Abstract
A highly efficient protocol for the synthesis of 1,2,3,4-tetrahydropyridines in the presence of nano-sphere silica sulfuric acid (NS-SSA) was used for good yields by one-pot multicomponent reaction (MCRs). The reagent nano-sphere silica sulfuric acid (NS-SSA) has several advantages, such as easy workup, nontoxicity, convenience and high yields of products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the basic and essential heterocycles are tetrahydropyridines that have used to synthesize pharmaceutical compounds [1–7]. They have high biological activities associated with antiparasitic, antimicrobial, antiviral, antimalarial, anticancer, herbicidal and antihypertensive properties [8–25]. In addition, some of the derivatives of these compounds are used in a drug administered for the cause of permanent Parkinson’s disease [26–28]..
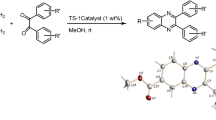
In recent decades, the use of solid-supported catalysts such as silica, alumina and titania has gained considerable attention both in industrial and academia research due to their unique properties, such as selectivity, efficiency and straightforward workup [29–36]. Considering the above points and along the line of our studies in design and application of new heterogeneous catalysts in chemical transformations [37–44], we report the synthesis, characterization and catalytic application of nano-sphere silica sulfuric acid (NS-SSA) that can be easily prepared from commercially available materials, for the synthesis of tetrahydropyridines by the one-pot multi-component reactions. This method shows high atom economy and high selectivity and is environmentally friendly as it reduces the number of synthesis steps [45, 46]. (Scheme 1).
Results and discussion
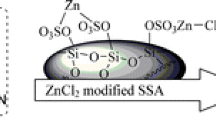
Initially, the nano-sphere silica was prepared according to the reported procedures.43 Then, the catalyst was synthesized by the reaction of nano-sphere silica with chlorosulfonic acid in excellent yield (Scheme 2).
Nano-sphere silica sulfuric acid was characterized by transmission electron microscopy (TEM), FT-IR, XRD, EDS, N2 adsorption–desorption techniques and thermal analysis [43].
To optimize the reaction conditions for the synthesis of tetrahydropyridine compounds, the reaction of 4-bromoaniline, benzaldehyde and methyl acetoacetate was selected as a model reaction to provide compound 2c (Table 4, entry 3).
At first, the reaction was examined in the presence of 20 mol % of different catalysts during 2 h. Higher yield of product was obtained when nano-sphere silica sulfuric acid was utilized as catalyst (Table 1, entry 5). The results are summarized in Table 1.
In the next step, the model reaction was tested using different amounts of NS-SSA at the same temperature (Table 2). As it can be seen in Table 2, the best amount of the catalyst was 0.05 g. Moreover, the product yield was not changed by increasing the amount of the catalyst. The best results were obtained when the reaction was performed at 65 °C. Increasing the reaction temperature did not improve the results (Table 2).
In another study, we studied the synthesis of tetrahydropyridines in a variety of solvents. The results showed that acetonitrile is the best solvent in terms of time and product yield (Table 3).
In the next part, the generality and efficiency of nano-sphere silica sulfuric acid in the synthesis of tetrahydropyridines were explored under the optimized reaction conditions by the reaction of various anilines and arylaldehydes with a broad range of electron-releasing substituents, electron-withdrawing substituents and halogens on their aromatic rings, and different β-ketoester in the acetonitrile. As it can be seen in Table 4, all benzaldehyde derivatives, anilines and different β-ketoester afforded the desired tetrahydropyridines in high to excellent yields. All the target compounds were completely characterized by IR, 1HNMR, 13CNMR.
In summary, we have developed a method for the synthesis of nano-sphere silica sulfuric acid (NS-SSA) as an efficient and heterogeneous catalyst via the reaction of nano-sphere silica with chlorosulfonic acid. NS-SSA showed powerful activity in the one-pot multicomponent reaction leading to tetrahydropyridines in good to high yield.
