Abstract
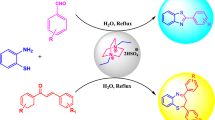
In this study, [H2-DABCO][HSO4]2 is applied as an acidic ionic liquid to exclude some disadvantages of former methods reported for the synthesis of 3-amino-1-aryl-1H-benzo[f]chromene-2-carbonitrile, 1-(benzothiazolylamino)phenylmethyl-2-naphthol, and 1-(benzoimidazolylamino)phenylmethyl-2-naphthol derivatives. In the presence of this catalyst, all reactions are performed under neat conditions during short reaction times in good-to-high yields. Ease of preparation and recyclability of the catalyst are the other important advantages of this method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to global environmental concern and green chemistry roles, its important issue to using less hazardous chemical solvents, reduce waste materials, and apply catalysis in organic reactions. One of the powerful synthetic tools that use to develop preparation of some organic compounds is multi-component reactions (MCRs). The importance of this category of reactions is that they can facilitate synthesis of some important heterocyclic and bioactive compounds in just one straightforward step; moreover, these reactions are in correlation with some green chemistry principles [1]. 2-Amino-4H-chromene, and benzimidazole and benzothiazole derivatives are two important classes of heterocyclic compounds that can be synthesized via this strategy.
Compounds, which possess 2-amino-4H-chromene structure moiety, are important heterocycles because of their remarkable biological and pharmacological properties such as antibacterial [2], anticancer [3,4,5], antimicrobial [6, 7], antiviral [8, 9], and central nervous system (CNS) [10] activities.
Benzimidazole and benzothiazole derivatives are a group of attractive heterocyclic pharmacophores which demonstrate a wide range of biological properties, including antimicrobial, antibacterial, antitumor, anticancer, anti-inflammatory, and analgesic [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] activities. These compounds also are known in drugs designed for targeting DNA and DNA-associated processes [26]. In addition, benzimidazole nucleus exists in some natural bioactive compounds like vitamin B12.
Because of the above-mentioned important properties many of methods have been reported for the synthesis of 2-amino-4H-chromene, benzimidazole and benzothiazole derivatives [27,28,29,30,31,32,33]. These methods are also useful, but most of them suffer from disadvantages such as harsh reaction conditions, waste primary materials, use of toxic solvents, and long reaction conditions.
Nowadays, due to the significant properties of ionic liquids, scientists have developed the applications of these compounds as green solvents and potential catalysts in various fields. Ionic liquids have attracted attentions because that their unique physical properties such as low viscosity, non-volatile, high solubility capability, and ease of preparation made them different from ordinary solvents [34, 35]. In addition, they can be prepared easily from commercially available materials. In this study and in continuation of our research group successive efforts in the synthesis of novel ionic liquid catalysts [36,37,38,39,40,41] and to solve the restrictions which have been observed in the synthesis of 3-amino-1-aryl-1H-benzo[f] chromene-2-carbonitrile, 1-(benzothiazolylamino)phenylmethyl-2-naphthol, and 1-(benzoimidazolylamino)phenylmethyl-2-naphthol derivatives, we wish to report the applicability of our recently introduced acidic DABCO-based ionic liquid [42] in the acceleration of the synthesis of these types of compounds.
Experimental
General
All the chemicals were used in this procedure including aldehydes, 2-naphthol, malononitrile, 2-aminobenzimidazole, and 2-aminobenzothiazole were purchased from Merck Chemical Company (Munich) and were used without further purification. The purity determination of the substrates and reactions was monitored by thin-layer chromatography (TLC) on silica-gel polygram SILG/UV 254 Plates.
DABCO (CAS: 280-57-9, MW: 112.17 g/gmol, assay ≥ 99% w/w %) and sulfuric acid (CAS: 7664-93-9, MW: 98.08 g/gmol, assay ≥ 99.99 v/v %) were purchased from Sigma-Aldrich Company (Mumbai). Both assays were reported by company and were not examined more. All solvents were prepared from Merck Chemical Company (Munich) and were kept in isolated conditions as well to minimize the absorption of atmosphere moisture. Moreover, they had gotten distilled before being used. Products were characterized by their physical constants, comparison with reported in the literature samples and IR and NMR spectroscopy.
