Abstract
There are many medicinal plants in traditional medicine which are used to prevent, control, and treat anemia. One of these plants is Allium saralicum R.M. Fritsch. The purpose of our research was to investigate the effect of aqueous extract of A. saralicum leaf in the treatment of hemolytic anemia. In this study, 60 rats were used. Induction of hemolytic anemia was done by three injections of Phenylhydrazine in 50 animals. Then, the rats were divided into six subgroups, including negative healthy control, untreated negative control, and four groups receiving the A. saralicum at 25, 50, 100, and 200 mg/kg concentrations. At the end of day 15 of treatment, the animals of all groups were weight and then sacrificed. The blood, liver and spleen samples were drawn immediately to analyze the hematological, biochemical and histological parameters. All groups of A. saralicum (especially AS200) significantly (p ≤ 0.05) reduced the raised concentrations of Fe, ferritin, erythropoietin, ALP, AST, ALT, GGT, cholesterol, LDL, triglyceride, total and conjugated bilirubin, urea, and creatinine and increased the levels of body weight, HDL, total protein, albumin, WBC, lymphocyte, monocytes, platelet, RBC, Hb, PCV, MCV, MCH, and MCHC as compared to the untreated group. Also, A. saralicum at all doses prevented pathological changes in the liver and spleen. In conclusion, because of aqueous extract of A. saralicum leaf anti-anemic property, it can be used as a medical supplement or drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood disorders are one of the most important systemic disorders that cause many deaths each year. Anemia is a common blood disorder in which there are no sufficient red blood cells or hemoglobin. One kind of anemia is hemolytic anemia. It is caused by hemolysis (destruction of red blood cells) (Qaseem et al. 2013). The common causes of hemolytic anemia are enlarged spleen, hepatitis, Epstein-Barr virus, typhoid fever, E. coli toxin, leukemia, lymphoma, tumors, lupus and etc (Sato et al. 2012). The most usual symptoms of hemolytic anemia include weakness, irregular or fast heartbeat, lightheadedness or mild vertigo, pale mucosal membrane and pale skin and nail bed, short nail, chest pain, numbness or coldness of the extremities, and headache (Telford et al. 2003). Hemolytic anemia is usually followed by raised bilirubin and hematopoiesis in bone marrow and jaundice, due to hemolysis (Telford et al. 2003). Use of ethno medicinal plants along with chemical drugs has always been taken into account for prevention, control, and treatment of hemolytic anemia (Zangeneh et al. 2018b; Boretti et al. 2009; Holley and Patel 2005; McKie et al. 2004). Use of plants to treat diseases has a history longer than human history (Han 2000; Farzaei et al. 2018; Hamelian et al. 2018; Sayyedrostami et al. 2018; Zhaleh et al. 2018). Plants do not have or have fewer side effects like those of chemical drugs. In Iranian traditional medicine, there are many plants for prevention, control, and treatment of hemolytic anemia. A list of these plants with their family are given below; Ananas comosus (Bromeliaceae), Allium sativum (Alliaceae), Centaurea cyanus (Asteraceae), Brassica rapa (Brassicaceae), Citrus latifolia (Rutaceae), Cucumis melo (Cucurbitaceae), Cucumis melo var.inodorus (Cucurbitaceae), Ficus carica (Moracee), Daucus carota (Apiaceae), Matricaria chamomilla (Asteraceae), Foeniculum vulgare (Apiaceae), Petroselinum crispum (Apiaceae), Oriyganum vulgare (Lamiaceae), Phaseolus vulgaris (Fabaceae), Raphanus sativus (Brassicaceae), Prunus armeniaca (Rosaceae), Rosmarinus officinalis (Lamiaceae), Rheum officinale (Polygonaceae), Solanum tuberosum L. (Solanaccae), Solanum lycopersicum (Solanaceae), Spinacia oleracea L. (Chenopdiaceae), Urtica dioica (Urticaceae), and Thymus vulgaris (Lamiaceae) (Cheraghi and Asadi-Samani 2016). One of the most important herbal medicines which are widely used for the treatment of blood disorders is Allium saralicum R.M. Fritsch from Plantae kingdom, Liliopsida class, Asparagales order, Amaryllidaceae family, and Allium genus (Sherkatolabbasieh et al. 2017). It is widely distributed in Iran, Iraq, and Turkey. A. saralicum is a good source of low-cost food and is a perfect part of Iranian diet (Zangeneh et al. 2018a). It has been used for its anti-inflammatory, antibacterial, antifungal, immunostimulatory, nephroprotective, hepatoprotective, and anti-hyperlipidemic effects (Sherkatolabbasieh et al. 2017; Goodarzi et al. 2017, 2018; Zangeneh et al. 2018a). A. saralicum has a long history of use in Iranian traditional medicine for treatment of hemolytic anemia (Zangeneh et al. 2018c). The high prevalence of hemolytic anemia in the whole world has drawn the attention of researchers to finding preventive and remedial methods to control this disease. In this regard, we attempted to study the potential of aqueous extract of A. saralicum leaf on the treatment of hemolytic anemia in rats.
