Abstract
A simple method for the oxidative aromatization of Hantzsch 1,4-dihydropyridines to the corresponding pyridines is described using hydrogen peroxide as green oxidant and silica vanadic acid as catalyst in acetonitrile at room temperature. The catalyst can be easily recovered and reused for 15 reaction cycles without considerable loss of activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Development of heterogeneous catalysts for fine chemicals synthesis has become a major area of research. One of the major drawbacks of the homogeneous catalysts is the difficulty in separating the relatively expensive catalysts from the reaction mixture at the end of the process. The possible contamination of catalyst in the product also restricts their use in industry. Efficient anchoring of these catalysts on supports may overcome these drawbacks. Since the catalytic action occurs at specific sites on the solid surface, often called “active sites”, the uniform dispersion of metal catalysts is highly desirable for significant improvement of catalytic action. These types of catalysts have used various support materials. One of the useful examples of support materials is silica gel. This inorganic supporter has some advantageous properties such as excellent stability, good accessibility and high surface area; this will be beneficial to the enhancement of loading amount and dispersion of catalytic action [1]. Also, the design of silica-supported transition metal oxides represents a rapidly growing field that has been significantly applied in industry. Among the various transition metals, vanadium, an early member of the 3d transition metal series, exists on the surface of the earth more abundantly and catalytic systems based on the supported vanadium oxide exhibit interesting catalytic properties for the partial oxidation of alkanes, aromatics, alcohols, and alkenes and dehydrogenation of different hydrocarbons [2–4].
Hantzsch 1,4-dihydropyridines are easily prepared from the Hantzsch reaction or its modifications [5–8]. In recent years, it has been found that drugs such as nifedipine and niguldipine undergo redox processes due to the catalysis of cytochrome P-450 in the liver during their metabolism [9]. Therefore, the oxidation of 1,4-dihydropyridines has attracted considerable attention. Several oxidation reagents have been reported for the oxidation of 1,4-dihydropyridines, such as SeO2 [10], lead(IV) tetraacetate [11], FeCl3/KMnO4 [12], Pd/C [13], nicotinium dichromate [14], silica-gel-supported ferric nitrate (silfen), N2O3, MCl x /NaNO2/wet SiO2 and silica chloride/NaNO2 wet SiO2 [15, 16], H2O2/Co(OAc)2 [17], NaHSO4/Na2Cr2O7/wet SiO2 [18], peroxydisulfate-cobalt(II) [19], Zr(NO3)4 [20], Co(II)-catalyzed auto oxidation) [21], KBrO3/MCl n [22, 23], (n-Bu)4IO4/[Mn(TPP)Cl] [24], Mn(III)-salophen/NaIO4 [25], oxone [26], M(NO3)2.XH2O [27], NaClO2 [28], graphite oxide [29], and Solid acid/NaNO2 [30]. In this paper, we want to apply silica vanadic acid in the oxidation of dihydropyridines.
Experimental
Synthesis of silica vanadic acid
In a 100 mL round-bottomed flask, n-hexane (25 mL) and silica gel (4.0 g) were stirred for 10 min and then vanadiumoxytrichloride (4.0 g) was dissolved in n-hexane (25 mL) and added drop wise to the silica gel suspension (15–20 min). After addition of VOCl3, the red-brown mixture was stirred for 10 h. Then, solvent was evaporated and the residue (the red-brown powder (II) was obtained after the grafting process) was filtered, washed with dry n-hexane several times to remove unreacted VOCl3, dried under vacuum, and stirred in the air for 72 h to promote the hydrolysis of V–O–Cl bonds. At this time the red-brown powder changed to dark green powder (III) slowly (Scheme 1) [31, 32]. Typical loading of vanadium was determined using X-ray fluorescence (XRF) and showed a loading 8 ± 0.1 mmol g–1.
General procedure for the oxidation of 1,4-dihydropyridines
A suspension of compound 1, silica vanadic acid, and H2O2 (5 mmol) in CH3CN (5 mL) was stirred at room temperature for a specific time (molar ratio of reagents and reactions times are indicated in Table 1). The progress of the reaction was monitored by thin-layer chromatography (TLC). After the spot of starting material 1 on TLC disappeared (Scheme 2), the reaction mixture was filtered off. The solvent was removed, and pure product 2 was obtained.
Results and discussion
In continuation of our studies on the synthesis of new inorganic silica-based resins [33], we found that silica gel reacts with vanadiumoxytrichloride to give silica vanadic acid.
Based on our previous studies on the use of silica vanadic acid as catalyst for carrying out organic reactions [31, 32], and continuing our interest in aromatization of Hantzsch 1,4-dihydropyridines [34], we examined the possibility of using a silica vanadic acid as a oxidation agent in the presence of H2O2 on the oxidation aromatization of Hantzsch 1,4-dihydropyridines.
A good range of dihydropyridines(1) was subjected to the oxidation reaction in the presence of silica vanadic acid and H2O2, in acetonitrile (Scheme 2).