Experimental section
General procedure for the synthesis of tetrahydropyridines
β-ketoester (1.0 mmol), aniline (2 mmol) and 0.05 g nano-sphere silica sulfuric acid (NS-SSA) in 10 ml CH3CN was stirred at 65 °C for 20 min, aldehyde (2.0 mmol) was then added and stirring was continued until the formation of a solid. Then the reaction mixture was filtered and the solid so obtained was washed with acetonitrile. Since the solid does not solve in chloroform, it was separated from nano-sphere silica sulfuric acid (NS-SSA) by the addition of CHCl3. Finally, a colorless powder resulted with filtering of solution and evaporation.
Methyl 1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2a): White powder, mp 217–220 °C; IR(KBr): υ = 3325, 3086, 3063, 2949, 2868, 1651, 1600, 1504, 626 cm−1. 1HNMR (600 MHz; CDCl3) = 2.709–2.665 (1H, m, J = 18 Hz), 2.87–2.82 (1H, m, J = 3 Hz), 3.937 (3H, s), 5.1–5.09 (1H, d, J = 6 MHz), 6.176–6.154 (2H, d, J = 12 Hz), 6.434-6.412 (2H, t, j = 12 Hz), 10.185 (S, 1H).13CNMR (150 MHz; CDCl3) = 33.4, 51.2, 55.27, 58.3, 98.4, 114, 121.1, 126.2, 127, 128.7, 129, 131.4, 136, 142, 143.2, 145.4, 168.4.
Methyl 1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-2,6-di-p-tolyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2b): White powder, mp 211–213 °C; IR(KBr): υ = 3248, 3087, 3023, 2950, 2857, 1651, 1605, 1585 cm−1. 1HNMR (600 MHz; CDCl3) = 2.34–2.31(6H, d, j = 18 Hz), 2.69–2.6 (1H, m, j = 18 Hz), 3.92 (3H, s), 5.06–5.05 (1H, d, J = 6 Hz), 6.19–6.17 (2H, d, j = 12 Hz), 6.43–6.41 (2H, d, j = 12 Hz), 10.18 (1H, s). 13CNMR (600 MHz; CDCl3) = 20.99, 33.47, 51.1, 55, 58, 98.1, 113, 121, 126.2, 127, 128, 131, 136, 137, 139, 140.1, 168.4.
Methyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2c): White powder, mp 245-248 °C; IR(KBr): υ = 3256, 3084, 2948, 1651, 1599, 1578 cm−1. 1HNMR (600 MHz; CDCl3) = 2.72–2.67 (1H, m, j = 3 Hz), 2.87–2.82 (1H, m, j = 3 Hz), 3.93 (3H, s), 5.1–5.09 (1H, d, j = 6 Hz), 6.11–6.09 (2H, d, j = 12 Hz), 6.39–6.37 (3H, d, j = 12 Hz), 10.17 (1H, s). 13CNMR (600 MHz; CDCl3) = 33.4, 51.2, 55.2, 58.2, 98.5, 108.4, 114.5, 119, 126.2, 127, 128.8, 131.5, 132, 136.8, 142.1, 143, 145.8, 168.4.
Methyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-di-p-tolyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2d): White powder, mp 226-229 °C; IR(KBr): υ = 3240, 3091, 2951, 2861, 1650, 1603, 1586 cm−1. 1HNMR (600 MHz; CDCl3) = 2.33–2.31 (6H, d, j = 12 Hz), 2.71–2.67 (1H, m, j = 24 Hz), 3.92 (3H, s), 5.06–5.04 (1H, d, j = 6 Hz), 6.13–6.11 (2H, d, j = 12 Hz), 6.39–6.36 (2H, d, j = 18 Hz), 10.17 (1H, s). 13CNMR (600 MHz; CDCl3) = 21, 33.4, 51.1, 55, 57.9, 98.6, 108.2, 114.5, 119, 126.2, 127.2, 129, 131, 136.1, 137, 139, 140, 145.9, 155.4, 168.4.
