Abstract
1,8-Dioxo-octahydro-xanthenes are easily prepared via the condensation of aldehydes with 1,3-cyclohexadione and/or dimedone using N-sulfonated DABCO as a new and efficient catalyst. This reagent is also efficiently able to catalyze the condensation of aldehydes with barbituric acid leading to 5-arylmethylene-pyrimidine-2,4,6-triones. The structure of the products was characterized by their IR, 1H NMR, and 13C NMR spectroscopy. The present methodology offers several advantages such as ease of preparation and handling of the catalyst, high yields, simple and green procedure, low cost, short reaction times, easy work-up, and preformation of the reaction in the absence of solvent or in water as a green solvent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, ionic liquids have received recognition as green media in organic synthesis due to their favorable properties, such as good solvating capability, wide liquid range, negligible vapor pressure, tunable polarity, high thermal stability, and ease of recyclability [1–3]. Although ionic liquids were initially introduced as an alternative green reaction medium, today they have marched far beyond this border, showing their significant role in controlling the reaction as catalysts [4–6]. So, the development and application of so-called “task-specific” ionic liquids are desirable. In addition, the synthesis of task-specific ionic liquids, which have a functional group in their framework, may expand the application of ionic liquids in organic chemistry [7–9]. Recently, ionic liquids have been successfully used in multi-component reactions [8–12].
Xanthenes are an important class of organic compounds that find use as dyes, fluorescent materials for visualization of biomolecules, and in laser technologies due to their useful spectroscopic properties [13–15].
Xanthenes have also received significant attention from many pharmaceutical and organic chemists essentially because of the broad spectrum of their biological and pharmaceutical properties such as their antiviral [16], antibacterial [17], antinociceptive [18], and anti-inflammatory activities [19], as well as their efficiency in photodynamic therapy [20].
Barbituric acids and their derivatives have been widely used as sedative, hypnotic, anesthetic, anticonvulsant, antiosteoporosis, as well as antitumor agents. Arylidene barbituric acids as well as their 2-thio analogues are useful intermediates in the synthesis of heterocyclic compounds, benzyl barbituric derivatives [21], oxadiazaflavines [22], and unsymmetrical disulphides [23]. Additionally, some of them have been recently studied as non-linear optical materials and dyes.
There are several reports in the literature for the synthesis of 1,8-dioxo-octahydroxanthene and 5-arylidene barbituric acids derivatives; these include use of [Hbim]BF4 [24], [bmim]HSO4 [25], p-dodecylbenzenesulphonic acid [26], p-toluenesulfonic acid [27], [TMPSA]HSO4 [28], [Hmim]TFA [29], N-sulfonic acid poly(4-vinylpyridinium) chloride (NSPVPC) [30], succinimide-N-sulfonic acid (SuSA) [31], 1,3-dibromo-5,5-dimethylhydantoin (DBH), benzyltriphenylphosphonium tribromide (BTPTB) [32] (for the synthesis 1,8-dioxo-octahydro-xanthene), nickel nanoparticles [33], sodium p-toluene sulfonate (NaPTSA) [34], basic alumina [35], IR lamp [36] and Ce1MgxZr1−xO2 (CMZO) [37] (for the preparation of 5-arylidene barbituric acids derivatives). However, many of these methods tolerate many disadvantages such as low yields, long reaction times, harsh reaction conditions, tedious work-up, and requirement of excess amounts of reagents or catalysts. Therefore, it is important to find more convenient methods for the synthesis of these types of compounds.
Experimental
Materials
All chemicals were purchased from Merck, Aldrich, and Fluka Chemical Companies and used without further purification. All the products were separated and characterized by their physical constant and comparison with authentic samples. The purity determination of the substrates and reaction monitoring were accompanied by TLC using silica gel SIL G/UV 254 plates.
Characterization techniques
Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes. The FT-IR spectra were recorded a Perkin-Elmer spectrum BX series spectrophotometer. In all the cases, the 1H NMR and 13C NMR spectra were recorded with BrukerAvance 400-MHz instrument. All chemical shifts are quoted in parts per million (ppm) relative to TMS using deuterated solvent.
Catalyst preparation [DABCO](SO3H)2(Cl)2 [38]
A round-bottomed flask (100 ml) was charged with a solution of 1,4-diazabicyclo[2]octane, DABCO (0.56 g, 5 mmol) in dry CH2Cl2 (50 ml), and then chlorosulfonic acid (1.21 g, 10.4 mmol) was added dropwise over a period of 10 min at room temperature. After the addition was completed, the reaction mixture was left 1 h. In this period of time, a white solid was produced. Afterward, the CH2Cl2 was decanted. The residue was triturated with dry diethyl ether and dried under vacuum to give [DABCO](SO3H)2(Cl)2 as a very viscous colorless oil at 98 % yield.