We preferred hydrogen peroxide as oxidant over other available oxidizing agent since it is cheap, operationally safe, environmentally friendly and easy to handle while it produces only water as a by-product, which reduces purification requirements.
In order to get the best reaction condition, the efficiency of a variety of silica vanadic acid (SVA) in the oxidation of diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate as a model reaction was studied. The best catalytic activity was optimized to be 0.01 g for 1 mmol of starting material in the model reaction and using the catalyst excess (0.05 g) does not show further increase in the conversion and yield of the product. Also, in the absence of catalyst or oxidant, the reaction goes slow or is not complete (Table 2).
Solvent effect on oxidation reaction, in ethanol, methanol, chloroform, ethylacetate, water, n-hexane, acetonitrile and dichloromethane was investigated. Although these organic mediums were found unsuitable for this oxidative aromatization, SVA/H2O2 system worked quite efficiently in acetonitrile. The results of this study are summarized in Table 3.
A series of 1,4-DHPs were aromatized using the optimized reaction conditions (as shown in Table 1). The reaction proceeded smoothly with various 1,4-DHPs bearing electron rich as well as electron deficient aromatic substituents at C-4. The reaction is quite general and is successfully applied to 1,4-DHPs having aliphatic as well as, substituents at C-4.
The reaction was mild and the workup was straightforward, requiring simple evaporation of the solvent; then, highly pure pyridine derivatives 2 could be obtained if the product is passed through a short pad of silica gel and evaporation of the solvent. The results and reaction conditions are given in Table 1.
In order to recover the catalyst, CH3CN was evaporated under reduced pressure and removing the product by extracting with ether from the reaction mixture and charging the reaction vessel with fresh substrate and H2O2 30 % and then repeating the experiment. The recovered catalyst was reused fifteen times in the oxidation of 3,5-diacetyl-1,4-dihydro-2,6-dimethylpyridine (1a) with H2O2, and smooth loss of catalytic activity was observed from the 11th time of reuse (Table 4).
To assess the capability and efficiency of our catalyst with respect to the reported catalysts for oxidation of dihydropyridines, the results of the application of these catalysts for the preparation of some pyridines are tabulated in Table 5. The described method has many advantages over other methods, such as, high recycling, short reaction time, high TON and TOF of catalyst.
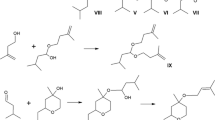
A plausible mechanism of these oxidizing systems is shown in Scheme 3. The catalytic cycle involves, as fundamental step, the formation in the active site of a peroxovanadium species (III) [31, 32], which is a much stronger oxidant than H2O2. This is followed by proton transfer from SVA to generate peroxidic species (V) [38, 39]. The nitrogen atom of 1,4-DHP attacked peroxidic oxygen(V) to produce hydroxylammonium ion (VI), then proton transfer from nitrogen to hydroxy group gives protonated N-hydroxyl-1,4-dihydropyridine, and finally dehydration and deprotonation of protonated N-hydroxyl-1,4-dihydropyridine afford the pyridine as depicted in scheme 3 [40].
In conclusion, our goal in undertaking this line of work was fourfold: (a) to simplify operation due to the heterogeneous nature of reaction; (b) to develop a high yielding conversion of 1,4-dihydropyridines to their corresponding pyridines; (c) to apply the catalytic system with high recycling by using a novel synthetic silica-based oxidant; (d) to apply room temperatures for these conversions.
References
M.R. Maurya, A. Kumar, J.C. Pessoa, Coord. Chem. Rev. 255, 2315 (2011)
B.M. Weckhuysen, D.E. Keller, Catal. Today 78, 25 (2003)
R. Neumann, M. Leavin-Elad, Appl. Catal. A 122, 85 (1995)
A. Bottino, G. Capannelli, A. Comite, S. Storace, R. Di, Felice. Chem. Eng. J. 94, 11 (2003)
