Abstract
Nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA) as two nanostructured novel, green and heterogeneous catalysts were designed, synthesized and fully characterized by FT-IR, energy-dispersive X-ray spectroscopy, X-ray diffraction patterns, scanning electron microscopy, transmission electron microscopy and thermal gravimetric analysis. Then their catalytic applications were studied in the Biginelli-type reaction for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via one-pot three-component condensation reaction between several aldehydes, ethyl acetoacetate and urea or thiourea. To further study catalytic properties of NMSDSA and NMSMSA, they were used in the synthesis of polyhydroquinoline and 2,3-dihydroquinazolin-4(1H)-one derivatives under same reaction conditions. NMSDSA and NMSMSA have advantages such as cost-effectiveness, cleaner reaction profile, benign and heterogeneous characters, reusability of the catalysts and being in agreement with the green chemistry protocols. The described nanostructured catalysts have natural-based acids and potential for industrial production.
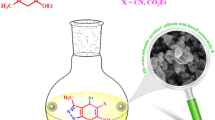
Graphical Abstract
Synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via Biginelli-type reaction, polyhydroquinolines and 2,3-dihydroquinazolin-4(1H)-ones using NMSDSA and NMSMSA as two novel nanostructured catalysts.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Knowledge-based developments of task-specific and natural-based catalysts are more demanded because catalytic systems have played an excellent role in the pollution prevention in our environment [1]. In this regard, a wide range of catalysts and their supported heterogeneous forms are reported in the last few years for various chemical and biochemical processes and extensively have been reviewed. Among the reported solid catalysts, solid inorganic and natural-based acids have been also used widely for various organic functional group transformations [2]. The outstanding potential and environmentally benign properties of silica make it the best choice, either as major support (core) or as coating agent for the synthesis of a wide variety of heterogeneous catalysts [3, 4]. One of the most important strategies to combine economic aspects with the environmental concerns is the use of silica or its corresponding derivatives as a core or coating agent in the synthesis of porous materials and nanocatalysts [5]. Silica sulfuric acid (SSA) as a familiar example has been prepared via reaction between silica gel and chlorosulfonic acid at room temperature [6]. SSA and its different forms as a superior proton source of all of the reported acidic solid supports or acidic resins have been widely used for a various functional groups transformations [7–10] and extensively reviewed [11].
Conversely, reduction in the amount of required sulfuric acid and/or overview in handling processes is needed for risk reduction, environment conservation and economic improvement [12]. Furthermore, heterogeneous systems attract attention and are studied owing to the significance they have in industry and developing knowledge [13]. In continuation of our researches on the application of solid acids [6–11] and inorganic acidic salts [14], we found that sodium metasilicate reacts with one or two equivalents of chlorosulfonic acid to synthesize nanometasilica monosulfuric acid sodium salt (NMSMSA) and nanometasilica disulfuric acid (NMSDSA), respectively. It is fascinating to note that the reaction is simple and safe without any work-up process because NaCl salt is performed as a solely by-product (Scheme 1).
The Biginelli-type reaction, as a significant and valuable MCRs, offers an efficient technique to give multi-functionalized 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) and linked heterocyclic compounds [15]. One of the noteworthy approaches for synthesis of this valuable type of compound is the one-pot condensation of an aldehyde, β-ketoester and urea under acidic conditions, first studied by Biginelli. Nonetheless, this reaction suffered from strict condition, frequent low yield and long reaction time [16]. Lately, 3,4-dihydropyrimidin-2(1H)-one (DHPM) framework has been under lots of research [17–19] as it has a wide pharmacological qualities, for instance anti-inflammatory [20], antihypertensive agents [21], antitumor agents [22], antiviral [23], antifungal [24] and calcium channel modulators [25].
Another one of the most noteworthy and conventional MCRs is the synthesis of dihydropyridine (DHP) and its derivatives which is attributed to Arthur Hantzsch who discovered them in 1881 [26]. Recently, great attention has been paid to the synthesis of Hantzsch polyhydroquinoline derivatives owing to its biological and physiological properties [27]. Although dihydropyridines were mainly developed as cardiovascular agents, they are antihypertensive, antiatherosclerotic, bronchodilator, vasodilator, geroprotective, antitumor, antimutagenic, hepatoprotective and antidiabetic agents [28]. Their broad pharmacological properties have interested the researchers to find novel derivatives which are more selective, effective and maybe with several modes of action [29].