Methyl 1,2,6-tris(4-bromophenyl)-4-((4-bromophenyl)amino)-1,2,5,6-tetrahydropyridine-3-carboxylate (2e): White powder, mp 226–229 °C; IR(KBr): υ = 3233, 3200, 2989, 1714, 1655, 1582 cm−1. 1HNMR (600 MHz; CDCl3) = 2.1 (5H, s), 2.7–2.65 (1H, m, j = 3 Hz), 2.81–2.79 (1H, m, j = 18 Hz), 3.92 (3H, s), 5.03–5.02 (1H, d, j = 6 Hz), 6.30–6.26 (5H, m, j = 24 Hz), 7.13–7.11 (4H, m, j = 12 Hz), 10.18 (1H, s). 13CNMR (600 MHz; CDCl3) = 30.93, 33.4, 51.3, 54.9, 57.4, 98, 109, 114.5, 117.6, 119, 120.5, 121.3, 128, 131.8, 132, 136.5, 140.7, 142, 145, 155.1, 168.1.
Methyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-bis(4-fluorophenyl)-1,2,5,6-tetrahydropyridine-3-carboxylate (2f): white powder, mp 239-241 °C; IR(KBr): υ = 3233, 3200, 2989, 1714, 1655, 1582 cm−1. 1HNMR (600 MHz; CDCl3) = 2.68 (2H, s), 2.82–2.64 (1H, m, j = 18 Hz), 3.82 (3H, s), 5.03–52.02 (3H, d, J = 6 Hz), 6.25 (1H, S), 6.32-6.30 (4H, m, j = 12 Hz), 10.18 (1H,s). 13CNMR (600 MHz; CDCl3) = 30.9, 33.5, 51.3, 54.9, 57.4, 97.9, 114, 120, 121.2, 126.9, 128, 129, 131.5, 136, 140.8, 142.9, 144.1, 155, 168.16.
methyl 2,6-bis(3-bromophenyl)-1-(4-bromophenyl)-4-((4-bromophenyl)amino)-1,2,5,6-tetrahydropyridine-3-carboxylate (2 g): white powder, mp 223–226 °C; IR(KBr): υ = 3233, 3200, 2989, 1714, 1655, 1582 cm−1. 1HNMR (600 MHz; CDCl3) = 1.56 (4H, s), 2.71-2.66 (1H, m, j = 3 Hz), 2.83–2.78 (1H, m, j = 3 Hz), 3.93 (3H, s), 5.05–5.04 (1H, d, j = 6 Hz), 6.31–6.28 (2H, d, j = 18 Hz), 7.16–7.13 (5H, m, j = 18 Hz), 10.17 (1H, s). 13CNMR (600 MHz; CDCl3) = 33.3, 51.3, 55, 57.7, 97.6, 109.2, 114.6, 119.8, 122.7, 125, 127.7, 129.2, 130, 131, 132, 136.5, 144.2, 145.2, 155.2, 168.1.
Methyl 1-(4-methoxyphenyl)-4-((4-methoxyphenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2 h): white powder, mp 292-294 °C; IR(KBr): υ = 3233, 3200, 2989, 1714, 1655, 1582 cm−1. 1HNMR (400 MHz; CDCl3) = 1.99 (3H, s), 2.14 (3H, s), 2.68–2.65 (1H, m, j = 12 Hz), 2.68–2.65 (3H, d, J = 12 Hz), 3.78 (3H, S), 5.25–5.24 (1H, d, J = 4 Hz), 6.18–6.15 (2H, d, j = 12 Hz), 6.25–6.21(3H, t, J = 12 Hz), 6.74–6.72 (2H, d, J = 8 Hz), 6.89–6.87 (2H, d, J = 8 Hz), 7.09–7.07(3H, d, J = 8 Hz), 7.22–7.21(7H, d, J = 4 Hz), 10.01(1H,s). 13CNMR (600 MHz; CDCl3) = 19.6, 20.33, 33.2, 50.9, 54, 56, 97, 112, 124, 124.9, 126, 126.2, 126.26, 128, 128.3, 129, 129.4, 134, 143, 144, 144.2, 155, 167.
Ethyl 1-(4-chlorophenyl)-4-((4-chlorophenyl)amino)-2,6-di-p-tolyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2i): white powder, mp 228-231 °C; IR(KBr): υ = 3230, 3173, 2979, 2864, 1646, 1604, 1504 cm−1. 1HNMR (600 MHz; CDCl3) = 1.47–1.43 (3H, t, j = 24 Hz), 2.7–2.67 (1H, m, j = 18 Hz), 2.86–2.81 (1H, m, J = 3 Hz), 2.33–2.31 (6H, d, j = 12 Hz), 4.34–4.29 (1H, m, j = 3 Hz), 5.06–5.05 (1H, d, j = 6 Hz), 6.43–6.4 (5H, m, j = 18 Hz). 13CNMR (600 MHz; CDCl3) = 14.7, 20.9, 21.1, 33.4, 55.5, 59.8, 98.8, 114, 121, 126, 128.9, 129, 131, 136, 137, 139, 140.2, 145, 155.3, 168.1.