Materials and methods
Extract preparation method
The leaves of A. saralicum was collected from Kemanshah city. For preparation of aqueous extract of A. saralicum, 200 g of leaves of A. saralicum were powdered and 200 g of the powder was extracted by maceration at room temperature (24 ± 3 °C) using 1000 mL of distilled water as solvent for 72 h. The extract was filtered and evaporated at reduced pressure to yield residues of 18.6% based on the dry plant material. The extract was stored at − 18 °C for experimental procedure. The powder of the obtained extract weighed as required depending on the dose and dissolved in normal saline. It was then administered to the rats by the oral catheter.
Experimental design
This experimental study was conducted on 60 Wistar male rats with the weight of 210 ± 5 g that were kept in individual cages for 10 days to adapt to the environment. During the experiments, the temperature of the animal house was adjusted at 22 ± 3 °C under a 12 h dark/light cycle. To induce hemolytic anemia, to all animals, except of negative healthy control, were injected intravenously (in the caudal vein) with Phenylhydrazine 20 mg/kg at three various times (Days 1, 3 and 5). Then, the animals were divided into six subgroups, including negative healthy control receiving distilled water, the untreated negative control receiving distilled water, and four groups receiving the A. saralicum aqueous extract at 25, 50, 100, and 200 mg/kg concentrations. One day after the last injection of the Phenylhydrazine, the rats underwent oral treatment of various doses of A. saralicum aqueous extract for 15 days (Days 6–21). On the 21st, 2 h after oral administration of various doses of A. saralicum aqueous extract, the rats were weighted and then sacrificed (Lee et al. 2014).
Determination of hematological parameters
After scarification, blood samples were taken from the rats’ heart to analyze hematological parameters including white blood cell (WBC), platelet, red blood cell (RBC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). For determination of hematological parameters, we used from Sysmex XP-300™ Automated Hematology Analyzer.
Determination of biochemical parameters
In our study, biochemical parameters including alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride, total and conjugated bilirubin, albumin, total protein, urea, creatinine, ferrous (Fe), ferritin, and erythropoietin determined by enzyme-linked immunosorbent assay and Pars-Azmoon kits.
Liver and spleen histology
For histopathological evaluations, a small piece of liver and spleen tissues were fixed with 10% formalin and embedded in paraffin. Serial sections (thickness of 5 µm) were processed and stained with Hematoxylin and Eosin (H&E) according to standard pathology laboratory procedures. Stained liver and spleen slices were evaluated under light microscopy.
Statistical analysis
All data were analyzed by SPSS-21 software using one-way ANOVA followed by Duncan’s test. Data were considered statistically significant at p ≤ 0.05.
Results
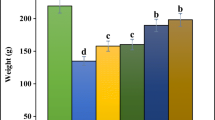
Effect of A. saralicum leaf aqueous extract on body weight
Body weight reduced significantly (p ≤ 0.05) in untreated animals compared to the control ones (Fig. 1). Administration of A. saralicum leaf aqueous extract at all doses significantly (p ≤ 0.05) enhanced body weight in comparison with the untreated group. There were no remarkable changes (p ≤ 0.05) among AS25, AS50, and AS100 groups. Among the treatment groups, the best result was found on AS200. In during of experiment, there wasn’t significant difference (p ≤ 0.05) in the food intake of all groups.
Effect of A. saralicum leaf aqueous extract on the concentrations of hematological parameters
The numbers of WBC, platelet, and RBC, the percentages of lymphocyte and monocyte, and the levels of Hb, PCV, MCV, MCH, and MCHC were significantly (p ≤ 0.05) decreased in the untreated group. The treatment with A. saralicum leaf aqueous extract significantly (p ≤ 0.05) raised the above parameters. No remarkable changes (p ≤ 0.05) were observed among all groups in the percentages of neutrophil, eosinophil, and basophil. There weren’t remarkable change (p ≤ 0.05) in percentages of lymphocyte, neutrophil, monocyte, eosinophil, and basophil among various doses of A. saralicum and control group (Figs. 2, 3, 4, 5, 6, 7, 8 and 9).
