Abstract
A very explicate and ligand-less ultrasound-vortex-assisted dispersive liquid–liquid microextraction (US-VA-DLLME) technique has been designed for the pre-concentration and extraction of ultra-trace amounts of cadmium ions, before its determination by graphite furnace atomic absorption spectrophotometry. In the proposed approach, a hydrophobic natural deep eutectic solvent (NADES), prepared from combination of salicylic acid (SA) and l-menthol (M), was studied as both the extraction solvent and the complexing agent for the extraction of cadmium ions. Some significant factors influencing the microextraction performance including pH, volume of DES, sonication time, and extraction temperature were carefully examined and optimized. The calibration curve, obtained under the optimal extraction and instrumental parameters for determination of cadmium, exhibited a wide linearity over the range of 0.001–7.5 µgL−1 (R2 = 0.9953). The proposed method provided low limits of detection (LOD) and quantification (LOQ) of 0.37 × 10−4 and 1.24 × 10−4 µgL−1, respectively. The relative standard deviation (RSD) and pre-concentration factor (PF) were also evaluated as 2.65% and 125, respectively. Finally, the developed US-VA-DLLME technique was favorably applied to the separation and determination of ultra-traces of cadmium in several waters and food samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals enter the environment from natural sources via different types of anthropogenic activities such as mining operation (Taylor et al. 2014), metallurgy (Shao et al. 2013), and electronic industry (Pecht et al. 2004). It is vital to check the level of heavy metals in the nourishment as they can accumulate in environment and living organisms, causing long-term toxic effects (Uluozlu et al. 2009; Mendil et al. 2009). One of these heavy metals is cadmium, which has a carcinogenic effect at low concentrations and is mainly accumulated in human organs including kidney, lung, liver, and reproductive organs. Cd(II) ion has been classified as a group B1 carcinomatous agents by the Environment Protection Agency (EPA). The acceptable value for Cd(II) concentration in drinking water has been set at 3.0 μg L−1 and 5.0 μg L−1 via the World Health Organization (WHO) and EPA, respectively (Chang et al. 2008; Liu et al. 2006; World Health Organization 2004; Waalkes 2000). Cd(II) ion is also considered as one of the main pollutions in plants owing to the use of phosphate fertilizers in husbandry (Kubier and Pichler 2019). In the case of the use of phosphate fertilizers, the agglomeration of cadmium is scaled up exceptionally in plants. Therefore, their consumption can be destructive to human health, through agglomeration into the food chain.
Nowadays, the design of selective and sensitive analytical methods for the determination of Cd(II) ions in various ecological and agricultural specimens is of increasing importance. Several analytical systems have been introduced for the determination of cadmium in complex matrices. These include flame atomic absorption spectrometry (FAAS) (Yilmaz et al. 2016), electrothermal vaporization atomic fluorescence spectrometry (ETVAFS) (Mao et al. 2013), inductively coupled plasma–mass spectrometry (ICP-MS) (Miedico et al. 2015), inductively coupled plasma–optical emission spectrometry (ICP-OES) (Bezerra et al. 2007), and graphite furnace atomic absorption spectrometry (GFAAS) (Shamsipur and Habibollahi 2010). Among these methods, GFAAS is established as a highly efficient technique for determination of concentration of various metal ion species, and is widely used owing to its small scale operational price, high accessibility, and high speed. Nevertheless, the drawback of this technique is its inability to detect ultra-traces of the analytes, because of their minute amounts and matrix interferences. In order to overcome this drawback, different sample preparation strategies have been introduced.
The liquid-phase microextraction method (LPME) is an effective technique for the pre-concentration of the metallic ions of interest and removal of the sample matrices. This technique is time-efficient and has the advantages in terms of miniaturization and simplicity (Shamsipur et al. 2015; Behbahani et al. 2014; Efendioglu et al. 2010). The new branches of LPME method include headspace liquid-phase microextraction (HS-LPME) (Shen and Lee 2003), dispersive liquid–liquid microextraction (DLLME) (Hibila et al. 2016; Pouyan et al. 2016; Zhang et al. 2013; Ragheb et al. 2021), ultrasound-assisted ionic liquid–based dispersive liquid–liquid microextraction (UA-IL-DLLME) (Unsal et al. 2015), vortex-assisted liquid–liquid microextraction (VA-LLME) (Hashemi et al. 2017; Chamsaz et al. 2013), ultrasound-assisted emulsification microextraction (USAEME) (Sereshti et al. 2012), and supramolecular solvent–based liquid-phase microextraction (Zohrabi et al. 2016; Jafarvand and Shemirani 2011).
In the late advances in liquid–liquid microextraction methods, it is also critical to select eco-friendly, low cost, and non-toxic extraction solvents for the improved pre-concentration of the pollutants in different specimens (Ali et al. 2020). In recent years, ionic liquids (ILs) received significant attention as environment-friendly alternatives to the conventional organic solvents, mainly due to their such excellent physicochemical characteristics as structural design-ability, insignificant vapor pressure, and high thermal stability, which lead to more efficient separations with less energy consumption (Han and Row 2010; Anderson et al. 2006). However, most of the ionic liquids possess several imperfections, including indigent biodegradability, high price, and toxicity. Thus, in order to get free of the disadvantages of ILs, the application of deep eutectic solvents (DESs) as a modern group of green and sustainable solvents have been developed (Zhang et al. 2012). These solvents are composed of several ingredients, specifically hydrogen bond donors (HBD) and hydrogen bond acceptors (HBA), which interacting by intramolecular hydrogen bonds to form liquids with melting points lower than those of their singular ingredients.
