrations were therefore investigated by testing 2.0 mL each of 0.50, 1.0, 2.0, 3.0, 4.0, and 5.0 M concentrations. A similar extraction was performed by directly placing a pellet of sodium hydroxide into the aqueous standard/switchable solution mixture and vortexed till complete dissolution. Added test (0.50 M) did not result in a distinct phase separation; 1.0 M resulted in a low upper volume while the higher concentrations recorded marginally higher upper phase volumes. The absorbance signal for 1.0 M was over two times higher than the signal for 2.0 M, with the higher concentrations recording lower signals as shown in Fig. 1. The pellet added test produced a signal higher than the concentrations between 2.0 and 5.0 M, but it was significantly lower than 1.0 M. The average weight of sodium hydroxide pellets used was 0.187 g and its concentration in the final extraction medium after dissolution was approximately 0.37 M.
Mixing type and mixing period
After the addition of switchable solvent to sample/standard solution, 5.0 s of vortexing was applied to facilitate its distribution throughout the aqueous medium. The addition of sodium hydroxide for deprotonation resulted in a cloudy solution and four types of mixing were performed to determine the best distribution efficiency for sodium hydroxide. Vortex, mechanical shaker, ultrasonication, and hand shaking were carried out for 1.0 min, and the highest absorbance signal was recorded for the vortex-assisted (VA) mixing. The absorbance values of three replicate measurements were very close to each other indicating high precision for mixing by vortex. The other mixing types recorded absorbance values slightly lower than the value of vortex, but hand shaking recorded the lowest absorbance and the highest percent relative standard deviation, indicating low precision. The optimum vortex mixing period was then determined by testing 0.25, 0.50, 1.0, and 2.0 min. A decreasing trend was observed for increasing mixing periods, but the precision was very consistent (RSD ≈ 4%) for all the mixing periods. The optimum vortexing period used for further studies was therefore set at 15 s.
Analytical performance of systems under optimum conditions
The optimum experimental parameters determined in this study are presented in Table 1, and these conditions were used to determine the analytical figures of merit for all systems given in Table 2. Calibration plots were developed for all systems over wide concentration ranges from which linear dynamic ranges were determined with regression coefficient values greater than 0.9996. The lowest concentration in each calibration plot was selected based on the signal being at least three times higher than the background noise. Seven replicate measurements of the lowest concentrations were recorded and their standard deviations (SDs) were calculated. The calculated standard deviations together with slope (m) from the linear calibration plots were used to determine the detection (LOD) and quantification (LOQ) limits by the expressions:
Improvement in detection limits with respect to the conventional FAAS was then calculated based on the LOD values determined. The increasing order of enhancement was approximately 4.0-, 9.0-, and 45-fold for SQT-FAAS, VA-SLLME-FAAS, and VA-SLLME-SQT-FAAS, respectively. The highest enhancement (45-fold) gave a 0.68 μg L−1 detection limit, which is comparable to limits recorded using cloud point extraction (0.44 μg L−1) (Golbedaghi et al. 2012) and task-specific ionic liquid-based dispersive liquid-liquid microextraction (1.2 μg L−1) (Mohamadi and Mostafavi 2011). The low percent relative standard deviation (%RSD) values calculated established the precision of both instrumental measurements and extraction procedure. The accuracy of the method was validated using a standard reference material (SRM 1633c), which was digested according to the microwave temperature program reported by Erarpat et al. (Erarpat et al. 2017). Due to the presence of trace and major elements in the reference material, standard addition calibration was used to quantify the amount of cadmium in the digested sample. The experimented value (0.735 ± 0.038 mg kg−1) was in good agreement with the certified value (0.758 ± 0.005 mg kg−1), and this confirmed that the method can be used for accurate quantification of cadmium. The method was then applied to nine water samples collected from both European and Anatolian regions of Turkey including three tap water samples, three lake water samples, one well water sample, and two wastewater samples. Cadmium was not determined in all samples according to the detection limit.
Interference studies
The ligand used in this study is not a selective ligand for only cadmium; thus, it can form coordinate covalent complexes with other metal ions that maybe present in the determination of cadmium. However, the optimum parameters obtained for cadmium could offer a certain degree of selectivity due to the fact that not all metal ions would efficiently form complexes under experimental conditions. To better understand this effect, common metals including Mg, Ca, Pb, and Fe were added to aqueous cadmium standard solutions at three different concentrations (Different analyte/interferant ratios). The interference study was performed with 20 μg L−1 aqueous cadmium standards, and the interferants were added at 20 μg L−1 (× 1), 200 μg L−1 (× 10), and 2.0 mg μg L−1 (× 100) concentrations. The effects of these metals on cadmium absorbance are graphically presented in Fig. 2. The most significant effect was observed for Fe at all three added standard concentrations according to the low percent absorbance recorded. For Pb, the 20 μg L−1 concentration reduced the absorbance signal of cadmium more than the higher concentrations. Ca presented a low interference for Cd (91–97%) next to Mg which presented the lowest interference (97–103). Interference is major problem in elemental analysis but methods such as matrix matching and standard addition can be used to overcome matrix related interferences.