On the other hand, 2,3-dihydro-2-aryl-4(1H)-quinazolinone derivatives are a type of fused heterocycles which have drawn much attention due to their potentially biological and pharmaceutical activities as diuretic, antitumor and herbicidal agents, in addition to plant growth regulators [30–34]. Three-component reaction of aldehyde, isatoic anhydride and amine [35–39], reduction cyclization of orthoformate and o-nitrobenzamides, aldehydes or ketones [40] and reduction of quinazolin-4(3H)-ones [41] are also studied for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones.
Consequently, the described novel catalysts were used for the well-known Biginelli-type reaction in the synthesis of 3,4-dihydropyrimidin-2(1H)-ones [42–63], Hantzsch polyhydroquinolines synthesis [64–70] and 2,3-dihydroquinazolin-4(1H)-ones [71–75] via one-pot condensation reaction under solvent-free benign conditions (Scheme 2).
Experimental
The materials were obtained from Merck, Fluka and Sigma-Aldrich and were applied without any additional purification. All reactions were observed via thin layer chromatography (TLC) on gel F254 plates. Spectrometer (1H NMR 400 MHz and 13C NMR 100) in pure deuterated DMSO with tetramethylsilane (TMS) was used as the internal standard. The synthesized catalysts were identified using IR, X-ray diffraction patterns (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis. X-ray diffraction (XRD) patterns of two catalysts were achieved on a APD 2000, Ital structure with Cu K_ radiation (k = 0.1542 nm) operating at 50 kV and 20 mA in a 2-h range of 10°–70° with step size 0.01° and time step 1.0 s to assess the crystallinity of the catalysts. Fourier transform infrared spectra of the samples were recorded on a PerkinElmer FT-IR spectrometer 17,259 by KBr disks. Thermal gravimetric analyses using a PerkinElmer TGA were carried out on catalysts. The SEM analyses were prepared with a TESCAN/MIRA with a maximum acceleration voltage of the primary electrons between 10 and 15 kV. Transmission electron microscope, TEM measurements were carried out on a Philips CM10 analyzer (operating at 120 kV). Semi-quantitative EDX (Röntec, Quantax/QX2) analysis was used for the characterization of element concentration.
General procedure for the synthesis of nanometasilica disulfuric acid (NMSDSA) as a green catalyst
To a round-bottomed flask (50 mL) including sodium metasilicate (5 mmol; 0.610 g) chlorosulfonic acid (10 mmol; 1.165 g; 0.665 mL) was added dropwise which in situ was reacted (with a ratio of 1:2) and was stirred over a period of 30 min at room temperature. Then, the yellow solid product was washed three times with diethyl ether and then dried under vacuum conditions (Scheme 1). Nanometasilica disulfuric acid (NMSDSA) with melting point of >380 °C (was decomposed) was fully characterized by FT-IR, energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis.
General procedure for the synthesis of nanometasilica monosulfuric acid sodium salt (NMSMSA) as a green catalyst
To a round-bottomed flask (50 mL) counting sodium metasilicate (5 mmol; 0.610 g) chlorosulfonic acid (5 mmol; 0.583 g; 0.333 mL) was added dropwise which in situ was reacted (with a ratio of 1:1) and was stirred over a period of 30 min at room temperature. Then, the white solid product was washed three times with diethyl ether and then dried under vacuum conditions (Scheme 1). Nanometasilica monosulfuric acid sodium salt (NMSMSA) with melting point of >394 °C (was decomposed) was fully characterized by FT-IR, energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis.
General procedure for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via Biginelli-type reaction
Nanometasilica disulfuric acid (NMSDSA) (10 mol %; 0.024 g) or nanometasilica monosulfuric acid sodium salt (NMSMSA) (10 mol %; 0.018 g) as a green mild catalyst was added to a mixture of aromatic aldehydes (1 mmol), ethyl acetoacetate (1 mmol; 0.132 g) and urea (1.5 mmol; 0.090) or thiourea (1.5 mmol; 0.114) in a round-bottomed flask, and the subsequent mixture was firstly stirred magnetically under solvent-free conditions at 80 °C. After completion of the reaction, as checked by TLC n-hexane/ethyl acetate (5:2), ethyl acetate (10 mL) was added to a reaction mixture, stirred and refluxed for 5 min, and then was washed with water (10 mL) and decanted to separate catalyst from the reaction mixture (the reaction mixture was soluble in hot ethyl acetate and NMSDSA or NMSMSA catalyst was soluble in water). The solvent of organic layer was evaporated, and the crude product was purified via recrystallization from ethanol/water (10:1).