The DESs possessed various advantages such as less toxicity, more biodegradability and reduced ecological impacts, compare to the ILs. Furthermore, the DESs are not difficult to provide, are greatly biocompatible, and have low-prices (Vilková and Płotka-Wasylka 2020; Smith et al. 2014). Currently, DESs have been favorably exerted to such diverse fields of research as organic synthesis (Niakan et al. 2021), CO2 absorption (Lin et al. 2021), electrochemistry (Li et al. 2020), and nanotechnology (Chen et al. 2021). Additionally, these solvents supply a variety of bonding abilities like hydrogen bonding, π–π interactions, and anion interchange with the analytes, which supply them a large capacity as clean solvents for the extraction and separation processes (Karimi et al. 2015; Yilmaz and Soylak 2015; Shahrezaei et al. 2020).
Cadmium (Cd) is a common and lateen toxic ecological pollutant, which originating chiefly from swift industrial operations, the overuse of fertilizers, composts and sewage sediments, and municipal activities (Shirani et al. 2019; Waalkes 2000). The essential goal of this study was the design of a powerful, green, and highly sensitive ligand-less ultrasound-vortex-assisted dispersive liquid–liquid microextraction (US-VA-DLLME) method for the separation and pre-concentration of cadmium ions from real samples. In this work, for the first time, a hydrophobic NADES composed of salicylic acid and l-menthol was used as an efficient extraction solvent and as a proper extraction agent in a novel microextraction procedure for the selective separation of trace magnitudes of cadmium ions. It is worth mentioning that salicylic acid (SA) is an everywhere plant phenolic combination that possesses a widespread effect on the growth and tolerance of rice seedlings to Cd stress. Therefore, SA addition to Cd contaminated soil can reduce toxicity in the rice cultivation (Majumdar et al. 2020; Liu et al. 2016). Thus, in this work, we used salicylic acid as a hydrogen bond donor and l-menthol as a hydrogen bond acceptor for the preparation of a novel deep eutectic solvent for successful ligand-less extraction of Cd(II) ion from different samples. To the best of our knowledge, there is no report on the utilization of a natural deep eutectic solvent based on salicylic acid and l-menthol for the microextraction of cadmium ion, in the literature.
Various experimental parameters, which potentially affecting the extraction process were carried out in the suggested procedure, in detail, and the analytical figures of merits for the suggested technique were investigated and compared with other methods. Also, the proposed methodology enabled us to monitoring the low concentration levels of Cd(II) ions in water, cereal products, and fruit samples, with excellent statistical consequences.
Materials and Methods
Reagents and Materials
Salicylic acid (≥ 99.0%), l-menthol (≥ 99.0%), Cd(NO3)2 (≥ 99.9%), and Pd(NO3)2 (≥ 99.9%) were purchased from Sigma-Aldrich (USA) and Merck (Germany). The stock solutions of Cd(II) were unlimbered by the dilution of a certified standard solution of 1000 mg L−1 (Merck, Darmstadt, Germany). The operative standards were prepared by subsequent dilutions of stocks solution with ultra-pure water (resistivity > 18 MΩ cm). The pH was adjusted by using acetate buffer.
Apparatus
Cadmium determination was performed with an atomic absorption spectrophotometer M5-Thermo series (Thermo, USA) equipped with an Analyst 95 graphite furnace and a deuterium (D2) background correction. The spectral band-pass and operating current of the cadmium hollow cathode lamp were regulated at 0.50 nm and 7.0 mA, respectively. The wavelength for analysis of Cd was set at 228.8 nm. Pd(NO3)2 was used as a matrix modifier and transferred to furnace by means of an auto sampler (AS-95). Highly pure argon gas with a flow speed of 200 mL min−1 was employed. A model 692 digital pH meter (Metrohm, Herisau, Switzerland) supplied by a glass electrode was applied for pH regulations. The digestion of solid specimen was attained using a closed vessel microwave apparatus (Milestone ETHOS UP, Sorisole BG, Italy). The operating conditions of microwave were adjusted, as suggested by the manufacturer.
The water content of the prepared DES was found to be lower than 0.01%, as examined by the Karl–Fischer titration method (720-KSS-Metrohm Herisau, Switzerland). FT-IR spectra were recorded with a Bruker PS-15 spectrometer (Germany), over the limits of 4000–400 cm‒1. 1H and 13C NMR spectra were taken on a Bruker SP-400 Avance spectrometer (Germany). The thermal properties of the DES were measured by differential scanning calorimetry (DSC) instrument (Mettler Toledo Instrument Model DSC 822 e, Switzerland), purging with nitrogen, over a temperature limit of − 40 to 40 °C with a heating rate of 10 °C min−1. Weight changes of NADES were obtained by (Mettler Toledo Instrument Model TGA/SDTA 851 e, Switzerland) in an open aluminum pan and heated at a rate of 10 °C/min from 50 to 700 °C, with a nitrogen purge. The information on speed of sound and density were examined using a mercantile sound and density velocity meter (Anton Paar DSA 5000 densitometer and a rate of sound analyzer) at a frequency of 3 MHz. The temperature was established to ± 0.01 K using a Peltier instrument. The degassed dual distilled water and dehydrated air were applied to calibrate the equipment at 298.15 K. The estimated precision was ± 5 × 10−6 g cm−3 and ± 0.5 m s−1 for density and velocity of sound, respectively.
