Abstract
There are many medicinal plants in Iranian traditional medicine which are used to treat anemia. One of these plants is Allium eriophyllum Boiss. The aim of this study was to investigate the anti-anemic potential of aqueous extract of A. eriophyllum leaf. After collection of the plant, its extract was obtained using Soxhlet extractor. In this study, 60 rats were used. Induction of hemolytic anemia was done by three injections of phenylhydrazine in 50 animals. After 1 day, the rats were divided into six subgroups, including negative healthy control, untreated negative control, and four groups receiving the A. eriophyllum at 20, 40, 80, and 160 mg/kg concentrations. At the end of day 15 of treatment, the animals of all groups were sacrificed, and blood samples were drawn immediately from the animals’ hearts to analyze the biochemical and hematological parameters. The data were analyzed by SPSS-21 software. Different doses of A. eriophyllum significantly (p ≤ 0.05) reduced the raised levels of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), urea, creatinine, ferrous, ferritin, and erythropoietin and increased the levels of body weight, white blood cell (WBC), lymphocyte, neutrophils, monocytes, platelet, red blood cell (RBC), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) as compared to the untreated group. Seemingly, aqueous extract of A. eriophyllum can be used for the treatment of hemolytic anemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is common blood disorder in which there are no sufficient red blood cells or hemoglobin. This state is classified on various bases, including red blood cell morphology and etiologic mechanisms. One type of anemia is hemolytic anemia. It is caused by hemolysis (destruction of red blood cells) (Qaseem et al. 2013). Hemolytic anemia is caused by dysfunction of red blood cells (like spherocytosis, elliptocytosis, fauvism, sickle cell disease, and thalassemia) or external causes like toxicity with chemicals, cold agglutinin syndrome, uremic hemolytic syndrome, self-immune hemolytic anemia, and long walk (Telford et al. 2003). The most important symptoms of hemolytic anemia are early fatigue and exhaustion. Other symptoms include weakness, pale mucosal membrane and pale skin and nail bed, irregular or fast heartbeat, short nail, chest pain, lightheadedness or mild vertigo, numbness or coldness of the extremities, and headache (Lippi et al. 2012). Anemia may be so mild at first that its symptoms are not realized. But severity of symptoms is increased with progress in anemia. Insomnia is also a symptom of hemolytic anemia (Lippi et al. 2012). This type of anemia is usually followed by increased bilirubin and hematopoiesis in bone marrow and jaundice, due to hemolysis. Renal impairments, splenomegaly, hepatomegaly, disturbed spermatogenesis, gallstones, and renal hypertension are also observed in this illness. Use of medicinal plants along with chemical drugs has always been taken into account for treatment of hemolytic anemia (Bratosin et al. 1998; Boretti et al. 2009). Herbal medicine is one of the most common alternative treatments for hemolytic anemia. Use of plants to treat diseases has a history longer than human history (Delaquis and Mazza 1995; Han 2000; Farzaei et al. 2018; Hamelian et al. 2018; Sayyedrostami et al. 2018; Zhaleh et al. 2018). Plants do not have or have less side effects than those of chemical drugs. The positive effects of many plants in the treatment of hemolytic anemia have been documented (Holley and Patel 2005; Kim et al. 1995). In Iranian traditional medicine, there are many plants for treatment of hemolytic anemia, and a list of these plants with their family and Persian names are given below; Allium sativum (Family: Alliaceae; Persian name: Sir), Ananas comosus (Bromeliaceae; Ananas), Brassica rapa (Brassicaceae; Shalgham), Centaurea cyanus (Asteraceae; Gole gandom), Citrus latifolia (Rutaceae; Limotorsh), Cucumis melo (Cucurbitaceae; Talebi), Cucumis melo var.inodorus (Cucurbitaceae; Kharbozeh), Daucus carota (Apiaceae; Havich), Ficus carica (Moracee; Anjir), Foeniculum vulgare (Apiaceae; Razianeh), Matricaria chamomilla (Asteraceae; Babouneh), Oriyganum vulgare (Lamiaceae; Marzanjoush), Petroselinum crispum (Apiaceae; Jafari), Phaseolus vulgaris (Fabaceae; Lobia), Prunus armeniaca (Rosaceae; Zardalou), Raphanus sativus (Brassicaceae; Torb), Rheum officinale (Polygonaceae; Rivas), Rosmarinus officinalis (Lamiaceae; Rosmari), Solanum lycopersicum (Solanaceae; Goje farangi), Solanum tuberosum L. (Solanaccae; Sibzamini), Spinacia oleracea L. (Chenopdiaceae; Esfenaj), Thymus vulgaris (Lamiaceae; Avishan), and Urtica dioica (Urticaceae; Gazaneh) (Cheraghi and Asadi-Samani 2016). One of the most important herbal medicines which are widely used for treatment of blood disorders is Allium eriophyllum Boiss from Plantae kingdom, Liliopsida class, Asparagales order, Amaryllidaceae family, and Allium genus. The plant is widely distributed in the western parts of Asia such as in Turkey, Iraq, and Iran (Mozafari et al. 2015; Foroughi et al. 2016). A. eriophyllum is a good source of low-cost food and is a perfect part of Iranian diet. It applied as a medicinal plant has been used for its anti-inflammatory, antioxidant, antimicrobial, antihypertensive, and antidiabetic activities (Mozafari et al. 2015; Foroughi et al. 2016; Janahmadi et al. 2015).