Ethyl 2,6-bis(4-fluorophenyl)-1-phenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate (2j): white powder, mp 215-218 °C; IR(KBr): υ = 3242, 3090, 3061, 3027, 2909, 1647, 1603, 1585, 1451 cm−1. 1HNMR (600 MHz; CDCl3) = 1.485–1.45 (3H, t, j = 18 Hz), 2.71–2.67 (1H, m, j = 18 Hz), 2.87–2.82 (1H, m, J = 3 Hz), 4.49–4.44 (1H, m, J = 3 Hz), 5.1–5.09 (1H, d, j = 6 Hz), 6.43–6.38 (1H, t, j = 3 Hz), 10.23 (1H, s). 13CNMR (600 MHz; CDCl3) = 14.7, 33.48, 51.14, 55.07, 58.01, 98.58, 113, 121, 126, 127, 128, 129, 131, 136.1, 137, 139, 140, 145, 155, 168.4.
Ethyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-di-p-tolyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2 k): white powder, mp 239–241 °C; IR(KBr): υ = 3234, 2922, 1647, 1603 cm−1. 1HNMR (600 MHz; CDCl3) = 1.47–1.43 (3H, d, j = 3 Hz), 2.33–2.31 (6H, d, j = 12 Hz), 2.71–2.67 (1H, t, j = 24 Hz), 2.85–2.83 (1H, m, j = 3 Hz), 4.34–4.29 (1H, m, j = 3 Hz), 5.06–5.05 (1H, d, j = 6 Hz), 6.14–6.12 (2H, d, j = 12 Hz), 6.39–6.37 (3H, m, j = 12 Hz), 10.22 (1H, s). 13CNMR (600 MHz; CDCl3) = 14.7, 20.9, 21.1, 33.4, 55, 58, 59.9, 98.9, 108.2, 114.5, 118.9, 126, 127.2, 129, 131.5, 136.1, 137, 139, 140, 145, 155, 168.1.
Ethyl 1-(4-bromophenyl)-4-((4-bromophenyl)amino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-carboxylate (2 l): white powder, mp 227-230 °C; IR(KBr): υ = 3256, 3084, 2948, 1651, 1599, 1578 cm−1. 1HNMR (600 MHz; CDCl3) = 2.686–2.681 (1H, d, j = 0.3 Hz), 2.84–2.82 (1H, d, j = 12 Hz), 4.33–4.31(1H, m, j = 12 Hz), 5.1–5.09 (1H, d, j = 12 Hz), 7.29–7.27 (7H, m, j = 12 Hz), 10.2 (1H, s). 13CNMR (600 MHz; CDCl3) = 14.7, 33.4, 55.2, 58.2, 59.9, 98.8, 108.4, 114.5, 119.1, 126.4, 127.2, 128.3, 131.6, 136.9, 142.1, 143.1, 145.5, 155.2, 168.1.
Ethyl 2,6-diphenyl-1-(p-tolyl)-4-(p-tolylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate (2 m): white powder, mp 223-226 °C; IR(KBr): υ = 3239, 3024, 2919, 2854, 1617, 1649, 15942 cm−1. 1HNMR (600 MHz; CDCl3) = 1.44 (3H, s), 2.25 (3H, S), 2.14 (3H, S), 2.74–2.7 (1H, m, J = 24 Hz), 4.32–4.31 (1H, m, j = 6 Hz), 4.44–4.43 (1H, m, J = 6 Hz), 6.16–6.14 (2H, d, j = 12 Hz), 6.88–6.85 (4H, m, j = 18 Hz), 10.19 (1H, s). 13CNMR (600 MHz; CDCl3) = 14.8, 20.1, 33.5, 55.1, 58.2, 59.5, 97.7, 112, 125, 126.1, 127, 128, 129.3, 135, 143, 144, 156.4, 168.2.