Melting points were measured by electrothermal IA9100 melting point apparatus in capillary tubes. The starting temperature of the approximate melting range was input via the keyboard and the melting point range was spotted visually. FT-IR spectra were recorded on a Perkin-Elmer spectrum BX series and KBr pellets were used for solid samples. 1H NMR and 13C NMR spectra were determined on Bruker AV-400 using TMS (0.00 ppm) as internal standard and DMSO-d6 as solvent.
Preparation of 1,4-diazabicyclo[2.2.2]octane-1,4-diium hydrogen sulfate {[H2-DABCO][HSO4]2} [39]
In a 100 mL round-bottomed flask, 1,4-diazabicyclo[2.2.2]octane (DABCO) (1.05 g, 9.4 mmol) was dissolved in 50 mL dry dichloromethane. Then, this mixture was stirred in an ice-bath for 1 min and a stoichiometric amount of sulfuric acid (99.99%, 1 mL, 18.8 mmol) was added drop-wise to it within 25 min. Then, the mixture was heated to reflux with constant stirring for 24 h. In continue, the solvent was decanted and the resulting white solid was frequently washed with diethyl ether (3 × 15 ml2) to obliterate non-ionic residues. After that, the obtained ionic liquid was dried under vacuum to achieve [H2-DABCO][HSO4]2 in 95% yield (2.74 g) (Scheme 1). The purity of the product was determined by melting point, Mass, FT-IR, 1H NMR, and 13C NMR.
Spectral data for [H2-DABCO][HSO4]2: white solid; M.p. 65 °C; MS: m/z = 308(M+); FT-IR (KBr, cm−1) vmax: 3400–2400, 1229, 1165, 849, 576; 1H NMR (400 MHz, DMSO-d6): δ (ppm) 3.59 (s, 6H), 6.64 (s, 1H, NH), 13.88 (s, 1H, HSO4); 13C NMR (100 MHz, DMSO-d6): δ (ppm) 43.4.
General procedure for the synthesis of 3-amino-1-aryl-1H-benzo[f]chromene-2-carbonitrile derivatives
In a 25 mL round-bottom flask, a mixture of the requested aromatic aldehyde 1 (1 mmol), 2-naphthol 2 (1 mmol), malononitrile 3 (1 mmol), and [H2-DABCO][HSO4]2 (0.030 g, 0.097 mmol) was stirred under solvent-free conditions at 100 °C for a certain time. After completion of the reaction, as identified by TLC [ethyl acetate:n-hexane (3:7)], the solid product was filtered and washed with water to separate the catalyst. Then, the precipitate was recrystallized from ethanol to obtain the pure product (5a–5l). The spectral data of the new compound are as follows.
3-Amino-1-(2-fluorophenyl)-1H-benzo[f] chromene-2-carbonitrile (5 l). Mp = 286–288 °C; IR (KBr) νmax/cm−1: 3440, 3342, 3089, 2921, 2180, 1645,1586, 1482, 1410, 1228, 1178, 810, 751; 1H NMR (400 MHz, DMSO-d6) δ = 7.95 (d, J = 9.2 Hz, 1H), 7.93 (d, J = 9.2 Hz, 1H), 7.77 (d, J = 8 Hz, 1H), 7.41–7.51 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 2H), 7.34 (d, J = 9.2 Hz, 1H), 7.16–7.25 (m, 3H), 7.09 (ddd, J1 = 13.4 Hz, J2 = 12 Hz, J3 = 1.2 Hz, 3H), 5.54 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ 160.6, 159.8 (d, 1JCF = 244 Hz), 147.5, 132.4, 132.3, 131.2, 130.5, 130.2, 130.1, 129.3(d, 3JCF = 7 Hz), 129.1, 127.8, 125.4, 123.1, 120.7, 117.2, 116.2 (d, 2JCF = 21 Hz), 114.7, 56.4, 32.9 ppm.
General procedure for the synthesis of 1-(benzothiazolylamino)phenylmethyl-2-naphthol and 1-(benzoimidazolylamino)phenylmethyl-2-naphthol derivatives.
To a mixture of the requested aromatic aldehyde 1 (1 mmol), 2-naphthol 2 (1 mmol) and 2-aminobenzimidazole/or 2-aminobenzothiazole 4 (1 mmol), [H2-DABCO][HSO4]2 (0.030 g, 0.097 mmol) was added, and then, the mixture was stirred at 120 °C for an appropriate time. The progress of the reaction was monitored by TLC [ethyl acetate: n-hexane (3:7)]. After completion of the reaction, the precipitate was filtered and washed with water to separate the catalyst. Finally, the crude products were purified by recrystallization from ethanol to obtain the pure product (6a–6m).