Spectral data of [DABCO](SO3H)2Cl2: 1HNMR (400 MHz, DMSO-d6): δ (ppm) 3.58 (s, 6H), 7.72 (s, 1H); 13CNMR (100 MHz, DMSO-d6): δ (ppm) 43.2; MS: m/z = 345 (M+). IR (KBr, cm−1) ν max: 3,500-2,800 (broad), 1,179, 881, 850.
Hammett acidity
An efficient method for evaluating the acidity of a Brønsted acidic ionic liquid is the Hammett acidity. The Hammett function is defined as:
where the pK (I)aq is the pK a value of aqueous solution of indicator, [IH+]s and [I]s are the molar concentrations of protonated and unprotonated forms of the indicator in the solvent, respectively. According to Lambert–Beer’s Law, the value of [I]s/[IH+]s can be determined and calculated through UV–visible spectrum.
For this reason, 4-nitroaniline (pK(I)aq = 0.99) as the basic indicator and CCl4 as the solvent were chosen. As can be seen in Fig. 1, the maximal absorbance of the unprotonated form of the indicator was observed at 328 nm in CCl4. When [DABCO](HSO3)2(Cl)2 as the ionic liquid catalyst was added to the indicator solution, the absorbance of the unprotonated form of the indicator decreased, which indicated that the indicator was partially in the form of [IH+]. These results, which are listed in Table 1, show the acidity strength of [DABCO](HSO3)2(Cl)2.
General procedure for the preparation of 5-arylmethylene-pyrimidine-2,4,6-trione derivatives
A mixture of the aldehyde (1 mmol), barbituric acid (1 mmol) and [DABCO](SO3H)2Cl2 (0.025 mmol, 0.009 g) in water (4 ml) was heated in an oil bath (70 °C). After completion of the reaction, as monitored by TLC, using n-hexane:EtOAc (1:4) as the eluent, the crude product was filtered off, washed with water, and recrystallized from ethanol to give a pure compound. The spectral data of the new compounds are as follow:
5,5′-(1,4-Phenylenebis(methanylylidene))bis(pyrimidine-2,4,6(1H,3H,5H)-trione) (Table 4, entry 15)
M.p. > 300 °C; FT-IR(KBr) ν = 3,495, 3,202, 3,085, 1,749, 1,685, 1,584, 808; 1HNMR (400 MHz, DMSO-d 6 ) (δ, ppm) = 8.05 (s, 4H), 8.29 (s, 2H, HC = C), 11.30 (NH, s, 2H), 11.45 (NH, s, 2H); 13CNMR (400 MHz, DMSO-d 6) (δ, ppm) = 120.9, 129.0, 132.3, 136.1, 150.6, 153.4, 161.9, 163.6.
5,5′-(1,3-Phenylenebis(methanylylidene))bis(pyrimidine-2,4,6(1H,3H,5H)-trione) (Table 4, entry 16)
M.p. > 300 °C; FT-IR(KBr) ν = 3,457, 3,201, 3,074, 1,757, 1,682, 1,571, 800, 682; 1HNMR (400 MHz, DMSO-d 6) (δ, ppm) = 7.55 (t, J = 7.6, 1H), 8.20 (dd, J 1 = 7.8, J 2 = 1.6, 2H), 8.29 (s, 2H, HC=C), 8.49 (s, 1H), 11.29 (NH, s, 2H), 11.44 (NH, s, 2H); 13CNMR (400 MHz, DMSO-d 6) (δ, ppm) = 120.3, 128.0, 132.9, 135.9, 137.6, 150.6, 153.9, 161.9, 163.6.
5-(2-Nitrobenzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione (Table 4, entry 17)
M.p. 276–278 °C; FT-IR(KBr) ν = 3,325, 3,197, 3,091, 1,735, 168, 1,572, 1,513, 1,345, 733; 1HNMR (400 MHz, DMSO-d6) (δ, ppm) = 7.58 (d, J = 7.6, 1H), 7.68 (dt, J 1 = 8, J 2 = 0.8, 1H), 7.79 (dt, J 1 = 7.6, J 2 = 1.2, 1H), 8.24 (dd, J 1 = 8, J 2 = 1.2, 1H), 8.61 (s, 1H, HC=C), 11.26 (NH, s, 1H), 11.51(NH, s, 1H); 13CNMR (400 MHz, DMSO-d6) (δ, ppm) = 120.9, 124.5, 130.6, 130.8, 132.1, 134.2, 146.7, 150.7, 152.9, 161.6, 162.8.