References
H.F. Jiang, J.H. Li, Z.W. Chen, L-Proline-catalyzed five-component domino reaction leading to multifunctionalized 1,2,3,4-tetrahydropyridines. Tetrahedron 66, 9721–9728 (2010)
H.J. Wang, L.P. Mo, Z.H. Zhang, Cerium Ammonium Nitrate-Catalyzed Multicomponent Reaction for Efficient Synthesis of Functionalized Tetrahydropyridines. ACS. Comb. Sci. 13, 181–185 (2011)
J. Safaei-Ghomi, A. Ziarati, An efficient FeCl3/SiO2 NPs as a reusable heterogeneous catalyzed five-component reactions of tetrahydropyridines under mild conditions. J. Iran. Chem. Soc. 10, 135–139 (2013)
S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, A.C. Willis, Trityl chloride as an efficient organic catalyst for one-pot, five-component and diastereoselective synthesis of highly substituted piperidines. Res. Chem. Intermed. 40, 723–736 (2014)
S. Verma, S. Kumar, S.L. Jain, B. Sain, Thiourea dioxide promoted efficient organocatalytic one-pot synthesis of a library of novel heterocyclic compounds. Org. Biomol. Chem. 9, 6943–6948 (2011)
A.T. Khan, T. Parvin, L.H. Choudhury, Effects of Substituents in the β-Position of 1,3-Dicarbonyl Compounds in Bromodimethylsulfonium Bromide-Catalyzed Multicomponent Reactions: A Facile Access to Functionalized Piperidines. J. Org. Chem. 73, 8398–8402 (2008)
X. Li, Y. Zhao, H. Qu, Z. Mao, X. Lin, Organocatalytic asymmetric multicomponent reactions of aromatic aldehydes and anilines with β-ketoesters: facile and atom-economical access to chiral tetrahydropyridines. Chem. Commun. 49, 1401–1403 (2013)
D.F. Yu, Y. Wang, P.F. Xu, Organocatalytic enantioselective multicomponent cascade reaction: facile access to tetrahydropyridines with C3 all-carbon quaternary stereocenters. Tetrahedron 67, 3273–3277 (2011)
R. Ramachandran, S. Jayanthi, Y.T. Jeong, One-pot synthesis of highly diversified tetrahydropyridines by tandem condensation of aldehydes, amines, and β-ketoesters. Tetrahedron 68, 363–369 (2012)
A.T. Khan, M.M. Khan, K.K.R. Bannuru, Iodine catalyzed one-pot five-component reactions for direct synthesis of densely functionalized piperidines. Tetrahedron 66, 7762–7772 (2010)
A.T. Khan, M. Lal, M.M. Khan, Synthesis of highly functionalized piperidines by one-pot multicomponent reaction using tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 51, 4419–4424 (2010)
S. Mishra, R. Ghosh, Efficient one-pot synthesis of functionalized piperidine scaffolds via ZrOCl2.8H2O catalyzed tandem reactions of aromatic aldehydes with amines and acetoacetic esters. Tetrahedron Lett. 52, 2857–2861 (2011)
W.B. Liu, H.F. Jiang, Z. Shi-Fa, W. Wang, Hydroalkylation leading to heterocyclic compounds (Part 2): practical synthesis of polysubstituted 1,2,3,4-tetrahydropyridines through multicomponent reactions (MCRs). Tetrahedron 65, 7985–7988 (2009)
A. Sambyal, R.K. Bamezai, T.K. Razdan, V.K. Gupta, Synthesis and Crystal Structure of (2S,6R) Ethyl 1,2,6-triphenyl-4-(phenylamino)-1,2,5,6-tetrahydropyridine-3-carboxylate. J. Chem. Crystallogr. 41, 868–873 (2011)
G. Harichandran, S.D. Amalraj, P. Shanmugam, A Facile and Efficient Solid Supported, One-Pot Synthesis of Functionalized Piperidine Derivatives Catalyzed by Amberlite IRA400-Cl Resin/I2/KI via Multicomponent Reaction. J. Heterocycl. Chem. 50, 539–543 (2013)
P.A. Clarke, A.V. Zaytzev, A.C. Whitwood, Pot, atom and step economic (PASE) synthesis of highly functionalized piperidines: a five-component condensation. Tetrahedron Lett. 48, 5209–5212 (2007)