Results and discussion
At the first step and to achieve the optimum reaction conditions, the synthesis of 3-amino-1-(4-chlorophenyl)-1H-benzo[f] chromene-2-carbonitrile (5b) and 1-benzothiazolylamino-(4-chlorophenylmethyl)-2-naphthol (6b) was selected as two model reactions in various conditions such as different amounts of the catalyst [[H2-DABCO][HSO4]2], and various solvents and temperatures. The results are summarized in Table 1. As it is clear from this table, the best yield and time for the synthesis of the compound 5b can be resulted in the presence of 0.030 g of the catalyst, in the absence of solvent at 100 °C, while, in the case of the compound 6b, the best results can be obtained in the presence of 0.030 g of the catalyst, under solvent-free conditions at 120 °C (Scheme 2). As shown in Table 1, for both of the reactions, a trace amount of the product is obtained when the reactions were proceeded in the absence of the catalyst.
After optimization of the reactions conditions and to improve these protocols, different types of aromatic aldehydes with electron-donating and electron-withdrawing functional groups were used for the synthesis of 3-amino-1-aryl-1H-benzo[f] chromene-2-carbonitrile, 1-(benzothiazolylamino)phenylmethyl-2-naphthol, and 1-(benzoimidazolylamino)phenylmethyl-2-naphthol derivatives under the optimized conditions. As represented in Table 2, various aldehydes with both electron-donating and electron-withdrawing substituents are converted to the corresponding products in short times and good-to-excellent yields.
To show the merits of our catalyst activity, in Table 3, the results obtained from the synthesis of 3-amino-1-aryl-1H-benzo[f]chromene-2-carbonitriles and 1-(benzothiazolylamino)phenylmethyl-2-naphthols catalyzed by [H2-DABCO][HSO4]2 are compared with other reported results in the literatures. As shown in this table, this method avoids from some of disadvantages associated with the other procedures such as low yields, long reaction times, hard conditions for the catalyst preparation, using ultrasonic irradiation, and high catalyst loading.
The proposed mechanism for these reactions is shown in Scheme 3. According to this mechanism, at first, carbonyl group of the aldehyde (1) is activated in the presence of [H2-DABCO][HSO4]2. After that, the mechanism is divided into two distinct routes. In route 1, this activated carbonyl group is attacked by nucleophilic compound (a) to give the cyanoolefin (b). In continue, compound (b) is attacked by 2-naphthol (2) to produce Michael-addition product. Then, after cyclization and tautomerization steps, the corresponding product (5a–5l) is produced.
In route 2, the activated carbonyl group can be attacked by 2-naphthol (2), to give compound (d). Then, compound (d) is attacked by 2-aminobenzimidazole/2-aminobenzothiazole (4) to produce compounds 6a–6m.
To investigate the recoverability of the catalyst, the reactions of 3-amino-1-(4-chlorophenyl)-1H-benzo[f] chromene-2-carbonitrile (5b) and 1-benzothiazolylamino-(4-chlorophenylmethyl)-2-naphthol (6b) were studied again. After completion of the reactions, the products were washed with water to remove the catalyst, and then, the filtrate was evaporated under vacuum up to 70 °C to obtain the recovered catalyst. Ultimately, the obtained catalyst was washed with diethyl ether, and dried and reused for the same reactions. This procedure was repeated four times for each reaction and the products were obtained by the recovered catalyst with the least change in the reaction times and yields. The results are demonstrated in Figs. 1 and 2.
Conclusion
In this study, [H2-DABCO][HSO4]2 is used as a novel acidic ionic liquid catalyst in the promotion of multi-component synthesis of 3-amino-1-aryl-1H-benzo[f]chromene-2-carbonitriles, 1-(benzothiazolylamino)phenylmethyl-2-naphthols and 1-(benzoimidazolylamino)phenylmethyl-2-naphthols. The most appealing feature of this catalyst is using accessible and inexpensive materials to synthesize the catalyst. Besides, the presented protocols represent some advantages such as avoiding the use of toxic solvents, ease of preparation of the catalyst, excellent yields, simple work-up, and recyclability of the catalyst. Moreover, the results clearly show that this method can tolerate a wide range of various substituted aldehydes including electron-withdrawing or electron-donating groups.