General procedure for the preparation of 1,8-dioxo-octahydro-xanthene derivatives
A mixture of dimedone and/or 1,3-cyclohexadione (2 mmol), aldehyde (1 mmol) and [DABCO](SO3H)2Cl2 (34 mg, 0.1 mmol) was stirred in an oil bath (80 °C) under solvent-free conditions. After completion of the reaction, as monitored by TLC, using n-hexane:EtOAc (5:1) as the eluent, water was added to separate the catalyst and the crude product was separated and recrystallized by EtOH (4 ml) to give the pure product.
Results and discussion
N-Sulfonic acids are efficient and important catalysts that could be easily prepared by simple reaction of N-substituted compounds with chlorosulfonic acid under mild conditions. In recent years, our research group prepared and introduced several types of these kinds of reagents of which saccharin sulfonic acid (SaSA) [39–42], melamine trisulfonic acid (MTSA) [43–46], succinimide sulfonic acid (SuSA) [31, 47, 48], and N-sulfonic acid poly(4-vinylpyridinium) chloride (NSPVPC) [30, 49, 50] are examples. Our obtained results showed that these reagents are useful catalysts that have been successfully utilized in all of the investigated reactions. Based on these studies, we were interested to investigate the applicability of N-sulfonated DABCO ([DABCO](SO3H)2Cl2) as a newly reported N-sulfonic acid [38] in the promotion of the synthesis of 5-arylmethylene-pyrimidine-2,4,6-trione and 1,8-dioxo-octahydro-xanthene derivatives. Our investigations showed that this reagent is efficiently able to catalyze the requested reactions under solvent-free conditions and in water, respectively.
In order to optimize the reaction conditions for each of the above-mentioned reactions, the condensation of 4-chlorobenzaldehyde (1 mmol) with barbituric acid (1 mmol) and/or 1,3-cyclohexadione was studied in the presence of [DABCO](SO3H)2Cl2, and the results are tabulated in Tables 2 and 3. On the basis of these results, the optimized conditions are selected as shown in Schemes 1 and 2.
After optimization of the reaction conditions and in order to study the efficiency of [DABCO](SO3H)2Cl2 in the preparation of 5-arylmethylene-pyrimidine-2,4,6-trione derivatives, various aromatic aldehydes were reacted with barbituric acid under the optimal reaction conditions to furnish the corresponding products in high yields and in short reaction times. The obtained results for the preparation of a variety type of 5-arylmethylene-pyrimidine-2,4,6-trione derivatives are summarized in Table 4. It seems that the presence of electron-withdrawing functional groups on the aromatic ring of the aldehyde increases the reaction time slightly.
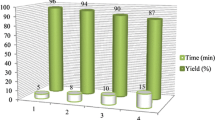
To investigate the recyclability of the catalyst in the synthesis of 5-arylmethylene-pyrimidine-2,4,6-trione derivatives, the reaction between 4-chlorobenzaldehyde and barbituric acid under optimized reaction conditions was studied again. When the reaction was completed, the catalyst was separated by filtration and dried under vacuum at 70 °C. The recovered catalyst was washed with diethyl ether, dried, and reused for the same reaction. This procedure was repeated for six runs, and each time the product was obtained by the recovered catalyst without much change in the reaction time and yield. The results are shown in Fig. 2.
After successful synthesis of a series of 5-arylmethylene-pyrimidine-2,4,6-trione derivatives, the efficiency of the same catalyst in the synthesis of 1,8-dioxo-octahydro-xanthene derivatives via the reaction of 1,3- cyclohexanedione and/or 5,5-dimethyl-1,3-cyclohexadione (dimedone) with aromatic aldehydes was studied under the selected conditions (Scheme 2). The results showed that this conversion also occurred with excellent yields in very short times (Table 5).
The recycling process was also performed for 1,8- dioxo-octahydroxanthene derivatives. This process was repeated for four runs and the results are presented in Fig. 3. This figure shows a small decrease in the efficiency of the catalyst after four runs.