General procedure for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation reaction
Nanometasilica disulfuric acid (NMSDSA) (10 mol %; 0.024 g) or nanometasilica monosulfuric acid sodium salt (NMSMSA) (10 mol %; 0.018 g) as a green mild catalyst was added to a mixture of aromatic aldehydes (1 mmol), ethyl acetoacetate (1 mmol; 0.132 g) or methyl acetoacetate (1 mmol; 0.116 g), dimedone (1 mmol; 0.140 g) or 1,3-cyclohexanedione (1 mmol; 0.112 g) and ammonium acetate (1 mmol; 0.077 g) in a round-bottomed flask, and the consequent mixture was first stirred magnetically under solvent-free conditions at 80 °C. After completion of the reaction, as identified by TLC n-hexane/ethyl acetate (5:2), ethyl acetate (10 mL) was added to a reaction mixture, stirred and refluxed for 5 min, and then was washed with water (10 mL) and decanted to separate catalyst from the reaction mixture (the reaction mixture was soluble in hot ethyl acetate and NMSDSA or NMSMSA catalyst was soluble in water). The solvent of organic layer was evaporated, and the crude product was purified via recrystallization from ethanol/water (10:1).
General procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-one
Nanometasilica disulfuric acid (NMSDSA) (10 mol %; 0.024 g) or nanometasilica monosulfuric acid sodium salt (NMSMSA) (10 mol %; 0.018 g) as a green mild catalyst was added to a mixture of aromatic aldehydes (1 mmol) and 2-aminobenzamide (1 mmol; 0.136 g) in a round-bottomed flask, and the subsequent mixture was initially stirred magnetically under solvent-free conditions at 80 °C. After completion of the reaction, as known by TLC n-hexane/ethyl acetate (5:2), ethyl acetate (10 mL) was added to a reaction mixture, stirred and refluxed for 5 min, and then was washed with water (10 mL) and decanted to separate catalyst from the reaction mixture (the reaction mixture was soluble in hot ethyl acetate and NMSDSA or NMSMSA catalyst was soluble in water). The solvent of organic layer was evaporated, and the crude product was purified via recrystallization from ethanol/water (10:1).
Ethyl 4-(3-ethoxy-4-hydroxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (Table 5, entry 10)
White solid; M.p: 192–194 °C; IR (KBr): υ 3427, 3188, 2991, 1667, 1585, 1476, 1333, 1284, 1194 cm−1; 1H NMR (400 MHz, DMSO-d 6 ): δppm 1.12 (t, 6H, J = 7.2 Hz, —CH3), 2.25 (s, 3H, —CH3), 3.99 (q, 4H, J = 7.2 Hz, —CH2), 5.11 (s, 1H, —CH aliphatic), 7.11 (d, 2H, J = 7.6 Hz, ArH), 7.13 (s, 1H, ArH), 7.63 (s, 2H, —NH), 9.10 (s, 1H, —OH); 13C NMR (100 MHz, DMSO-d 6 ): δppm 14.5, 18.1, 21.0, 54.1, 59.5, 60.3, 99.9, 100.1, 126.5, 129.3, 1368, 142.4, 148.5, 152.6, 165.8, 169.3.
Ethyl 4-(3-ethoxy-4-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (Table 5, entry 20)
White solid; M.p: 213–215 °C; IR (KBr): υ 3416, 3264, 3127, 2983, 1702, 1654, 1602, 1474, 1277, 1098 cm−1; 1H NMR (400 MHz, DMSO-d 6 ): δppm 1.12 (t, 3H, J = 7.6 Hz, —CH3), 1.33 (t, 3H, J = 7.6 Hz, —CH3), 2.23 (s, 3H, —CH3), 4.02 (q, 4H, J = 7.6 Hz, —CH2), 5.04 (s, 1H, —CH aliphatic), 6.72 (d, 2H, J = 8.0 Hz, ArH), 6.78 (s, 1H, ArH), 7.56 (s, 1H, —NH), 8.75 (s, 1H, —NH), 9.05 (s, 1H, —OH); 13C NMR (100 MHz, DMSO-d 6 ): δppm 14.5, 15.2, 18.1, 54.0, 59.5, 64.4, 100.1, 112.9, 115.8, 118.9, 136.3, 146.6, 146.7, 148.2, 152.6, 165.9.
Results and discussion
In continuation of our previous investigations linked to the design, synthesis, applications and knowledge-based improvement in novel solid acids [6–11] for organic functional group transformations, herein, we wish to study synthesis, characterization and applications of NMSMSA and NMSDSA as two elegant and full-fledged catalysts with silica sulfuric acid moieties.
Characterization of novel silica-based catalyst with silica sulfuric acid moieties: nanometasilica disulfuric acid (NMSDSA) as a catalyst
The structure of nanometasilica disulfuric acid (NMSDSA) as a green mild and full-fledged catalyst with silica sulfuric acid moiety was considered and fully characterized by FT-IR, EDX, XRD, SEM, TEM and TG analysis.