Preparation of Hydrophobic Natural Deep Eutectic Solvent
There are a variety of hydrogen bond acceptors and hydrogen bond donors which can be applied to the production a different of DESs (Zhang et al. 2012; Han and Row 2010; Anderson et al. 2006). The hydrophobic natural deep eutectic solvent used in this work was first reported by our research group (Abri et al. 2019). Generally, a mixture of l-menthol crystal and salicylic acid powder, in a 4:1 molar proportion, was warmed at 40 °C for 30 min to appear a clear solution. When a homogenous water-immiscible solution was formed, the stirring was slap down and the mixture was permitted to cool down. The water content of the prepared NADES was found to be lower than 0.01%. Finally, the purity and structure of the prepared NADES were studied by the FT-IR and NMR spectroscopies.
General Procedure for VE-UA-DLLME
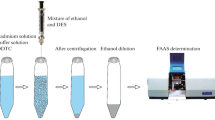
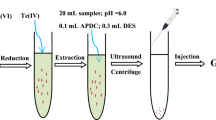
In this work, 200 µL of the l-menthol-salicylic acid NADES as a green hydrophobic extraction solvent was rapidly injected into 25 mL of a standard solution including 1.0 μg L−1 of Cd(II) and 1.0 mL acetate buffer of pH 6.0, and then vortexed for 5 min. A water/NADES cloudy solution was then formed in the extraction vessel. In this stage, the Cd(II) from the aqueous sample was extracted into the fine droplets of NADES. The mixture was then put into an ultrasonic bath at 40 °C for 5 min and the NADES aggregates were gradually fragmented into nano-sized species under ultra-sonication process. Then, 20 μL of the extracted higher thin layer was taken, and together with 10 μL of Pd(NO3)2 modifier solution, were injected into GFAAS.
Sample Collection and Pretreatment
Different water and food samples were collected from Kurdistan Province, Iran. The collected water samples were passed through a filter paper, in order to efface any impurity materials, and were kept at 8 °C, until analysis. The other samples were cleaned by deionized water and, after drying at room temperature, they were digested by a microwave system.
Microwave-assisted Digestion of Agricultural Samples
The microwave digestion procedure was used to digest several agricultural samples. For effective microwave digestion of samples, a mixture of hydrogen peroxide and nitric acid in an optimal proportion of 1:4 was added to 0.5 g of each specimen and then heated with microwave energy for 15 min. The temperature was risen to 200 °C at 20 bar pressure and 1800 W power. After cooling to ambient temperature, the samples were transferred into different tubes and the contents were diluted to 25 mL with deionized water for subsequent cadmium determination.
Results and Discussion
Spectroscopic and Thermal Characterization of the Prepared Natural Deep Eutectic Solvent
The natural deep eutectic solvents have been prosperously used for the pre-concentration of environmental contaminations, essential and nonessential elements, food additives and have received an increasing attention, due to their attributed green chemistry (Zhang et al. 2012; Han and Row 2010; Anderson et al. 2006). In this work, we afforded the application of the abovementioned NADES as extraction solvent for the pre-concentration and separation of Cd(II) ions from rice, wheat, watermelon, and drinking water samples.
The composition and purity of the prepared NADES was first intensely explored using FT-IR and nuclear magnetic resonance (1HNMR, 13CNMR) spectroscopies. To this aim, the FT-IR spectra of salicylic acid:l-menthol at 1:4 ratio eutectic solvent and the specific ingredients were conducted and the results are shown in Fig. 1. As seen, upon mixing of the l-menthol with salicylic acid, the O–H stretching vibration band of l-menthol exhibited a blue shift from 3263 to 3360 cm−1, which can indicate the occurrence of hydrogen bond generation. It should additionally be noted that the blue shift from 1655 to 1668 cm−1 in the carbonyl band and red shift from 3423 to 3360 cm−1 in the O–H stretching vibration band of salicylic acid is also suggesting the generation of hydrogen bonding arrangement between l-menthol and salicylic acid as hydrogen bond acceptor and hydrogen bond donor, respectively.
The purity and configuration of the prepared l-menthol:salicylic acid deep eutectic solvent were studied with 1H and 13C NMR spectroscopies (Fig. 2a and b). As can be seen from Fig. 2, the peaks ascribed to the initial ingredients are obviously comprehended and no additional peaks were found, which indicated that no interaction occurred between the ingredients of the NADES. The thermal behavior of NADES was studied using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) and their curves were illustrated in Figs. 3a and b, respectively. The TGA thermogram reported of the l-menthol as basic component in NADES shows one-step thermal the composition pattern. The decomposition was completed at 115 °C. Therefore, NADES indicates rapid weight loss of ≥ 95% during this process. Based on DTG curve, the maximum thermal degradation temperature of the prepared NADES was determined. The DSC thermogram of salicylic acid showed a sharp endothermic peak at 173 °C. The DSC thermal curve of l-menthol:SA system presented a unique endothermic peak at 22.9 °C. The thermogram of NADES contained no peak from the alpha and beta polymorphs of l-menthol (i.e., ≈ 28 °C and ≈ 33 °C) and salicylic acid. The l-menthol basis NADES also displayed a decline in melting peak, compared to the individual components, which was perhaps owing to asymmetrical system in the NADES, based on mixing of l-menthol and salicylic acid.