In order to show the merit of this method, the efficiency of [DABCO](SO3H)2Cl2 with other catalysts in the synthesis of 5-(4-chlorobenzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione (Table 4, entry 2) and 3,3,6,6-tetramethyl-9-(4-chlorophenyl)-1,8-dioxo-octahydroxanthene (Table 5, entry 14), is compared in Tables 6, 7, respectively. On the basis of these comparisons, we can say that the present method is more efficient in decreasing of the reaction times, increasing of the products yield and/or in modulation of the reaction conditions.
In a plausible mechanism, at first, the aldehyde is activated by the proton from [DABCO](SO3H)2Cl2. Next, the carbonyl carbon is attacked by the nucleophilic cyclohexanedione derivatives or barbituric acid to form the related Knoevenagel products (1) or (2), respectively. The subsequent addition of (1) to cyclohexane derivatives, gives the acyclic adduct intermediate, which undergoes intramolecular cyclization with participation of two hydroxyl groups to afford the xanthene derivatives. The Knoevenagel product at the barbituric acid (2) is not able to continue the reaction leading to 5-arylmethylene-pyrimidine-2,4,6-trione derivatives (Scheme 3).
Conclusions
In summary, we have introduced a novel and efficient method for the synthesis of 5-arylmethylene-pyrimidine-2,4,6-trione and 1,8-dioxo-octahydro-xanthene derivatives in the presence of a catalytic amount of [DABCO](SO3H)2Cl2 as an acidic ionic liquid. This method has several advantages such as ease of preparation and handling of the catalyst, simplicity and easy work-up, high reaction rates, excellent yields, and eco-friendly procedure. In addition, simplicity of the preparation of this catalyst is valuable point for the presented method.
References
T. Welton, Chem. Rev. 99, 2071 (1999)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772 (2000)
T. Welton, Coord. Chem. Rev. 248, 2459 (2004)
X. Mi, S. Luo, J.P. Cheng, J. Org. Chem. 70, 2338 (2005)
R.V. Hangarge, D.V. Jarikoteb, M.S. Shingare, Green Chem. 4, 266 (2002)
V. Singh, S. Kaur, V. Sapehiyi, Catal. Commun. 6, 57 (2005)
Y. Ishida, D. Sasaki, M. Miyauchi, K. Saigo, Tetrahedron Lett. 45, 9455 (2004)
J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J. Bazureau, P. Hamelin, J. Catal. Commun. 3, 185 (2002)
V. Singh, S. Kaur, V. Sapehiyia, J. Singh, G.L. Kad, Catal. Commun. 6, 57 (2005)
N. Gupta, G.L.S. Kad, J. Singh, Catal. Commun. 8, 1323 (2007)
A.R. Hajipour, L. Khazdooz, A.E. Ruoho, Catal. Commun. 9, 89 (2007)
M. Dabiri, P. Salehi, M. Baghbanzadeh, M. Shakouri, S. Otokesh, T. Ekrami, R. Doosti, J. Iran. Chem. Soc. 4, 393 (2007)
S.M. Menchen, S.C. Benson, J.Y.L. Lam, W. Zhen, D. Sun, B.B. Rosenblum, S.H. Khan, M.U.S. Taing, Patent 6. Chem. Abstr. 139, p54287f (2003)
R.C. Hunter, T. Beveridge, J. Appl. Environ. Microibiol. 71, 2501 (2005)
M. Ahmad, T.A. King, D.K. Ko, B.H. Cha, J. Lee, J. Phys. D Appl. Phys. 35, 1473 (2002)
R.W. Lambert, J.A. Martin, J.H. Merrett, K.E.B. Parkes, G .Thomas, J. PCT Int. Appl. WO 9706178 (1997); Chem. Abstr. 126, p212377y (1997)
T. Hideo, Jpn. Tokkyo Koho 56,005,480, (1981); Chem. Abstr. 95, 80922b (1981)
E.F. Llama, C.D. Campo, M. Capo, M. Anadon, Eur. J. Med. Chem. 24, 391 (1989)