Effects of A. saralicum leaf aqueous extract on the concentrations of biochemical parameters
The results of the biochemical parameters are revealed in Figs. 10, 11, 12, 13, 14, 15, 16 and 17. Phenylhydrazine-induced toxicity reduced the concentrations of HDL, total protein, and albumin, and enhanced Fe, ferritin, erythropoietin, ALP, AST, ALT, GGT, cholesterol, LDL, triglyceride, total and conjugated bilirubin, urea, and creatinine significantly (p ≤ 0.05) as compared to the control group. Various doses of A. saralicum leaf aqueous extract significantly (p ≤ 0.05) ameliorated above parameters as compared to the untreated group. There weren’t remarkable change (p ≤ 0.05) between AS200 and control groups in the concentrations of LDL and HDL. Administration of AS100 and AS200 significantly (p ≤ 0.05) reduced the levels of GGT and triglyceride similar to the control group. No remarkable changes (p ≤ 0.05) were found among all groups of A. saralicum and control group in the levels of total and conjugated bilirubin. In general, among the treatment groups, the best result of biochemical parameters was found on AS200.
The levels of ALP, AST, ALT, and GGT in various groups. C Control, U untreated, AS Allium saralicum R.M. Fritsch, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyl transferase. Non-like letters show a remarkable change between the various groups (p ≤ 0.05)
Effects of A. saralicum leaf aqueous extract on the histological parameters
The histopathologic sections of the liver of the untreated rats showed degenerative changes in the hepatocytes represented by disorganization of the hepatic cords, enlargement and congestion of the central veins with mild hepatocellular necrosis and the sinusoids were infiltrated by mild nonspecific inflammatory cells. The hepatocytes of the untreated rats showed morphological change such as pyknosis, karyorrhexis, chromatolysis and cytoplasmic vacuolization (Fig. 18). However, the liver of the treated rats with A. saralicum leaf aqueous extract at all doses revealed slight improvement in the structure of the hepatic tissue compared to those of the untreated ones, except for a few mildly degenerated hepatocytes around the central vein of the A. saralicum treated rats which still had some cytoplasmic vacuoles, other hepatocytes and portal and sinusoidal areas were almost normal (Fig. 18). The liver of the normal control rats had normal structure.
The histopathology of the spleen associated with hemolytic anemia is shown in Fig. 19. There were no particular changes in the spleen of the control and A. saralicum groups. In untreated group, congestion and enlargement of spleen vessels, an excessive accumulation of RBCs resulting from the enhanced sequestration and phagocytosis of them, was observed in the red pulp of spleen.
Discussion
Phenylhydrazine is toxic for the body and impairs various tissues while entering the body (Sato et al. 2012). Studies have revealed that Phenylhydrazine causes oxidative stress, production of free radicals, lipid peroxidation, oxidative degradation of spectrum cell membrane, and lysis of red blood cells (Zangeneh et al. 2018c; Sato et al. 2012). It indicated that Phenylhydrazine caused the conversion of hemoglobin to methemoglobin, therefore it plays a major role in forming the Hains bodies (McKie et al. 2004). Studies have shown Phenylhydrazine, by increasing hemolysis (increased the levels of ferrous, ferritin, and erythropoietin), causes reduction of mean number of testicular sperms (through atrophy of testicular structure), spleen enlargement and chronic failure (through hypertrophy of spleen cells), chronic and acute renal failure (by the destruction of structures such as proximal and distal renal cells), and liver enlargement and chronic failure (through hypertrophy of liver cells) (Zangeneh et al. 2018c; Goorani et al. 2018; Shukla et al. 2012). In a study reported that Phenylhydrazine with degenerating of proximal convoluted tubules, distal convoluted tubules, glomeruli, and hepatocytes, increased the biochemical parameters of the kidneys and liver in the blood. Also, in the previous study revealed that Phenylhydrazine reduced body weight and increased the weight and volume of the adrenal glands, kidneys, liver, and spleen (Diallo et al. 2008; Ryu and Yook 2001). According to the above studies, in our study indicated that Phenylhydrazine significantly (p ≤ 0.05) decreased the levels of body weight, total protein, albumin, WBC, neutrophils, monocytes, platelet, RBC, Hb, PCV, MCV, MCH, and MCHC, and enhanced the concentrations of ALP, AST, ALT, GGT, Fe, ferritin, erythropoietin, cholesterol, LDL, triglyceride, total and conjugated bilirubin, urea, and creatinine. Also, A. saralicum at all doses inhibited pathological changes in the liver and spleen. In spite of hematotoxicity, hepatotoxicity, and splenotoxicity effects of Phenylhydrazine, the treatment with various doses of aqueous extract of A. saralicum leaf significantly (p ≤ 0.05) ameliorated the levels of above parameters. In a study, A. saralicum decreased the raised volume of the liver, hepatocytes, and sinusoids and also the levels of hepatic biochemical parameters (ALP, ALT, and AST) as compared to the CCl4-treated group (Goodarzi et al. 2017). In other study indicated that A. saralicum had strong nephroprotective effect against CCl4, so that it reduced the volume of kidneys and their subcomponents include proximal convoluted tubules, vessels, and interstitial tissues in comparison with the untreated group. Also in the previous study reported that A. saralicum with raising the clearance rate of the kidney, decreased the concentrations of urea and creatinine (Sherkatolabbasieh et al. 2017).