The FT-IR spectrum of the nanostructured NMSDSA displayed two abroad peaks at 3480 and 3417 cm−1 which can be related to O–H stretching on –SO3H group. Moreover, two peaks at 1283 and 1176 cm−1 are linked to vibrational modes of O–SO2 bonds. The absorption peak connected to S=O bond vibration appeared at 1055 cm−1. Also, two absorption peaks of the SiO3 at 1098 and 518 cm−1 are related to the stretching vibration of Si–O groups (Fig. 1).
The energy-dispersive X-ray spectroscopy (EDX) of nanostructured NMSDSA has revealed the presence of oxygen (O), silicon (Si) and sulfur (S) composition in the pure catalyst (Fig. 2). It is clearly shown that the synthesized NMSDSA catalyst involves only O, Si and S and elements, which is presented in Fig. 2. No extra peak linked with any impurity has been known in the SEM coupled EDX, which approves that the NMSDSA catalyst is composed of just with O (78.59), Si (4.21) and S (17.21) which is exposed in elemental analysis.
The structure of NMSDSA was studied via X-ray diffraction (XRD) pattern (Fig. 3). Peak width (FWHM), size and inter-planer distance linked to XRD pattern of NMSDSA were studied in the 19.00°–29.60°, and the achieved results are summarized in Table 1. For instance, assignments for the highest diffraction line 29.40° offered that an FWHM of 0.58, a crystalline size of the catalyst of ca. 14.16 nm via the Scherrer equation [D = Kλ/(βcosθ)] (where D is the mean size of the arranged (crystalline) domains, which may be smaller or equal to the grain size. K is a dimensionless shape factor. The shape factor has a model value of about 0.9. λ is the X-ray wavelength. β is the line width at half the maximum intensity (FWHM), after subtracting the instrumental line width, in radians. θ is the Bragg diffraction angle in degree, and an inter-planer distance of 0.303439 nm (the similar highest diffraction line at 29.40°) was investigated via the Bragg equation: [dhkl = λ/(2sinθ)], [(λ:Cu radiation (0.154,178 nm)] were achieved. Achieving crystalline sizes from several diffraction lines through the Scherrer equation were found to be in the nanometer range (9.71–68.49 nm), which is specially in close agreement with the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 4). To determine the morphology and the size of the nanostructured NMSDSA catalyst, SEM and TEM were considered. This image of the nanostructured NMSDSA catalyst shows that the size of these particles is about 70 nm (Fig. 4).
The thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis of nanometasilica disulfuric acid (NMSDSA) as an efficient catalyst displays the mass loss of organic materials as they decompose upon heating (Fig. 5). The main weight loss from the NMSDSA catalyst (room temperature to 110 °C) is due to the removal of physically adsorbed water and organic solvents, which were used in making the catalyst. The weight loss is about 2 %. The weight loss (13 %) between 110 and 185 °C is qualified mainly to the thermal decomposition sulfuric acid moiety of catalyst. NMSDSA catalyst demonstrates two-step weight loss behavior. Weight loss of catalyst seems about 13 % at 185–400 °C, which is contributed to the thermal decomposition of the catalyst. The thermal gravimetric analysis of the NMSDSA catalyst offered remarkable loss in two steps and decomposed after 400 °C.
Characterization of novel catalyst with silica sulfuric acid moiety: nanometasilica monosulfuric acid sodium salt (NMSMSA) as a catalyst
The structure of nanometasilica monosulfuric acid sodium salt (NMSMSA) as an elegant and benign catalyst with silica sulfuric acid moiety was studied and fully identified by FT-IR, EDX, XRD, SEM, TEM and TG analysis.
The FT-IR spectrum of the nanostructured NMSMSA shows an abroad peak at 3439 cm−1 which can be connected to O–H stretching on –SO3H group. Furthermore, two peaks at 1035 and 967 cm−1 are related to vibrational modes of O–SO2 bonds. The absorption peak linked to S=O bond vibration appeared at 897 cm−1. Additionally, two absorption peaks of the SiO3 at 897 and 526 cm−1 are correlated with the stretching vibration of Si–O groups (the peaks of Si–O group overlaps with the peaks of S=O bond and is shielded due to the ionic structure of catalyst) (Fig. 6).
Energy-dispersive X-ray spectroscopy (EDX) from the attained nanostructured NMSMSA provided the presence of the probable elements in the structure of the catalyst, for instance Na, O, Si, S and Cl (Fig. 7).