References
H.G.O. Alvim, E.N. da Silva Júnior, B.A. Neto, RSC Adv. 4, 54282 (2014)
M. Kidwai, S. Saxena, M.K.R. Khan, S.S. Thukral, Bioorg. Med. Chem. Lett. 15, 4295 (2005)
J. Skommer, D. Wlodkowic, M. Mättö, M. Eray, J. Pelkonen, Leuk. Res. Treat. 30, 322 (2006)
J.L. Wang, D. Liu, Z.J. Zhang, S. Shan, X. Han, S.M. Srinivasula, C.M. Croce, E.S. Alnemri, Z. Huang, PNAS. 97, 7124 (2000)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
M.M. Khafagy, A.H.A. El-Wahab, F.A. Eid, A.M. El-Agrody, ІІ Farmaco 57, 715 (2002)
V. Jeso, K.C. Nicolaou, Tetrahedron Lett. 50, 1161 (2009)
P.W. Smith, S.L. Sollis, P.D. Howes, P.C. Cherry, I.D. Starkey, K.N. Cobley, H. Weston, J. Scicinski, A. Merritt, A. Whittington, P. Wyatt, J. Med. Chem. 41, 787 (1998)
A. Martínez-Grau, J. Marco, Bioorg. Med. Chem. Lett. 7, 3165 (1997)
F. Eiden, F. Denk, Arch. Pharm. 324, 353 (1991)
S.O. Podunavac-Kuzmanović, D.M. Cvetković, L.S. Vojinović, Acta Period. Technol. 35, 239 (2004)
Z. Ates-Alagoz, S. Yildiz, E. Buyukbingol, Chemotherapy 53, 110 (2007)
S.O. Podunavac-Kuzmanović, D.M. Cvetković, J. Serb. Chem. Soc. 75, 459 (2007)
N.U. Perisic-Janjic, S.O. Podunavac-Kuzmanovic, J.S. Balaz, D. Vlaovic, J. Planar. Chromatogr. Mod. TLC. 13, 123 (2000)
R.S. Keri, C.K. Rajappa, S.A. Patil, B.M. Nagaraja, Pharmacol. Rep. 68, 1254 (2016)
S.T. Asundaria, K.C. Patel, Pharm. Chem. J. 45, 725 (2012)
B. Soni, M.S. Ranawat, R. Sharma, A. Bhandari, S. Sharma, Eur. J. Med. Chem. 45, 2938 (2010)
A. Gellis, H. Kovacic, N. Boufatah, P. Vanelle, Eur. J. Med. Chem. 43, 1858 (2008)
H.M. Refaat, Eur. J. Med. Chem. 45, 2949 (2010)
K. Starčević, M. Kralj, K. Ester, I. Sabol, M. Grce, K. Pavelić, G. Karminski-Zamola, Bioorg. Med. Chem. 15, 4419 (2007)
S. Saeed, N. Rashid, P.G. Jones, M. Ali, R. Hussain, Eur. J. Med. Chem. 45, 1323 (2010)
D. Havrylyuk, L. Mosula, B. Zimenkovsky, O. Vasylenko, A. Gzella, R. Lesyk, Eur. J. Med. Chem. 45, 5012 (2010)
R. Caputo, M.L. Calabrò, N. Micale, A.D. Schimmer, M. Ali, M. Zappalà, S. Grasso, Med. Chem. Res. 21, 2644 (2012)
D. Evans, T.A. Hicks, W.R.N. Williamson, W. Dawson, S.C.R. Meacock, E.A. Kitchen, Eur. J. med. Chem. 31, 635 (1996)
M. Gaba, D. Singh, S. Singh, V. Sharma, P. Gaba, Eur. J. med. Chem. 45, 2245 (2010)
S. Bhattacharya, P. Chaudhuri, Curr. Med. Chem. 15, 1762 (2008)
M.M. Heravi, K. Bakhtiari, V. Zadsirjan, F.F. Bamoharram, O.M. Heravi, Bioorg. Med. Chem. Lett. 17, 4262 (2007)
M.M. Heravi, T. Hosseinnejad, Z. Faghihi, M. Shiri, M. Vazinfard, J. Iran. Chem. Soc. 14, 823 (2017)
M.T. Maghsoodlou, M. Karima, M. Lashkari, B. Adrom, J. Aboonajmi, J. Iran. Chem. Soc. 14, 329 (2017)