Of the herbs that are antioxidant, A. saralicum has strong antioxidant property. In a study indicated that A. saralicum collected in Iran were rich in antioxidant compounds includes 1,4,8,11-tetraazacyclotetradecane, hexanedioic acid, 3,7,11,15-tetramethyl, 2-hexadecene, n-ethyl-1,3-dithioisoindoline, eicosane, γ-tocopherol, hexatriacontane, n-tetracosane, ethanol, 2-tetradecyloxy, vitamin E, hexadecanoic acid, 2-phenyl-5-methylindole, neophytadiene, phytol, and especially linolenic acid, methyl ester (Goodarzi et al. 2018). Antioxidants can play a key role in destruction of free radicals and toxic materials and maintenance of hemostasis because free radicals interfere with biological cell membrane such as red blood cells through peroxidation of unsaturated fatty acids and bring about pathological changes (Onyeabo et al. 2017; Bjelakovic et al. 2004). In study of Zangeneh et al. (2018c) revealed that the aqueous extract of Allium eriophyllum Boiss leaf (Other specious of Allium genus) had anti-anemic effect against phenylhydrazine-induced hemolytic anemia in Wistar male rats. In previous study, anti-anemic potential of Allium eriophyllum Boiss related to the antioxidant compounds such as neophytadiene, phytol, and especially linolenic acid, methyl ester. Hence, it can be assumed that A. saralicum is rich of antioxidant compounds especially linolenic acid, methyl ester and plays a pivotal role in the prevention, control, and treatment of several disorders such as hemolytic anemia by the destruction of free radicals.
Conclusion
As shown in the findings, aqueous extract of A. saralicum leaf improved the hematological, biochemical and histological parameters, and raise the decreased level of body weight in Phenylhydrazine-induced anemic animals. It is offered that clinical trials be conducted to achieve the remedial property in human.