Nanometasilica monosulfuric acid sodium salt (NMSMSA) as a nanostructured catalyst was investigated by X-ray diffraction (XRD) pattern (Fig. 8), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 9). In this investigation, to confirm the structure of NMSMSA, firstly its XRD pattern was studied. As exposed in Fig. 8, the XRD patterns of nanostructured organocatalyst show peaks at 2θ ≈ 18.30°, 19.50°, 25.30°, 25.60°, 27.30°, 27.50°, 28.60°, 28.90°, 30.30°, 31.50°, 39.00°, 48.30° and 52.20°, respectively; this was approved by the described value of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 9). Peak width (FWHM), size and inter-planar distance related to XRD pattern of nanostructured catalyst were investigated in the 18.30°–52.20° and the attained results are summarized in Table 2. The average crystallite size D was calculated via the Scherrer formula: D = Kλ/(βcosθ); K is the Scherrer constant, λ being the X-ray wavelength, β is the half-maximum peak width, and θ is the Bragg diffraction angle. Consequently, the average size of the nanostructured catalyst achieved from this equation was found to be about 10.31–67.26 nm, which is mainly in good accordance with the scanning electron microscopy and transmission electron microscopy (Fig. 9).
The thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis exposed that NMSMSA catalyst was very stable and there was no evident mass loss below 390 °C (see Fig. 10). The significant weight loss (4 %) between 115 and 198 °C is qualified chiefly to the thermal decomposition of sulfuric acid moiety. Also the weight loss (14 %) happened in the range of 198–415 °C, is qualified to the loss of chief NMSMSA catalyst. The thermal gravimetric analysis of the catalyst presented noteworthy loss in two steps, and decomposed above 415 °C.
Application of nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA) as two elegant and full-fledged catalysts with silica sulfuric acid moieties
Initially, to optimize the reaction conditions, the condensation reaction of 4-methylbenzaldehyde, ethyl acetoacetate and urea was selected as a typical reaction and different amounts of NMSDSA and NMSMSA as two nanostructured catalysts at the range of 25–100 °C were studied under solvent-free conditions (Table 3). As revealed in Table 1, the worthy results were achieved when the reaction was attained in the presence of 10 mol % of nanostructured catalysts at 80 °C (Table 3, entry 12). No improvement was identified in the yield of reaction by increasing the amount of the catalysts and temperature (Table 3, entries 13 and 14). Table 1 obviously shows that in the absence of catalysts, the product was produced in a low yield (Table 3, entries 1 and 2). A slight increase of the urea was known to be desirable, and therefore, the molar ratio of ethyl acetoacetate and 4-methylbenzaldehyde to urea was investigated at 1:1:1.5 (the yield of the reaction 84 % using 1 mmol of urea was increased to 90 % using 2 mmol of urea).
To compare the effect of the solution in comparison with solvent-free conditions, a mixture of 4-methylbenzaldehyde, ethyl acetoacetate and urea as typical reaction, using 10 mol % of nanostructured catalysts in numerous solvents, for instance H2O, C2H5OH, CH3CN, CH3CO2Et and CH2Cl2 were studied at 80 °C. The results are summarized in Table 4. The results show that solvent-free condition was the best choice in this reaction (Table 4, entry 1).
After detecting the optimized reaction conditions, the study was followed through performing the reaction between several aldehydes, ethyl acetoacetate and urea or thiourea. To display the general applicability of this process, various aldehydes were capably reacted with ethyl acetoacetate and urea or thiourea in the related conditions. These results stimulated us to consider the scope, limitations and the overview of this synthetic procedure for numerous aldehydes under optimized conditions. As exposed in Table 5, a series of aromatic aldehydes underwent electrophilic substitution reaction with ethyl acetoacetate and urea or thiourea to afford a different range of substituted 3,4-dihydropyrimidin-2(1H)-ones via Biginelli-type reaction with worthy to excellent yields. The nature and electronic properties of the substituents on the aromatic ring affect the alteration rate, and aromatic aldehydes having electron-withdrawing groups on the aromatic ring react similarly as electron-donating groups do (in terms of reaction time and isolated yields). As it is shown in Table 5, the attained results (lower reaction time and higher isolated yields) in the presence of urea were more successful than the results of thiourea in this reaction conditions. Correspondingly, the attained results displayed that the NMSDSA as a nanostructured catalyst was better than the results of NMSMSA as a nanostructured catalyst (owing to production of desired products with higher yields and shorter reaction time).