P.K. Sahu, P.K. Sahu, D.D. Agarwal, RSC Adv. 5, 69143 (2015)
S. Sadjadi, M.M. Heravi, V. Zadsirjan, M. Ebrahimizadeh, Res. Chem. Intermed. 43, 5467 (2017)
M. Zendehdel, M.A. Bodaghifard, H. Behyar, Z. Mortezaei, Micropor. Mesopor. Mater. 266, 83 (2018)
F. Tamaddon, D. Azadi, J. Iran. Chem. Soc. 14, 2077 (2017)
T. Welton, Coord. Chem. Rev. 248, 2459 (2004)
C. Chiappe, C.S. Pomelli, Eur. J. Org. Chem. 6120 (2014) (2014)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, J. Mol. Liq. 234, 268 (2017)
F. Shirini, A. Yahyazadeh, K. Mohammadi, Res. Chem. Intermed. 41, 6207 (2015)
F. Shirini, A. Yahyazadeh, K. Mohammadi, N.G. Khaligh, C. R. Chim. 17, 370 (2014)
O. Goli-Jolodar, F. Shirini, M. Seddighi, Chin. J. Catal. 38, 1245 (2017)
O. Goli-Jolodar, F. Shirini, J. Iran. Chem. Soc. 13, 1077 (2016)
F. Shirini, M.S.N. Langarudi, O. Goli-Jolodar, Dyes Pigm. 123, 186 (2015)
F. Shirini, M.S.N. Langarudi, N. Daneshvar, M. Mashhadinezhad, N. Nabinia, J. Mol. Liq. 243, 302 (2017)
A.R. Moosavi-Zare, M.A. Zolfigol, O. Khaledian, V. Khakyzadeh, M.H. Beyzavi, H.G. Kruger, Chem. Eng. J. 248, 122 (2014)
M.G. Dekamin, M. Eslami, A. Maleki, Tetrahedron. 69, 1074 (2013)
B. Sadeghi, I. Zarepour, J. Nanostruct. Chem. 5, 305 (2015)
F.K. Behbahani, S. Maryam, J. Korean. Chem. Soc. 57, 357 (2013)
M. Seddighi, F. Shirini, M. Mamaghani, C. R. Chim. 18, 573 (2015)
M. Yarie, M.A. Zolfigol, S. Baghery, D.A. Alonso, A. Khoshnood, M. Kalhor, Y. Bayat, A. Asgari, New. J. Chem. 41, 4431 (2017)
M.R. Naimi-Jamal, S. Mashkouri, A. Sharifi, Mole. Divers. 14, 473 (2010)
H. Eshghi, S. Damavandi, G.H. Zohuri, Inorg. Met Org. Nano-Met. Chem. 41, 1067 (2011)
S. Balalaie, S. Ramezanpour, M. Bararjanian, J.H. Gross, Synth. Commun. 38, 1078 (2008)
S.M. Baghbanian, N. Rezaei, H. Tashakkorian, Green Chem. 15, 3446 (2013)
M.M. Heravi, B. Baghernejad, H.A. Oskooie, J. Chin. Chem. Soc. 55, 659 (2008)
S. Javanshir, A. Ohanian, M.M. Heravi, M.R. Naimi-Jamal, F.F. Bamoharram, J. Saudi Chem. Soc. 18, 502 (2014)
A. Shaabani, A. Rahmati, E. Farhangi, Tetrahedron Lett. 48, 7291 (2007)
L. Yang, J. Chem. 9, 2424 (2012)
A. Kumar, M.S. Rao, V.K. Rao, Aust. J. Chem. 63, 1538 (2010)
Acknowledgements
We are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nabinia, N., Shirini, F., Tajik, H. et al. An affordable DABCO-based ionic liquid efficiency in the synthesis of 3-amino-1-aryl-1H-benzo[f] chromene-2-carbonitrile, 1-(benzothiazolylamino)phenylmethyl-2-naphthol, and 1-(benzoimidazolylamino)phenylmethyl-2-naphthol derivatives. J IRAN CHEM SOC 15, 2147–2157 (2018). https://doi.org/10.1007/s13738-018-1408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1408-x