Some of the resulting physicochemical properties of the prepared NADES, such as density, speed of sound, and molar mass, are given in Table 1.
Optimization of Experimental Factors
Finally, the experimental factors influencing the efficiency of the analytical method including pH, proportion of deep eutectic solvent, vortex, and ultrasonic times were studied and optimized in detail. The optimization studies were carried out by using 25-mL standard aqueous solutions, containing 1.0 μgL−1 of Cd(II), and all experiments were repeated for three times.
Choice of Extracting Solvent
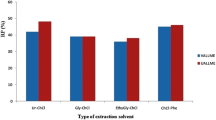
The selection of a suitable extraction solvent is all-significant to improve the extraction efficiency and selectivity. In the current research, the appropriate formulation of the NADES was chosen from several important properties such as low toxicity, hydrophobic nature, and low solubility in aqueous phases and selectivity. Considering these properties, six types of DESs containing choline chloride–ethylene glycol (Chcl-EG), menthol-ethylene glycol (M-EG), methyl tert-butyl ether-ethylene glycol (MTB-EG), choline chloride-urea (Chcl-U), l-menthol-salicylic acid (M-SA), and l-menthol-camphor sulfonic acid (M-CSA) were studied by the adding of 0.5 mL of these DESs to the 1.0 μg L−1 of Cd(II) sample solution. As shown in Fig. 4, among the investigated solvents examined, the best extraction efficiency was obtained with the l-menthol-salicylic NADES. In the combination of l-menthol-salicylic acid, salicylic acid is the ingredient that is complexed with cadmium ion and, subsequently, removed it from the aqueous phase by the NADES used, as it is insoluble in water. Therefore, the l-menthol-salicylic acid NADES was selected as the best extraction solvent for additional analyses.
Effect of pH
The pre-concentration/separation of metallic ions is significantly affected with pH of the sample solution. In addition, pH performs a significant affect in both the chemical stability of DES and its interaction with the metal ions. Hence, the impact of sample pH on the extraction efficiency of cadmium was examined over the range of 3.0–8.0, and the results are displayed in Fig. 5a. As is clearly observed, at low pHs, the absorbance signal and extraction efficiency of the cadmium ion are low. This may be attributed to the interaction of DES with hydronium ions rather than the Cd(II) ions. The extraction efficiency and sensitivity increased with increasing of pH, until reaching a maximum at pH 6.0. However, upon further increase in the solution pH, a decrease was observed in the analytical signal and the extraction efficiency, most presumably owing to the hydrolysis of cadmium ion in basic solutions. As it can be seen, the highest extraction efficiency of cadmium was attained at pH 6.0, when an acetate buffer was used. Thus, a solution of pH 6.0 was selected for further studies.
A Effect of solution pH on pre-concentration of Cd(II) ions by US-VA-DLLME–GFAAS (conditions: volume of extraction solvent, 200 µL; sample volume, 25.0 mL; Cd(II), 1.0 μg L−1; sonication time, 5 min). B Effect of volume of DES on pre-concentration of Cd(II) ions by US-VA-DLLME–GFAAS (conditions: pH, 6.0; sample volume, 25.0 mL; Cd(II), 1.0 μg L−1; sonication time, 5 min). C Effect of temperature on pre-concentration of Cd(II) ions by US-VA-DLLME–GFAAS (conditions: pH, 6.0; extraction solvent, 200 µL; sample volume, 25.0 mL; Cd(II), 1.0 μg L−1; sonication time, 5 min). D Effect ultrasonic time on pre-concentration of Cd(II) ions in US-VA-DLLME–GFAAS (conditions: pH, 6.0; volume of extraction solvent, 200 µL; sample volume, 25.0 mL, Cd(II): 1.0 μg L−1)
Effect of Volume of NADES
In order to achieve an appropriate pre-concentration factor, high extraction efficiency, and also a minimum toxicity to the environment, the volume of extraction solvent was studied. The results for the influence of the NADES solvent on the extraction of Cd(II) ion was examined over the limits of 50–350 μL and the results are shown in Fig. 5b. It is clearly seen that the highest absorbance signal was recorded for 200 μL. With further addition in volume of the extraction solvent, no noteworthy difference in absorbance signal and percentage recovery of cadmium ion was observed. Thus, a volume of 200 μL of DES was selected as the best solvent volume for furthermore studies.
Effect of Temperature
It is well known that the temperature plays a significant role in increasing kinetics and performance of the extraction processes, via its influence on the viscosity of extraction solvent and the diffusion coefficients of analytes. At low temperatures, the viscosity of DES is high and, consequently, the diffusion rate of the analyte and dispersion of the extraction solvent is low. It is expected that both the diffusion coefficients and mass transfer rates enhance at higher temperatures. Thus, the change of temperature was investigated between 25 (room temperature) and 70 °C, and the results are given in Fig. 5c. It is seen that the extraction efficiency increased gradually with increasing temperature up to 40 °C and then decreased with further increase in temperature, most probably due to the increased solubility of the analyte in the aqueous phase, compared with that in NADES. Hence, a temperature of 40 °C was chosen for furthermore studies.