J.P. Poupelin, G. Saint-Rut, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, I. Eur, J. Med. Chem. 13, 67 (1978)
R.M. Ion, D. Frackowiak, A. Planner, K. Wiktorowicz, Acta Biochim. Pol. 45, 833 (1998)
Y. Frangin, C. Guimbal, F. Wissocq, H. Zamarlik, Synthesis 44, 3241 (1986)
J.D. Figueroa-Villar, C.E. Rangel, L.N. Dos Santos, Synth. Commun. 22, 1159 (1992)
K. Tanaka, X. Cheng, F. Yoneda, Tetrahedron 44, 3241 (1988)
K. Venkatesan, S.S. Pujari, R.J. Lahoti, K.V. Srinivasan, Ultra. Sonochem. 15, 548 (2008)
K. Niknam, M. Damya, J. Chin. Chem. Soc. 56, 659 (2009)
T.S. Jin, J.S. Zhang, J.C. Xiao, A.Q. Wang, T.S. Li, Synlett 5, 866 (2004)
A.R. Khosropour, M.M. Khodaei, H. Moghannian, Synlett 6, 955 (2005)
D. Fang, K. Gong, Z.L. Liu, Catal. Lett. 127, 291 (2009)
M. Dabiri, M. Baghbanzadeh, E. Arzroomchilar, Catal. Commun. 9, 939 (2008)
F. Shirini, M. Abedini, R. Pourhasan, Dyes & Pig. 99, 250 (2013)
F. Shirini, N.G. Khaligh, Dyes & Pig. 95, 789 (2012)
F. Shirini, G.H. Imanzadeh, M. Abedini, M.A. Dokhte-Ghaziani, P.G. Ghasemabadi, M.S. Langroodi, Iranian. J. Catal. 2, 115 (2012)
J.M. Khurana, K. Vij, Catal. Lett. 138, 104 (2010)
S. Kamble, G. Rashinkar, A. Kumbhar, K. Mote, R. Salunkhe, Arch. Appl. Sci. Res. 2, 217 (2010)
A. Khalafi-Nezhad, A. Hashemi, Iran J. Chem. Chem. Eng. 20, 9 (2001)
G. Alcerreca, R. Sanabria, R. Miranda, G. Arroyo, J. Tamariz, F. Degado, Synth. Commun. 30, 1295 (2000)
S.B. Rathod, A.B. Gambhire, B.R. Arbad, M.K. Lande, Bull. Korean Chem. Soc. 31, 339 (2010)
F. Nemati, S.G. Alizadeh, J. Chem. 2013, 1 (2013)
A. Zare, M. Mokhlesi, A.R. Hasaninejad, T. Hekmat-Zadeh, E. J. Chem. 9, 1854 (2012)
F. Shirini, M.A. Zolfigol, M. Abedini, Monatsh. Chem. 140, 61 (2009)
F. Shirini, M.A. Zolfigol, M. Abedini, Monatsh. Chem. 140, 1495 (2009)
F. Shirini, M.A. Zolfigol, M. Abedini, J. Iran. Chem. Soc. 7, 603 (2010)
F. Shirini, M.A. Zolfigol, J. Albadi, Synth. Commun. 40, 910 (2010)
F. Shirini, M.A. Zolfigol, A.R. Aliakbar, J. Albadi, Synth. Commun. 40, 1022 (2010)
F. Shirini, M.A. Zolfigol, J. Albadi, Chin. Chem. Lett. 22, 318 (2011)
F. Shirini, M.A. Zolfigol, J. Albadi, J. Iran. Chem. Soc. 7, 895 (2010)
F. Shirini, N.G. Khaligh, Phosph. Sul. Sil. 186, 2156 (2011)
F. Shirini, N.G. Khaligh, Monatsh. Chem. 143, 631 (2012)
F. Shirini, N.G. Khaligh, O. Goli-Jolodar, Chem. Soc. 10, 181 (2013)
F. Shirini, O. Goli-Jolodar, J. Mol. Catal. A Chem. 356, 61 (2012)
C.S. Reddy, A. Nagaraj, P. Jalapathi, Indian J. Chem. 46B, 660 (2007)
S. Kokkirala, N.M. Sabbavarapu, V.D.N. Yadavalli, Eur. J. Chem. 2, 272 (2011)
A.P. Chavan, J. Korean Chem. Soc. 53, 415 (2009)
M. Bayat, H. Imanieh, S.H. Hossieni, Chin. J. Chem. 27, 2203 (2009)
G.H. Mahdavinia, J. Iran. Chem. Res. 1, 11 (2008)
A. John, P.J.P. Yadav, S. Palaniappan, J. Mol. Catal. A: Chem. 248, 121 (2006)
Acknowledgments
The authors are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirini, F., Langarudi, M.S.N., Seddighi, M. et al. Bi-SO3H functionalized ionic liquid based on DABCO as a mild and efficient catalyst for the synthesis of 1,8-dioxo-octahydro-xanthene and 5-arylmethylene-pyrimidine-2,4,6-trione derivatives. Res Chem Intermed 41, 8483–8497 (2015). https://doi.org/10.1007/s11164-014-1905-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1905-1