G. Brahmachari, S. Das, Bismuth nitrate-catalyzed multicomponent reaction for efficient and one-pot synthesis of densely functionalized piperidine scaffolds at room temperature. Tetrahedron Lett. 53, 1479–1484 (2012)
X. Zhang, Y. Wan, L. Pang, H. Wang, L. Zhao, C. Wang, S.Y. Hang, G.X. Liu, L.F. Chen, H. Wu, Silica Sulfuric Acid-Catalyzed Five-component Efficient Synthesis of Five-Substituted Tetrahydropyridines. J. Heterocycl. Chem. 51, 442–449 (2014)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, A.C. Willis, One-pot five-component synthesis of highly functionalized piperidines using oxalic acid dihydrate as a homogenous catalyst. Chin. Chem. Lett. 23, 569–572 (2012)
B. Umamahesh, V. Sathesh, G. Ramachandran, M. Sathishkumar, K. Sathiyanarayanan, LaCl3.7H2O as an Efficient Catalyst for One-Pot Synthesis of Highly Functionalized Piperidines via Multi-component Organic Reactions. Catal. Lett. 142, 895–900 (2012)
P. Kar, B.G. Mishra, S.R. Pradhan, Polyphosphoric acid–zirconia pillared clay composite catalytic system for efficient multicomponent one pot synthesis of tetrahydropyridines under environmentally benign conditions. J. Mol. Catal. A: Chem. 387, 103–111 (2014)
A. Javidan, A. Ziarati, J. Safaei-Ghomi, Simultaneous sonication assistance for the synthesis of tetrahydropyridines and its efficient catalyst ZrP2O7 nanoparticles. Ultrason. Sonochem. 21, 1150–1154 (2014)
R. Ghorbani-Vaghei, H. Shahbazi, Highly efficient one-pot synthesis of tetrahydropyridines. C. R. Chimie. 16, 1047–1054 (2013)
H.R. Shaterian, K. Azizi, Acidic ionic liquids catalyzed one-pot, pseudo five-component, and diastereoselective synthesis of highly functionalized piperidine derivatives. J. Mol. Liq. 180, 187–191 (2013)
S.S. Sajadikhah, N. Hazeri, M.T. Maghsoodlou, S.M. Habibi-Khorassani, A. Beigbabaei, A.C. Willis, Al(H2PO4)3 as an efficient and reusable catalyst for the multi-component synthesis of highly functionalized piperidines and dihydro-2-oxypyrroles. J. Iran. Chem. Soc. 10, 863–871 (2013)
M. Misra, S.K. Pandey, V.P. Pandey, J. Pandey, R. Tripathi, R.P. Tripathi, Organocatalyzed highly atom economic one pot synthesis of tetrahydropyridines as antimalarials. Bioorg. Med. Chem. 17, 625–633 (2009)
M.R. Mousavi, J. Aboonajmi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, M. Safarzaei, La(NO3)3.6H2O Catalyzed One-pot Highly Diastereoselective Synthesis of Functionalized Piperidines. Lett. Org. Chem. 10, 171–177 (2013)
S. Khaksar, S.M. Vahdat, M. Alipour, Cerium(IV) triflate-catalyzed domino annulation approaches to the synthesis of highly substituted piperidines. C. R. Chimie. 16, 1024–1028 (2013)
B.G. Trewyn, J.A. Nieweg, Y. Zhao, V.S.Y. Lin, Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. J. Chem. Eng. 137, 23–29 (2008)
B. Sreedhar, P. Radhika, B. Neelima, N. Hebalkar, Regioselective ring opening of epoxides with amines using monodispersed silica nanoparticles in water. J. Mol. Catal. A. 272, 159–163 (2007)
M. Najafi, Y. Yousefi, A.A. Rafati, Synthesis, characterization and adsorption studies of several heavy metal ionson amino-functionalized silica nano hollow sphere and silica gel. Sep. Pur. Tech. 85, 193–205 (2012)