Effects of Ultra-sonication and Extraction Time
The dispersion of extraction solvent and the mass transfer phenomena are largely influenced by the ultra-sonication time. The impact of this parameter on extraction efficiency was evaluated over the limit of 1–7 min, under unchanged ultrasound energy. From the results shown in Fig. 5d, it can be seen that a linear increase in analytical signal was obtained as the ultra-sonication time was enlarged up to 5 min and then persisted unchanged. The observed increase in analytical signal with increasing of the sonication time may be due to the great contact region among the aqueous phase and the extraction solvent which provides a better mass transfer and higher extraction efficiency. Based on this observation, a sonication time of 5 min was selected for further studies.
The extraction time is also another significant parameter that can influence the efficiency of extraction process. In this study, the extraction time was interpreted as the time interval between injection of NADES solvent into the aqueous solution and the appearance of phase separation. It should be noted that, following the formation of a cloudy solution, the surface region among fine droplets of extraction solvent and aqueous sample solution is indefinitely great and, accordingly, the mass transfer from the aqueous solution to the extraction solvent is very fast. Hence, a quick equilibrium state is established and the extraction time is acutely small. This is a remarkable benefit of the technique used in this work.
Analytical Figures of Merit
The operation analysis of the proposed US-VA-DLLME technique was evaluated under optimum experimental conditions. A linear calibration curve was achieved ranging from 0.001 to 7.5 μg L−1, with a correlation coefficient (R2) of 0.9953. The precision of the method (expressed as RSD%), was investigated over six replicate spikes at a cadmium ion concentration of 0.1 μg L−1, and it was found to be 2.65%, indicating a quite good precision for the proposed technique. The limit of detection (LOD) and limit of quantification (LOQ) were computed with 3Sb/m and 10Sb/m equations, respectively, where Sb is the standard deviation for five repetitive analyses of the blank solutions and m is the slope of calibration curve after pre-concentration with the proposed procedure. The resulting LOD and LOQ were then estimated as 0.37 × 10−4 µgL−1 and 1.24 × 10−4 µgL−1, respectively. The pre-concentration factor (PF), which represents the ratio of volumes of the aqueous sample solution (25 mL) and the organic extraction solvent (200 µL), was found to be 125 for determination of Cd(II) ion.
Interference Study
After the optimization of the experimental parameters, the interference effects of several anions, cations, and some trace metal ions on the determination of Cd(II) ions were analyzed. The tolerance limit was established as the amount of added ions causing less than 5% relative error in the analytical signal. The results shown in Table 2 clearly indicated the pre-concentration of cadmium ion was not obviously affected by any of the foreign ions. It is quite obvious that the proposed technique possesses a high selectivity for Cd(II) ions and is, therefore, appropriate for the analysis of Cd(II) ion within the complex matrices.
Analysis of Real Samples
To demonstrate the utility of the technique in actual samples, it was used to the quantitation of Cd(II) ions in rice, wheat, watermelon, and drinking water samples. The standard addition method was used to assess the matrix effects. For this purpose, all real samples were spiked with standard solution of Cd(II) ions at various levels. The obtained results for the prediction of the concentrations of Cd(II) ions are shown in Table 3. The good extraction recoveries of spiked samples and high precision obtained indicated the fact that the matrix of real samples has no measureable impress on the extraction efficiency of the proposed method.
Conclusion
In this research, an efficient, green, and inexpensive ligand-less ultrasound-vortex-assisted liquid-phase microextraction procedure based on the use of a novel deep eutectic solvent coupled with GFAAS has been established for the extraction and determination of traces of Cd(II) ions. The deep eutectic solvents are biodegradable and environment-friendly solvents that have attracted a broad attention of analytical researchers because of their essential specifications. In this study, a hydrophobic natural deep eutectic solvent composed of salicylic acid and l-menthol (at 1:4 molar proportion) was used as both the complexing agent and the extraction solvent for the microextraction of Cd(II) ions. The solvent is provided from inexpensive raw materials without any significant instrument and difficult process. A comparison of the analytical performances achieved from the present technique and the previously reported techniques for the extraction and determination of Cd(II) ions (Ali et al. 2020; Sorouraddin et al. 2020; Altunay et al. 2019; Unutkan et al. 2019; Yilmaz and Soylak 2015; Jalbani and Soylak 2014; Chamsaz et al. 2013; Zeng et al. 2012; Karim-Nezhad et al. 2011; Mahpishanian and Shemirani 2010; Li et al. 2009; Ma et al. 2009) is presented in Table 4. As it is seen, the proposed US-VA-DLLME method possesses good analytical performance, low detection limit, and suitable precision with a wide linear range, as compared to other techniques. The chief benefits of the suggested technique are simplicity, rapidity, superior sensitivity, and low usage of reagents. Importantly, the present method does not require any chelating agent for the separation of cadmium ions from different actual samples, including drinking water, cereal products (wheat and rice), and watermelon.
Data Availability
Data will be available on reasonable request.