Recovery tests
Three spiked concentrations within the linear calibration plot of the optimum analytical method (VA-SLLME-SQT-FAAS) were tested on lake water and wastewater to determine the applicability and accuracy of the method to real samples. Even though there were no visible particulates in the lake water sample, it was filtered consecutively through a 125-mm filter paper and 0.45-μm syringe filter, similarly to wastewater. Triplicate blank determinations were made for each sample, and Cd was not detected according to the detection limit of this method. The samples were then spiked to final concentrations of 10, 20, and 30 μg L−1. Calibration standards were prepared in deionized water and used to determine the percent recovery of the samples as presented in Table 3. The recovery results for the lake water sample at the three concentrations were close to 100%, indicating compatibility of the method and little to no interference from the sample matrix. Wastewater is usually characterized by a very complex matrix which hinders the determination of most analytes. In order to improve upon analyte recovery and obtain accurate quantitation, methods such as standard addition and matrix matching are used to overcome matrix effects. But, recovery results for wastewater in this study were appreciably high enough for a reliable quantification of Cd when present in wastewater. There was no need to use standard addition and matrix matching experiments to get high recovery results.
Conclusions
In this study, a simple and efficient analytical method was developed for the determination of Cd at trace levels in complex matrices. Slotted quartz tube was used to increase the dwell time of Cd atoms in the light source path, after concentrating into measurable amounts by switchable liquid-liquid microextraction. The miscibility of the extraction solvent with water was facilitated by protonation with dry ice and reverted to the immiscible state by deprotonation with an optimum amount of sodium hydroxide. Optimized parameters were used to obtain a very low detection limit, and the repeatability of the entire method was established by low percent relative standard deviation values. The method was also applicable over a wide concentration range. The accuracy and applicability of the method to real samples was tested by spiking experiments for lake and wastewater samples at different concentrations, and the results obtained were satisfactory, which was the indication of no matrix effect on analyte signal. The method presented in this study is simple, economical, high yielding, and environmentally friendly.
References
Akkaya, E., Chormey, D. S., & Bakırdere, S. (2017). Sensitive determination of cadmium using solidified floating organic drop microextraction-slotted quartz tube-flame atomic absorption spectroscopy. Environmental Monitoring and Assessment, 189(10), 513. https://doi.org/10.1007/s10661-017-6232-8.
Al-Saidi, H. M., & Emara, A. A. A. (2014). The recent developments in dispersive liquid–liquid microextraction for preconcentration and determination of inorganic analytes. Journal of Saudi Chemical Society, 18(6), 745–761. https://doi.org/10.1016/j.jscs.2011.11.005.
Amorello, D., Barreca, S., Bruno, M., Milia, A., Orecchio, S., & Pettignano, A. (2016). Chemical characterization of ancient liturgical vestment (chasuble) by inductively coupled plasma–optical emission spectrometry (ICP–OES). Microchemical Journal, 129(Supplement C), 305–309, https://doi.org/10.1016/j.microc.2016.07.011.
Bakirdere, S., Aydin, F., Bakirdere, E. G., Titretir, S., Akdeniz, İ., Aydin, I., Yildirim, E., & Arslan, Y. (2011). From mg/kg to pg/kg levels: A story of trace element determination: A review. Applied Spectroscopy Reviews, 46(1), 38–66. https://doi.org/10.1080/05704928.2010.520179.
Borkowska-Burnecka, J., Szymczycha-Madeja, A., & Żyrnicki, W. (2010). Determination of toxic and other trace elements in calcium-rich materials using cloud point extraction and inductively coupled plasma emission spectrometry. Journal of Hazardous Materials, 182(1), 477–483. https://doi.org/10.1016/j.jhazmat.2010.06.057.
Büyükpınar, Ç., Maltepe, E., Chormey, D. S., San, N., & Bakırdere, S. (2017). Determination of nickel in water and soil samples at trace levels using photochemical vapor generation-batch type ultrasonication assisted gas liquid separator-atomic absorption spectrometry. Microchemical Journal, 132(Supplement C), 167–171. https://doi.org/10.1016/j.microc.2017.01.024.
Corazza, M. Z., & Tarley, C. R. T. (2016). Development and feasibility of emulsion breaking method for the extraction of cadmium from omega-3 dietary supplements and determination by flow injection TS-FF-AAS. Microchemical Journal, 127(Supplement C), 145–151. https://doi.org/10.1016/j.microc.2016.02.021.
Čundeva, K., Stafilov, T., Pavlovska, G., Karadjova, I., & Arpadjan, S. (2003). Preconcentration procedures for trace cadmium determination in natural aqueous systems prior to zeeman etaas. International Journal of Environmental Analytical Chemistry, 83(12), 1009–1019. https://doi.org/10.1080/03067310310001621051.
Erarpat, S., Özzeybek, G., Chormey, D. S., & Bakırdere, S. (2017). Determination of lead at trace levels in mussel and sea water samples using vortex assisted dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Chemosphere, 189, 180–185. https://doi.org/10.1016/j.chemosphere.2017.09.072.