Z.Z. Li, L.X. Wen, L. Shao, J.F. Chen, J. Cont, Fabrication of porous hollow silica nanoparticles and their applications in drug release control. Release. 98, 245–254 (2004)
B. Sreedhar, D. Yada, P. Surendra Reddy, Nanocrystalline Titania-Supported Palladium(0) Nanoparticles for Suzuki–Miyaura Cross-Coupling of Aryl and Heteroaryl Halides. Adv. Synth. Catal. 353, 2823–2836 (2011)
B. Sreedhar, A. Suresh Kumar, D. Yada, CuFe2O4 nanoparticles: a magnetically recoverable and reusable catalyst for the synthesis of 5-substituted 1H-tetrazoles. Tetrahedron Lett. 52, 3565–3569 (2011)
L. He, A.F. Dexter, A.P.J. Middelberg, Biomolecular engineering at interfaces. Chem. Eng. Sci. 61, 989–1003 (2006)
B. Sreedhar, A. Suresh Kumar, P. Surendra Reddy, Magnetically separable Fe3O4 nanoparticles: an efficient catalyst for the synthesis of propargylamines. Tetrahedron Lett. 51, 1891–1895 (2010)
M.A. Zolfigol, Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron 57, 9509–9511 (2001)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H. Khavasi, R. Chem, Hantzsch reaction on free nano-Fe2O3 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability. Commun. 47, 9230–9232 (2011)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Rapid synthesis of 1-amidoalkyl-2-naphthols over sulfonic acid functionalized imidazolium salts. Appl. Catal. A: General 400, 70–81 (2011)
P. Salehi, M.A. Zolfigol, F. Shirini, M. Baghbanzadeh, Silica Sulfuric Acid and Silica Chloride as Efficient Reagents for Organic Reactions. Curr. Org. Chem. 10, 2171–2189 (2006)
M.A. Zolfigol, P. Salehi, M. Shiri, A. Sayadi, A. Abdoli, H. Keypour, M. Rezaeivala, K. Niknam, E. Kolvari, A simple and efficient route for the synthesis of di and tri(bis(indolyl) methanes) as new triarylmethanes. Mol. Divers. 12, 203–207 (2008)
M.A. Zolfigol, A. Khazaei, M. Safaiee, M. Mokhlesi, R. Rostamian, M. Bagheri, M. Shiri, H.G. Kruger, J. Mole, Application of silica vanadic acid as a heterogeneous, selective and highly reusable catalyst for oxidation of sulfides at room temperature. Catal. A: Chem. 370, 80–86 (2013)
M.A. Zolfigol, A. Khazaei, M. Mokhlesi, F. Derakhshan-Panah, J. Mole, Synthesis, characterization and catalytic properties of monodispersed nano-sphere silica sulfuric acid. Catal. A: Chem. 370, 111–116 (2013)
A. Khazaei, M.A. Zolfigol, M. Mokhlesi, R. Rostamian, Nano-sphere silica sulfuric acid: novel and efficient catalyst in the one-pot multi-component synthesis. J. Iran. Chem. Soc. 10, 1297–1301 (2013)
A. Khazaei, M.A. Zolfigol, M. Mokhlesi, A. Zare, F. Derakhshan-Panah, M. Merajoddin, H. Keypour, A.A. Dehghani-Firouzabadi, Pyrazinium Di(hydrogen sulfate) as a Novel, Highly Efficient and Homogeneous Catalyst for the Condensation of Enolizable Ketones with Aldehydes, Acetonitrile and Acetyl Chloride. J. Chin. Chem. Soc. 58, 199–207 (2011)
J. Zhu, H. Bienayme, Multicomponent Reactions (Wiley-VCH, Weinheim, Germany, 2005)
Acknowledgments
The authors acknowledge the Bu-Ali Sina University Research Council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for providing support to this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daraei, M., Zolfigol, M.A., Derakhshan-Panah, F. et al. Synthesis of tetrahydropyridines by one-pot multicomponent reaction using nano-sphere silica sulfuric acid. J IRAN CHEM SOC 12, 855–861 (2015). https://doi.org/10.1007/s13738-014-0548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0548-x