Furthermore, reusability of the NMSDSA and NMSMSA as two worthy nanostructured catalysts was approved in the condensation of 4-methylbenzaldehyde, ethyl acetoacetate and urea. At the end of the reaction, ethyl acetate was added to the reaction mixture and heated to extract product and remained starting materials (the product and remained starting materials are soluble in hot ethyl acetate but catalysts is insoluble in ethyl acetate). It is known that the catalytic activities of the catalysts were restored within the limits of the experimental errors for eight continuous runs (Table 6). The recycled catalyst was also characterized by FT-IR spectrum after its application in the reaction. The deactivations of the catalysts are low. The reaction was scaled up to 10 mmol of 4-methylbenzaldehyde and ethyl acetoacetate and 15 mmol of urea in the presence of 100 mol % of catalysts at 80 °C. The results are summarized in Table 6.
To investigate more about the scope and limitations of catalytic activities of the described nanostructured catalysts in the other work, the Hantzsch synthesis of polyhydroquinoline and 2,3-dihydroquinazolin-4(1H)-one derivatives were studied under the same reaction conditions. After optimization of the reaction conditions, to study the efficacy and the scope of the offered process, various polyhydroquinoline derivatives (via one-pot four component condensation between 1,3-diketone, β-ketoester, aromatic aldehydes and ammonium acetate) and 2,3-dihydroquinazolin-4(1H)-one derivatives (via condensation between aromatic aldehydes and 2-aminobenzamide) were synthesized using 10 mol % of NMSDSA and NMSMSA as catalyst under solvent-free reaction conditions. The results are shown in Tables 7 and 8. The effects of substituents on the aromatic rings were estimated strong in terms of yields under these reaction conditions. Both classes of aromatic aldehydes containing electron-releasing and electron-withdrawing substituents in their aromatic rings gained the appropriate products in high to excellent yields in short reaction times. The reaction times of aromatic aldehydes having electron-withdrawing groups and electron-donating groups had rather same results. Similarly, the achieved results exhibited that the NMSDSA as a nanostructured catalyst was better than the results of NMSMSA as a nanostructured catalyst (because of synthesis of preferred products with higher yields and shorter reaction time).
Conclusion
In summary, two benign, efficient and full-fledged nanostructured catalysts with silica sulfuric acid moieties, namely nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA), were designed and synthesized, and their catalytic application was studied in the synthesis of 3,4-dihydropyrimidin-2(1H)-one, polyhydroquinoline and 2,3-dihydroquinazolin-4(1H)-one derivatives under solvent-free benign conditions. NMSDSA and NMSMSA were fully characterized by FT-IR, energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction patterns (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and thermal gravimetric analysis (TGA). The suggested mechanism exposed that the buffer ability of NMSDSA and NMSMSA probably plays a noteworthy and dual catalytic role in the defined reaction. Finally, main advantages of the offered methodology and/or investigation are reasonably simple work-up, high yield, short reaction time, low cost, commercial availability of the starting materials of catalysts, recoverability and reusability of the catalysts and cleaner reaction profile which makes it in close accordance with the green chemistry disciplines. Also, the achieved results show that the NMSDSA nanostructured catalyst was more effective than NMSMSA due to higher yields and shorter reaction time. Finally, described nanostructured catalysts have a natural base and potential for industrial production.