References
Abri A, Babajani N, Moshtaghi Zonouz A, Shekaari H (2019) Spectral and thermophysical properties of some novel deep eutectic solvent based on l-menthol and their mixtures with ethanol. J Mol Liq 285:477–487. https://doi.org/10.1016/j.molliq.2019.04.001
Ali J, Tuzen M, Kazid TG (2020) Green and innovative technique develop for the determination of vanadium in different types of water and food samples by eutectic solvent extraction method. Food Chem 306:125638. https://doi.org/10.1016/j.foodchem.2019.125638
Altunay N, Elik A, Gürkan R (2019) Monitoring of some trace metals in honeys by flame atomic absorption spectrometry after using natural deep eutectic solvent. Microchem J 147:49–59. https://doi.org/10.1016/j.microc.2019.03.003
Anderson JL, Armstrong DW, Wei GT (2006) Ionic liquids in analytical chemistry. Anal Chem 78:2892–2902. https://doi.org/10.1021/ac069394o
Behbahani M, Esrafili A, Bagheri S, Radfar S, Bojdi MK, Bagheri A (2014) Modified nanoporous carbon as a novel sorbent before solvent-based de-emulsification dispersive liquid–liquid microextraction for ultra-trace detection of cadmium by flame atomic absorption spectrophotometry. Measurement 51:174–181. https://doi.org/10.1016/j.measurement.2014.02.010
Bezerra MA, Nascimento MSM, Oliveira EP, Carvalho MDFB, Santelli RE (2007) Internal standardization for the determination of cadmium, cobalt, chromium and manganese in saline produced water from petroleum industry by inductively coupled plasma optical emission spectrometry after cloud point extraction. Spectrochim Acta Part B 62:985–991. https://doi.org/10.1016/j.sab.2007.06.009
Chamsaz M, Atarodi A, Eftekhari M, Asadpour S, Adibi M (2013) Vortex-assisted ionic liquid microextraction coupled to flame atomic absorption spectrometry for determination of trace levels of cadmium in real samples. J Adv Res 4:35–41. https://doi.org/10.1016/j.jare.2011.12.002
Chang X, Luo H, Cui Y, Zhu XG, Zhai Y, Hu Z, He Q (2008) ICP-OES determination of trace metal ions after pre-concentration by 4-(8-hydroxy-5-quinolylazo) naphthalenesulfonic acid modified silica gel. J Mol Struct 891:45–49. https://doi.org/10.1016/j.molstruc.2008.02.037
Chen J, Wei X, Tang H, Claude Munyemana J, Guan M, Zhang S, Qiu H (2021) Deep eutectic solvents-assisted synthesis of ZnCo2O4 nanosheets as peroxidase-like nanozyme and its application in colorimetric logic gate. Talanta 222:121680–121687. https://doi.org/10.1016/j.talanta.2020.121680
Efendioglu A, Asci MY, Bati B (2010) Preconcentration of Cu (II), Cd (II) and Pb (II) on Amberlite XAD-4 resin6 (3) functionalized with N, N′-Bis (o-vanillinidene)-ethylenediamine and their determination by FAAS in water samples. Anal Sci 26:1283–1288. https://doi.org/10.2116/analsci.26.1283
Habila MA, Yilmaz E, AlOthman ZA, Soylak M (2016) Combination of dispersive liquid liquid microextraction and multivariate optimization for separation enrichment of traces lead by flame atomic absorption spectrometry. J Ind Eng Chem 37:306–311. https://doi.org/10.1016/j.jiec.2016.03.037
Han D, Row KH (2010) Recent applications of ionic liquids in separation technology. Molecules 15:2405–2426. https://doi.org/10.3390/molecules15042405
Hashemi M, Zohrabi P, Torkejokar M (2017) Forced vortex assisted liquid phase microextraction for preconcentration and spectrophotometric determination of mefenamic acid in biological samples. Sep Purif Technol 176:126–133. https://doi.org/10.1016/j.seppur.2016.11.073
Jafarvand S, Shemirani F (2011) Supramolecular-based dispersive liquid-liquid microextraction: a novel sample preparation technique utilizes coacervates and reverse micelles. J Sep Sci 34:455–461. https://doi.org/10.1002/jssc.201000630
Jalbani N, Soylak M (2014) Ligandless surfactant mediated solid phase extraction combined with Fe3O4 nano-particle for the preconcentration and determination of cadmium and lead in water and soil samples followed by flame atomic absorption spectrometry: Multivariate strategy. Ecotoxicol Environ Saf 102:174–178. https://doi.org/10.1016/j.ecoenv.2013.11.018
Karimi M, Dadfarnia S, Shabani AMH, Tamaddon F, Azadi D (2015) Deep eutectic liquid organic salt as a new solvent for liquid-phase microextraction and its application in ligandless extraction and preconcentraion of lead and cadmium in edible oils. Talanta 144:648–654. https://doi.org/10.1016/j.talanta.2015.07.021
Karim-Nezhad G, Ahmadi M, Zare-Dizajdizi B (2011) Background corrected dispersive liquid-liquid microextraction of cadmium combined with flame atomic absorption spectrometry. J Braz Chem Soc 22:1816–1822. https://doi.org/10.1590/S0103-50532011000900026
Kubier A, Pichler T (2019) Cadmium in groundwater−a synopsis based on a large hydrogeochemical data set. Sci Total Environ 689:831–842. https://doi.org/10.1016/j.scitotenv.2019.06.499