References
Bjelakovic G, Nikolova D, Simonetti RG, Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364:1219–1228
Boretti FS, Buehler PW, D’Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ (2009) Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119(8):2271–2280
Cheraghi M, Asadi-Samani M (2016) Hematopoietic medicinal plants based on ethnobotanical documents of Iran: a strategy to develop nature-based drugs effective on anemia. Pharm Lett 8(5):393–399
Diallo A, Gbeassor M, Vovor A, Eklu-Gadegbeku K, Aklikokou K, Agbonon A, Abena AA, de Souza C, Akpagana K (2008) Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats. Fitoterapia 79(5):332–336
Farzaei MH, Zangeneh MM, Goodarzi N, Zangeneh A (2018) Stereological assessment of nephroprotective effects of Trachyspermum ammi essential oil against carbon tetrachloride-induced nephrotoxicity in mice. Int J Morphol 36(2):750–757
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4-induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Goodarzi N, Zangeneh MM, Zangeneh A (2018) The effect of ethanolic extract of Allium saralicum R.M. Fritsch on diabetic hepatopathy in male mice. Sci Res J Shahed Univ 25:21–30
Goorani S, Koohi MK, Zangeneh A, Zangeneh MM, Moradi R (2018) Pharmacological evaluation of anti-anemic property of aqueous extracts of Falcaria vulgaris leaf in rats. Comp Clin Path. https://doi.org/10.1007/s00580-018-2839-6
Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H (2018) Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organometal Chem 32(9):e4458
Han GH (2000) Antimicrobial food packaging. J Food Technol 54(3):56–65
Holley RA, Patel D (2005) Improvement in shelf life and safety of perishable foods by plant essential oils and smoke antimicrobials. J Food Microbiol 22(4):273–292
Lee HW, Kim H, Ryuk JA, Kil KJ, Ko BS (2014) Hemopoietic effect of extracts from constituent herbal medicines of Samul-tang on phenylhydrazine-induced hemolytic anemia in rats. Int J Clin Exp Pathol 7(9):6179–6185
McKie AT, Barrow D, Latunde DadaGO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S (2004) Tissue specific changes in iron metabolism genes in mice following Phenylhydrazine-induced haemolysis. Biochim Biophys Acta 14(2):169–176
Onyeabo C, Achi NK, Ekeleme-Egedigwe CA, Ebere CU, Okoro CK (2017) Haematological and biochemical studies on Justicia carnea leaves extract in phenylhydrazine induced-anemia in albino rats. Acta Sci Pol Technol Aliment 16(2):217–230
Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P (2013) Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 159(11):770–779
Ryu JH, Yook CS (2001) The effects of sa-multang, a traditional chines medicine, on phenylhydrazine-induced anemic rats. J Appl Pharmacol 9:1–6
Sato H, Sakari T, Fujimura H, Sugimoto J, Kume E, Kitamura K, Takahashi K (2012) Hematological and morphological investigation of thrombogenic mechanisms in the lungs of phenylhydrazine treated rats. Exp Toxicol Pathol 65(4):457–462
Sayyedrostami T, Pournaghi P, Ebrahimi Vosta-Kalaeea S, Zangeneh MM (2018) Evaluation of the wound healing activity of Chenopodium botrys leaves essential oil in rats (a short-term study). J Essent Oil Bear Pl 21(1):164–174
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Shukla P, Yadav NK, Singh P, Bansode FW, Singh RK (2012) Phenylhydrazine induced toxicity: a review on its hematotoxicity. Int J Basic Appl Med Sci 2(2):86–91
Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA (2003) Footstrike is the major cause of hemolysis during running. J Appl Physiol 94(1):38–42
Zangeneh MM, Goodarzi N, Zangeneh A, Tahvilian R, Najafi F (2018a) Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp Clin Path 27(4):861–867
Zangeneh MM, Zangeneh A, Salmani S, Jamshidpour R, Kosari F (2018b) Protection of phenylhydrazine-induced hematotoxicity by aqueous extract of Ocimum basilicum in Wistar male rats. Comp Clin Path. https://doi.org/10.1007/s00580-018-2845-8
Zangeneh MM, Zheleh M, Salmani S, Zangeneh A, Almasi M, Khedri MR, Rasidi K (2018c) Assessment of the anti-anemic effect of aqueous extract of Allium eriophyllum Boiss leaf in phenylhydrazine-treated Wistar male rats. Comp Clin Path. https://doi.org/10.1007/s00580-018-2851-x
Zhaleh M, Sohrabi N, Zangeneh MM, Zangeneh A, Moradi R, Zhaleh H (2018) Chemical composition and antibacterial effects of essential oil of Rhus coriaria fruits in the west of Iran (Kermanshah). J Essent Oil Bear Pl 21(2):493–501
Acknowledgements
The authors would like to thank the Kermanshah University of Medical Sciences for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All institutional and national guidelines for the care and use of laboratory animals were followed. All animal procedures were approved by standards of Payame Noor University of Kermanshah-Iran (No. 01/Z/G 1395/12/01) on Humane Care and Use of Laboratory Animals, in accordance with the Research Ethics Committee of the Ministry of Health and Medical Education in Iran (adopted on April 17, 2006), based on the Helsinki Protocol (Helsinki, Finland, 1975).
Conflict of interest
This manuscript described has not been published before; not under consideration for publication anywhere else; and has been approved by all co-authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goorani, S., Shariatifar, N., Seydi, N. et al. The aqueous extract of Allium saralicum R.M. Fritsch effectively treat induced anemia: experimental study on Wistar rats. Orient Pharm Exp Med 19, 403–413 (2019). https://doi.org/10.1007/s13596-019-00361-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-019-00361-5