References
P. Gupta, S. Paul, Catal. Today 236, 153 (2014)
M. Kaur, S. Sharma, P.M.S. Bedi, Chin. J. Catal. 36, 520 (2015)
S. Rostamnia, E. Doustkhaha, J. Mol. Catal. A: Chem. 411, 317 (2016)
S. Rostamnia, E. Doustkhaha, Synlett 26, 1345 (2015)
R.K. Sharma, S. Sharma, S. Dutta, R. Zboril, M.B. Gawande, Green Chem. 17, 3207 (2015)
M.A. Zolfigol, Tetrahedron 57, 9509 (2001)
M. Daraei, M.A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H.G. Kruger, M. Mokhlesi, J. Iran. Chem. Soc. 12, 855 (2015)
D. Azarifar, S.M. Khatami, M.A. Zolfigol, R. Nejat-Yami, J. Iran. Chem. Soc. 11, 1223 (2014)
M. Safaiee, M.A. Zolfigol, M. Tavasoli, M. Mokhlesi, J. Iran. Chem. Soc. 11, 1593 (2014)
M.A. Zolfigol, A. Khazaei, M. Mokhlesi, F. Derakhshan-Panah, J. Mol. Catal. A: Chem. 370, 111 (2013)
P. Salehi, M.A. Zolfigol, F. Shirini, M. Baghbanzadeh, Curr. Org. Chem. 10, 2171 (2006)
J.M. Riego, Z. Sedin, J.M. Zaldivar. N.C. Marziano, C. Tortato. Tetrahedron Lett. 37, 513 (1996)
N.J. Turro, Tetrahedron 43, 1589 (1987)
M.A. Zolfigol, F. Shirini, P. Salehi, M. Abedini, Curr. Org. Chem. 12, 183 (2008)
P. Biginelli, Gazz. Chim. Ital. 23, 360 (1893)
P. Biginelli, Gazz. Chim. Ital. 26, 447 (1893)
K. Folkers, T.B. Johnson, J. Am. Chem. Soc. 55, 3784 (1933)
C.O. Kappe, J. Org. Chem. 62, 7201 (1997)
C.O. Kappe, Tetrahedron 49, 6937 (1993)
K.S. Atwal, G.C. Rovnyak, S.D. Kimball, D.M. Floyd, S. Moreland, B.N. Swanson, D.Z. Gougoutas, J. Schewartz, K.M. Smillie, M.F. Malley, J. Med. Chem. 33, 2629 (1990)
G.J. Groer, S. Dzwonczyk, D.M. Mcmullen, C.S. Normadinam, P.G. Sleph, S.J. Moreland, J. Cardiovasc. Pharmacol. 26, 289 (1995)
E. Klein, S. DeBonis, B. Thiede, D.A. Skoufias, F. Kozielski, L. Lebeau, Bioorg. Med. Chem. 15, 6474 (2007)
C.O. Kape, Acc. Chem. Res. 33, 879 (2000)
K.S. Atwal, B.N. Swanson, S.E. Unger, D.M. Floyd, S. Moreland, A. Hedberg, J. Med. Chem. 34, 806 (1991)
K.S. Atwal, S. Moreland, Bioorg. Med. Chem. Lett. 1, 291 (1991)
A. Hantzsch, Chem. Ber. 14, 1637 (1881)
R. Shan, C. Velazquez, E.E. Knaus, J. Med. Chem. 47, 254 (2004)
N. Edraki, A.R. Mehdipour, M. Khoshneviszadeh, R. Miri, Drug Disc. Today 14, 1058 (2009)
T.J. Cleophas, R. van Marum, Am. J. Cardiol 87, 487 (2001)
M.G. Biressi, G. Cantarelli, M. Carissimi, A. Cattaneo, F. Ravenna, Farmaco Ed. Sci. 24, 199 (1969)
P.R. Bhalla, B.L. Walworth, American Cyanamid Co., Eur. Pat. Appl. EP 58, 822. Chem. Abstr. 98, 1669 (1983)
P.R. Bhalla, B.L. Walworth, American Cyanamid Co., U.S. Patent 4, 431, 440, Chem. Abstr. 100, 174857 (1984)
E. Hamel, C.M. Lin, J. Plowman, H. Wang, K. Lee, K.D. Paull, Biochem. Pharmacol. 51, 53 (1996)
M. Hour, L. Huang, S. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel, K. Lee, J. Med. Chem. 43, 4479 (2000)
J. Chen, W. Su, H. Wu, M. Liu, C. Jin, Green Chem. 9, 972 (2007)
M. Dabiri, P. Salehi, M. Baghbanzadeh, M.A. Zolfigol, M. Agheb, S. Heydari, Catal. Commun. 9, 785 (2008)
J. Chen, D. Wu, F. He, M. Liu, H. Wu, J. Ding, W. Su, Tetrahedron Lett. 49, 3814 (2008)
M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgary, A.A. Mohammadi, Tetrahedron Lett. 46, 6123 (2005)
M.P. Surpur, P.R. Singh, S.B. Patil, S.D. Samant, Synth. Commun. 37, 1965 (2007)
D. Shi, L. Rong, J. Wang, Q. Zhuang, X. Wang, H. Hu, Tetrahedron Lett. 44, 3199 (2003)
S.W. Li, M.G. Nair, D.M. Edwards, R. Kisliuk, Y. Gaumont, I.K. Dev, D.S. Duch, J. Humphreys, G.K. Smith, R. Ferone, J. Med. Chem. 34, 2746 (1991)