Li R, Gao Q, Dong Q, Luo C, Sheng L, Liang J (2020) Template-free electrodeposition of ultra-high adhesive superhydrophobic Zn/Zn stearate coating with ordered hierarchical structure from deep eutectiv solvent. Surf Coat Technol 403:126267–126276. https://doi.org/10.1016/j.surfcoat.2020.126267
Li S, Cai S, Hu W, Chen H, Liu H (2009) Ionic liquid-based ultrasound-assisted dispersive liquid–liquid microextraction combined with electrothermal atomic absorption spectrometry for a sensitive determination of cadmium in water samples. Spectrochim Acta B 64:666–671. https://doi.org/10.1016/j.sab.2009.05.023
Lin H, Gong K, Hykys P, Chen D, Ying W, Sofer Z, Yan Y, Li Z, Peng X (2021) Nanoconfined deep eutectic solvent in laminated MXene for efficient CO2 separation. Chem Eng J 405:126961–126971. https://doi.org/10.1016/j.cej.2020.126961
Liu YW, Chang X, Guo Y, Meng SM (2006) Biosorption and preconcentration of lead and cadmium on waste Chinese herb Pang Da Hai. J Hazard Mater 135:389–394. https://doi.org/10.1016/j.jhazmat.2005.11.078
Liu Z, Ding Y, Wang F, Ye Y, Zhu C (2016) Role of salicylic acid in resistance to cadmium stress in plants. Plant Cell Rep 35:719–731. https://doi.org/10.1007/s00299-015-1925-3
Ma JJ, Du X, Zhang JW, Li JC, Wang LZ (2009) Ultrasound-assisted emulsification-microextraction combined with flame atomic absorption spectrometry for determination of trace cadmium in water samples. Talanta 80:980–984. https://doi.org/10.1016/j.talanta.2009.08.029
Mahpishanian S, Shemirani F (2010) Preconcentration procedure using in situ solvent formation microextraction in the presence of ionic liquid for cadmium determination in saline samples by flame atomic absorption spectrometry. Talanta 82:471–476. https://doi.org/10.1016/j.talanta.2010.04.060
Majumdar S, Sachdev S, Kundu R (2020) Salicylic acid mediated reduction in grain cadmium accumulation and amelioration of toxicity in Oryza sativa L. cv Bandana. Ecotoxicol Environ Saf 205:111167–111178. https://doi.org/10.1016/j.ecoenv.2020.111167
Mao X, Liu J, Huang Y, Feng L, Zhang L, Tang X, Wang M (2013) Assessment of homogeneity and minimum sample mass for cadmium analysis in powdered certified reference materials and real rice samples by solid sampling electrothermal vaporization atomic fluorescence spectrometry. J Agric Food Chem 61:848–853. https://doi.org/10.1021/jf3045473
Mendil D, Uluözlü OD, Tüzen M, Soylak M (2009) Investigation of the levels of some element in edible oil samples produced in Turkey by atomic absorption spectrometry. J Hazard Mater 165:724–728. https://doi.org/10.1016/j.jhazmat.2008.10.046
Miedico O, Iammarino M, Pompa C, Tarallo M, Chiaravalle AE (2015) Assessment of lead, cadmium and mercury in seafood marketed in Puglia and Basilicata (Italy) by inductively coupled plasma mass spectrometry. Food Addit Contam B 8:85–92. https://doi.org/10.1080/19393210.2014.989281
Niakan M, Masteri-Farahani M, Shekaari H, Karimi S (2021) Pd supported on clicked cellulose-modified magnetite-graphene oxide nanocomposite for C-C coupling reactions in deep eutectic solvent. Carbohydr Polym 251:117109–117118. https://doi.org/10.1016/j.carbpol.2020.117109
Pecht M, Fukuda Y, Rajagopal S (2004) The impact of lead-free legislation exemptions on the electronics industry. IEEE Trans Electron Pack Manuf 27:221–232. https://doi.org/10.1109/TEPM.2004.843150
Pouyan M, Bagherian G, Goudarzi N (2016) Determination of ultra-trace palladium (II) in water, soil, and food samples by dispersive liquid-liquid microextraction-atomic absorption spectrometry using 2-mercaptobenzimidazole as a complexing agent. Microchem J 127:46–51. https://doi.org/10.1016/j.microc.2016.02.003
Ragheb E, Shamsipur M, Jalali F, Sadeghi M, Babajani N, Mafakheri N (2021) Magnetic solid-phase extraction using metal–organic framework-based biosorbent followed by ligandless deep-eutectic solvent-ultrasounds-assisted dispersive liquid–liquid microextraction (DES-USA-DLLME) for preconcentration of mercury (II). Microchem J 166:106209–106218. https://doi.org/10.1016/j.microc.2021.106209
Sereshti H, Heravi YE, Samadi S (2012) Optimized ultrasound-assisted emulsification microextraction for simultaneous trace multielement determination of heavy metals in real water samples by ICP-OES. Talanta 97:235–241. https://doi.org/10.1016/j.talanta.2012.04.024
Shahrezaei F, Shamsipur M, Gholivand MB, Zohrabi P, Babajani N, Abri A, Moshtaghi Zonouz A, Shekaari H (2020) A highly selective green supported liquid membrane by using a hydrophobic deep eutectic solvent for carrier-less transport of silver ions. Anal Methods 12:4682–4690. https://doi.org/10.1039/D0AY01266A
Shamsipur M, Habibollahi S (2010) A highly sensitive procedure for determination of ultra-trace amounts of molybdenum by graphite furnace atomic absorption spectrometry after dispersive liquid-liquid microextraction. Microchim Acta 171:267–273. https://doi.org/10.1007/s00604-010-0421-2