M.A. Zolfigol, M. Safaiee, Synlett 5, 827 (2004)
M.A. Chari, K. Syamasundar, Catal. Commun. 6, 624 (2005)
M.A. Zolfigol, P. Salehi, M. Safaiee, Lett. Org. Chem. 3, 39 (2006)
G. Sabitha, G.S.K.K. Reddy, C.S. Reddy, J.S. Yadav, Tetrahedron Lett. 44, 4129 (2003)
A. Ghorbani-Choghamarani, M.A. Zolfigol, M. Hajjami, S. Rastgoo, S. Mallakpour, Lett. Org. Chem. 7, 249 (2010)
X. Cai, H. Yang, G. Zhang, Can. J. Chem. 83, 273 (2005)
M. Litvic, I. Cepanec, M. Filipan, K. Kos, A. Bartolincic, V. Druskovic, M.M. Tibi, V. Vinkovic, Heterocycles 65, 23 (2005)
J.J. Xia, G.W. Wang, Synthesis (14), 2379 (2005)
N. Nakamichi, Y. Kawashita, M. Hayashi, Org. Lett. 4, 3955 (2002)
M.M. Sadeghi, I. Mohammadpoor-Baltork, H.R. Memarian, S. Sobhani, Synth. Commun. 30, 1661 (2000). and references cited therein
M.A. Zolfigol, A. Ghorbani Choghamarani, S. Dialameh, M.M. Sadeghi, I. Mohammadpoor-Baltork, H.R. Memarian, J. Chem. Res-S. (2003) 18, and references cited therein
M.A. Zolfigol, F. Shirin, A. Ghorbani Choghamarani, I. Mohammadpoor-Baltork, Phosphorus. Sulfur. 178, 1709 (2003). and references cited therein
M.M. Hashemi, Y. Ahmadibeni, H. Ghafuri, Monatshefte Fur. Chem. 134, 107 (2003)
M.A. Zolfigol, M.M. Sadeghi, I. Mohammadpoor-Baltork, A. Ghorbani-Choghamarani, A. Taqian-Nasab, Asian J. Chem. 13, 887 (2001)
M. Anniyappan, D. Muralidharan, P.T. Perumal, Tetrahedron 58, 5069 (2002)
G. Sabitha, G.S.K. Kumar Reddy, C.S. Reddy, N. Fatima, J.S. Yadav, Synthesis 8, 1267 (2003)
S.P. Chavan, R.K. Kharul, U.R. Kalkote, I. Shivakumar, Synth. Commun. 38, 1333 (2003)
B. Zeynizadeh, K.A. Dilmaghani, A. Roozijoy, J. Chem. Res. (S). (2005) 675
B. Zeynizadeh, K.A. Dilmaghani, A. Roozijoy, Synth. Commun. 35, 557 (2005)
M. Nasr-Esfahani, M. Moghadam, S. Tangestaninejad, V. Mirkhani, Bioorg. Med. Chem. Lett. 15, 3276 (2005)
M. Nasr-Esfahani, M. Moghadam, S. Tangestaninejad, V. Mirkhani, A.R. Momeni, Bioorg. Med. Chem. 14, 2720 (2006)
Z. Liu, W. Yu, Li Yang, Z.L. Liu, Tetrahedron Lett. 48, 5321 (2007)
A.C. Shaikh, C. Chinpiao, Bioorg. Med. Chem. Lett. 20, 3664 (2010)
X. Liao, W. Lin, J. Lu, C. Wanga, Tetrahedron Lett. 51, 3859 (2010)
M. Mirza-Aghayan, R. Boukherroub, M. Nemati, M. Rahimifard, Tetrahedron Lett. 53, 2473 (2012)
M.A. Zolfigol, M. Bagherzadeh, K. Niknam, F. Shirini, I. Mohammadpoor-Baltork, A. Ghorbani, A. Ghorbani Choghamarani, M. Baghbanzadeh, J. Iran. Chem. Soc. 3, 73 (2006)
M.A. Zolfigol, A. Khazaei, M. Safaiee, M. Mokhlesi, R. Rostamian, M. Bagheri, M. Shiri, H.G. Kruger, J. Mol. Catal. A. Chem. 370, 80 (2013)
A. Khazaei, M.A. Zolfigol, M. Safaiee, M. Mokhlesi, E. Donyadari, M. Shiri, H.G. Kruger, Catal. Commun. 26, 34 (2012)
M.A. Zolfigol, P. Salehi, A. Ghorbani Choghamarani, M. Safaiee, M. Shahamirian, Synth. Commun. 37, 1817 (2007)
M.A. Zolfigol, A. Ghorbani Choghamarani, M. Shahamirian, M. Safaiee, I. Mohammadpoor-Baltork, S. Mallakpour, M. Abdollahi-Alibeik, Tetrahedron Lett. 46, 5581 (2005)
M.M. Heravi, H.A. Oskooie, R. Malakooti, B. Alimadadi, H. Alinejad, F.K. Behbahani, Catal. Commun. 10, 819 (2009)
M. Nasr-Esfehani, M. Moghadam, G. Valipour, Synth. Commun. 39, 3867 (2009)
M.M. Heravi, F.K. Behbahani, H.A. Oskooie, R. Hekmat Shoar, Tetrahedron Lett. 46, 2775 (2005)
V. Conte, A. Coletti, B. Floris, G. Licini, C. Zonta, Coord. Chem. Rev. 255, 2165 (2011)
O. Bortolini, V. Conte, J. Inorg. Biochem. 99, 1549 (2005)
Z.Y. Chen, W. Zhang, Chin. Chem. Lett. 18, 1443 (2007)
Acknowledgments
Financial support for this work by the Center of Excellence of Development of Chemical Methods (CEDCM) of Bu Ali Sina University, Hamedan, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safaiee, M., Zolfigol, M.A., Tavasoli, M. et al. Application of silica vanadic acid [SiO2-VO(OH)2] as a heterogeneous and recyclable catalyst for oxidative aromatization of Hantzsch 1,4-dihydropyridines at room temperature. J IRAN CHEM SOC 11, 1593–1597 (2014). https://doi.org/10.1007/s13738-014-0431-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-014-0431-9