Fırat, M., Bakırdere, S., Fındıkoğlu, M. S., Kafa, E. B., Yazıcı, E., Yolcu, M., Büyükpınar, Ç., Chormey, D. S., Sel, S., & Turak, F. (2017). Determination of trace amount of cadmium using dispersive liquid-liquid microextraction-slotted quartz tube-flame atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 129(Supplement C), 37–41. https://doi.org/10.1016/j.sab.2017.01.006.
Golbedaghi, R., Jafari, S., Yaftian, M. R., Azadbakht, R., Salehzadeh, S., & Jaleh, B. (2012). Determination of cadmium(II) ion by atomic absorption spectrometry after cloud point extraction. Journal of the Iranian Chemical Society, 9(3), 251–256. https://doi.org/10.1007/s13738-011-0018-7.
Heydari, S. (2014). Extraction and microextraction techniques for the determination of compounds from saffron.
Hughes, D. J., Shimmield, T. M., Black, K. D., & Howe, J. A. (2015). Ecological impacts of large-scale disposal of mining waste in the deep sea. Scientific Reports, 5, 9985. https://doi.org/10.1038/srep09985.
IARC. (1993). Cadmium and cadmium compounds. International Agency for Research on Cancer Monographs, 58, 1993119–1993237.
Jancic, S. A., & Stosic, B. Z. (2014). Chapter fourteen—Cadmium effects on the thyroid gland. In G. Litwack (Ed.), Vitamins & Hormones (Vol. 94, pp. 391–425): Academic Press.
Memon, Z. M., Yilmaz, E., & Soylak, M. (2017). Switchable solvent based green liquid phase microextraction method for cobalt in tobacco and food samples prior to flame atomic absorption spectrometric determination. Journal of Molecular Liquids, 229(Supplement C), 459–464. https://doi.org/10.1016/j.molliq.2016.12.098.
Miller-Ihli, N. J., & Baker, S. A. (2017). Atomic spectroscopy, food and dairy products applications of A2 Lindon, John C. In G. E. Tranter, & D. W. Koppenaal (Eds.), Encyclopedia of Spectroscopy and Spectrometry (Third Edition) (pp. 82–88). Oxford: Academic Press.
Mohamadi, M., & Mostafavi, A. (2011). Flame atomic absorption determination of trace amounts of cadmium after preconcentration using a thiol-containing task-specific ionic liquid (Vol. 94).
Moreda-Piñeiro, J., & Moreda-Piñeiro, A. (2015). Recent advances in combining microextraction techniques for sample pre-treatment (Vol. 71).
Naeemullah, K., T, G., Tuzen, M., Shah, F., Afridi, H. I., & Citak, D. (2014). Development of a new green non-dispersive ionic liquid microextraction method in a narrow glass column for determination of cadmium prior to couple with graphite furnace atomic absorption spectrometry. Analytica Chimica Acta, 812, 59–64. https://doi.org/10.1016/j.aca.2013.12.034.
Newbigging, A. M., Yan, X., & Le, X. C. (2015). Cadmium in soybeans and the relevance to human exposure. Journal of Environmental Sciences, 37(Supplement C), 157–162. https://doi.org/10.1016/j.jes.2015.09.001.
Søndergaard, J., Asmund, G., & Larsen, M. M. (2015). Trace elements determination in seawater by ICP-MS with on-line pre-concentration on a Chelex-100 column using a ‘standard’ instrument setup. MethodsX, 2(Supplement C), 323–330. https://doi.org/10.1016/j.mex.2015.06.003.
Soylak, M., Khan, M., & Yilmaz, E. (2016). Switchable solvent based liquid phase microextraction of uranium in environmental samples: A green approach. Analytical Methods, 8(5), 979–986. https://doi.org/10.1039/C5AY02631H.
Titretir, S., Kendüzler, E., Arslan, Y., Kula, İ., Bakırdere, S., & Ataman, O. Y. (2008). Determination of antimony by using tungsten trap atomic absorption spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 63(8), 875–879. https://doi.org/10.1016/j.sab.2008.03.021.
Wu, P., He, S., Luo, B., & Hou, X. (2009). Flame furnace atomic absorption spectrometry: A review. Applied Spectroscopy Reviews, 44(5), 411–437. https://doi.org/10.1080/05704920903018585.
Zhong, W.-S., Ren, T., & Zhao, L.-J. (2016). Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. Journal of Food and Drug Analysis, 24(1), 46–55. https://doi.org/10.1016/j.jfda.2015.04.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
• VA-SLLME-SQT-FAAS was used for the first time in this study.
• Slotted quartz tube was used to further enhance the sensitivity of the FAAS system.
• A very low detection limit was obtained (0.68 ng mL−1).
• Appreciable recoveries were obtained for lake and waste water samples.
• A simple, economical, high yielding, and environmentally friendly method was developed.
Rights and permissions
About this article
Cite this article
Fırat, M., Bodur, S., Tışlı, B. et al. Vortex-assisted switchable liquid-liquid microextraction for the preconcentration of cadmium in environmental samples prior to its determination with flame atomic absorption spectrometry. Environ Monit Assess 190, 393 (2018). https://doi.org/10.1007/s10661-018-6786-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6786-0