Shamsipur M, Zohrabi P, Hashemi M (2015) Application of a supramolecular solvent as the carrier for ferrofluid based liquid-phase microextraction for spectrofluorimetric determination of levofloxacin in biological samples. Anal Methods 7:9609–9614. https://doi.org/10.1039/C5AY02330K
Shao X, Cheng H, Li Q, Lin C (2013) Anthropogenic atmospheric emissions of cadmium in China. Atmos Environ 79:155–160. https://doi.org/10.1016/j.atmosenv.2013.05.055
Shen G, Lee HK (2003) Headspace liquid-phase microextraction of chlorobenzenes in soil with gas chromatography-electron capture detection. Anal Chem 75:98–103. https://doi.org/10.1021/ac020428b
Shirani M, Habibollahi S, Akbari A (2019) Centrifuge-less deep eutectic solvent based magnetic nanofluid-linked airagitated liquid–liquid microextraction coupled with electrothermal atomic absorption spectrometry for simultaneous determination of cadmium, lead, copper, and arsenic in food samples and non-alcoholic beverages. Food Chem 281:304–311. https://doi.org/10.1016/j.foodchem.2018.12.110
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Sorouraddin SM, Farajzadeh MA, Okhravi T (2020) Application of deep eutectic solvent as a disperser in reversed-phase dispersive liquid-liquid microextraction for the extraction of Cd(II) and Zn(II) ions from oil samples. J Food Compos Anal 93:103590. https://doi.org/10.1016/j.jfca.2020.103590
Taylor MP, Mould SA, Kristensen LJ, Rouillon M (2014) Environmental arsenic, cadmium and lead dust emissions from metal mine operations: Implications for environmental management, monitoring and human health. Environ Res 135:296–303. https://doi.org/10.1016/j.envres.2014.08.036
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2009) Assessment of trace element contents of chicken products from Turkey. J Hazard Mater 163:982–987. https://doi.org/10.1016/j.jhazmat.2008.07.050
Unsal YE, Soylak M, Tuzen M (2015) Ultrasound-assisted ionic liquid-based dispersive liquid–liquid microextraction for preconcentration of patent blue V and its determination in food samples by UV–visible spectrophotometry. Environ Monit Assess 187:203–211. https://doi.org/10.1007/s10661-015-4427-4
Unutkan T, Tışlı B, Tekin Z, Çetin G, Bakırdere S (2019) Ultrasound assisted deep eutectic solvent based microextraction-slotted quartz tube-flame atomic absorption spectrometry for the determination of cadmium. Spectrochim Acta B 155:1–3. https://doi.org/10.1016/j.sab.2019.03.001
Vilková M, Płotka-Wasylka J (2020) The role of water in deep eutectic solvent-base extraction. J Mol Liq 304:112747. https://doi.org/10.1016/j.molliq.2020.112747
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79:241–244. https://doi.org/10.1016/S0162-0134(00)00009-X
World Health Organization (2004) Guidelines for drinking water quality. Recommendations, Vol. 1, Geneva.
Yilmaz E, Soylak M (2015) Switchable polarity solvent for liquid phase microextraction of Cd (II) as pyrrolidinedithiocarbamate chelates from environmental samples. Anal Chim Acta 886:75–82. https://doi.org/10.1016/j.aca.2015.06.021
Yilmaz E, Ocsoy I, Ozdemir N, Soylak M (2016) Bovine serum albumin-Cu(II) hybrid nanoflowers: an effective adsorbent for solid phase extraction and slurry sampling flame atomic absorption spectrometric analysis of cadmium and lead in water, hair, food and cigarette samples. Anal Chim Acta 906:10–117. https://doi.org/10.1016/j.aca.2015.12.001
Zeng C, Hu Y, Luo J (2012) Ionic liquid-based hollow fiber supported liquid membrane extraction combined with thermospray flame furnace AAS for the determination of cadmium. Microchim Acta 177:53–58. https://doi.org/10.1007/s00604-011-0748-3
Zhang Y, Duan J, He M, Chen B, Hu B (2013) Dispersive liquid liquid microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for the speciation of inorganic selenium in environmental water samples. Talanta 115:730–736. https://doi.org/10.1016/j.talanta.2013.06.040
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146. https://doi.org/10.1039/C2CS35178A
Zohrabi P, Shamsipur M, Hashemi M, Hashemi B (2016) Liquid-phase microextraction of organophosphorus pesticides using supramolecular solvent as a carrier for ferrofluid. Talanta 160:340–346. https://doi.org/10.1016/j.talanta.2016.07.036
Funding
The financial support of this work by Research Council of Razi University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Ethical of the manuscript is approved.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shamsipur, M., Mafakheri, N. & Babajani, N. A Natural Deep Eutectic Solvent–based Ultrasound-Vortex-assisted Dispersive Liquid–Liquid Microextraction Method for Ligand-less Pre-concentration and Determination of Traces of Cadmium Ions in Water and Some Food Samples. Food Anal. Methods 15, 1203–1213 (2022). https://doi.org/10.1007/s12161-021-02222-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02222-x