A. Domling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
B.B. Toure, D.G. Hall, Chem. Rev. 109, 4439 (2009)
V. Estevez, M. Villacampa, J.C. Menendez, Chem. Soc. Rev. 39, 4402 (2010)
A. Domling, Chem. Rev. 106, 17 (2006)
M. Shiri, Chem. Rev. 112, 3508 (2012)
A. Rahmatpour, Catal. Lett. 142, 1505 (2012)
P. Salehi, M. Dabiri, M.A. Zolfigol, M.A. Bodaghifard, Tetrahedron Lett. 44, 2889 (2003)
A.N. Dadhania, V.K. Patel, D.K. Raval, J. Braz. Chem. Soc. 22, 511 (2011)
P. Salehi, M. Dabiri, M.A. Zolfigol, M.A. Bodaghifard, Heterocycles 60, 2435 (2003)
H. Salehi, Q.X. Guo, Chin. J. Chem. 23, 91 (2005)
Z. Wang, L. Xu, C. Xia, H. Wang, Tetrahedron Lett. 45, 7951 (2004)
H.G.O. Alvim, T.B. de Lima, H.C.B. de Oliveira, F.C. Gozzo, J.L. de Macedo, P.V. Abdelnur, W.A. Silva, B.A.D. Neto, ACS Catal. 3, 1420 (2013)
L.M. Ramos, A.Y. Ponce de Leony, Tobio, M.R. dos Santos, H.C.B. de Oliveira, A.F. Gomes, F.C. Gozzo, A.L. de Oliveira, B.A.D. Neto. J. Org. Chem. 77, 10184 (2012)
L.M. Ramos, B.C. Guido, C.C. Nobrega, J.R. Correa, R.G. Silva, H.C.B. de Oliveira, A.F. Gomes, F.C. Gozzo, B.A.D. Neto, Chem. Eur. J. 19, 4156 (2013)
D.L. Da Silva, S.A. Fernandes, A.A. Sabino, A. De Fa, Tetrahedron Lett. 52, 6328 (2011)
M. Nasr-Esfahani, S.J. Hoseini, F. Mohammadi, Chin. J. Catal. 32, 1484 (2011)
E. Kolvari, N. Koukabi, O. Armandpour, Tetrahedron 70, 1383 (2014)
K. Folkers, H.J. Harwood, T.B. Johnson, J. Am. Chem. Soc. 54, 3751 (1932)
B. Zeynizadeh, K.A. Dilmaghani, M. Yari, Phosphor. Sulfur Silic. Relat. Elemen. 184, 2465 (2009)
D.L. Da Silva, F.S. Reis, D.R. Muniz, A.L.T.G. Ruiz, J.E. De Carvalho, A.A. Sabino, L.V. Modolo, A. De Fatima, Bioorg. Med. Chem. 20, 2645 (2012)
W.Y. Chen, S.D. Qin, J.R. Jin, Synth. Commun. 37, 47 (2007)
C.G.S. Lima, S. Silva, R.H. Gonalves, E.R. Leite, R.S. Schwab, A.G. Corra, M.W. Paixo, Chem. Cat. Chem. 6, 3455 (2014)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, H. Alinezhad, M. Norouzi, RSC Adv. 4, 57662 (2014)
M. Tajbakhsh, H. Alinezhad, M. Norouzi, S. Baghery, M. Akbari, J. Mol. Liq. 177, 44 (2013)
S.M. Vahdat, F. Chekin, M. Hatami, M. Khavarpour, S. Baghery, Z. Roshan-Kouhi, Chin. J. Catal. 34, 758 (2013)
A. Khazaei, M.A. Zolfigol, A.R. Moosavi-Zare, J. Afsar, A. Zare, V. Khakyzadeh, M.H. Beyzavi, Chin. J. Catal. 34, 1936 (2013)
A. Zare, F. Abi, A.R. Moosavi-Zare, M.H. Beyzavi, M.A. Zolfigol, J. Mol. Liq. 178, 113 (2013)
S.B. Sapkal, K.F. Shelke, B.B. Shingate, M.S. Shingare, Tetrahedron Lett. 50, 1754 (2009)
S.J. Ji, Z.Q. Jiang, J. Lu, T.P. Loh, Synlett 5, 831 (2004)
M. Hajjami, B. Tahmasbi, RSC Adv. 5, 59194 (2015)
J. Safari, S. Gandomi-Ravandi, J. Mol. Liq. 390, 1 (2014)
J. Safari, S. Gandomi-Ravandi, Compt. Rend. Chimie. 16, 1158 (2013)
Z.H. Zhang, H.Y. Lu, S.H. Yang, J.W. Gao, J. Comb. Chem. 12, 643 (2010)
S.B. Bharate, N. Mupparapu, S. Manda, J.B. Bharate, R. Mudududdla, R.R. Yadav, R.A. Vishwakarma, ARKIVOC, viii, 308 (2012)
Acknowledgments
We thank Bu-Ali Sina University, Iran National Science Foundation (INSF) (the Grant of Allameh Tabataba’i’s Award, Grant Number BN093) and National Elites Foundation for financial support to our research group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zolfigol, M.A., Ghaderi, H., Baghery, S. et al. Nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA) as two novel nanostructured catalysts: applications in the synthesis of Biginelli-type, polyhydroquinoline and 2,3-dihydroquinazolin-4(1H)-one derivatives. J IRAN CHEM SOC 14, 121–134 (2017). https://doi.org/10.1007/s13738-016-0964-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0964-1