High prevalence of hemolytic anemia in the whole world has drawn the attention of researchers to finding therapeutical and preventive methods to control this disease. In this regard, we made an attempt to study the effects of aqueous extract of A. eriophyllum as a treatment of hemolytic anemia in rats.
Materials and methods
Extract preparation method
In this empirical study, 1600 g of A. eriophyllum were collected in Kermanshah, Iran (geographical coordinates: 34.3277° N and 47.0778° E). Then, the leaves of the plant were dried in shadow, and after grinding, each time 200 g of the obtained powder was dissolved in 2000 cc distilled water and put in Soxhlet extractor for 8 h. The collected extract was filtered by Whatman filter paper no 1 and steamed into a glass container at the solvent temperature. The remaining dried extract was poured into a glass container and weighed. The powder of the obtained extract weighed as required depending on the dose and dissolved in saline. It was then administered to the rats by the oral catheter (Goodarzi et al. 2018).
Experimental design
This experimental study was conducted on 60 Wistar male rats with the weight of 205 ± 5 g that were kept in individual cages (each group in two separate cages) for 10 days to adapt to the environment. During the experiments, the temperature of the animal house was adjusted at 22 ± 3 °C under a 12-h dark/light cycle. All institutional and national guidelines for the care and use of laboratory animals were followed. To induce anemia, to all groups, with the exception of negative healthy control, phenylhydrazine 20 mg/kg at three different times (days 1, 3, and 5) were injected intravenously (in caudal vein). Then, the animals were divided into six subgroups, including negative healthy control receiving distilled water, the untreated negative control receiving distilled water, and four groups receiving the A. eriophyllum aqueous extract at 20, 40, 80, and 160 mg/kg concentrations. One day after the last injection of the phenylhydrazine, the rats underwent oral treatment of several doses of A. eriophyllum aqueous extract for 15 days (days 6–21). On the 21st, 4 h after oral administration of different doses of A. eriophyllum aqueous extract, the rats were sacrificed. Blood samples were taken from the rats’ heart to analyze biochemical and hematological parameters (Lee et al. 2014).
Statistical analysis
All data were analyzed by one-way variance analysis (ANOVA), using the SPSS 18 software package. Data were considered statistically significant at p ≤ 0.05.
Results
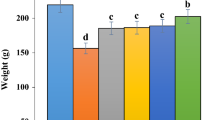
Effect of A. eriophyllum aqueous extract on body mass
As shown in Fig.1, the body weight was significantly (p ≤ 0.05) reduced in untreated rats compared to the control ones. Several groups of A. eriophyllum aqueous extract significantly (p ≤ 0.05) enhanced it. There were not remarkable changes (p ≤ 0.05) between AE80 and AE160 groups.
Effect of A. eriophyllum aqueous extract on levels of biochemical parameters
Phenylhydrazine-induced hematotoxicity increased significantly (p ≤ 0.05) the concentrations of ferrous, ferritin, erythropoietin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), urea, and creatinine as compared to the control group. Several doses of A. eriophyllum aqueous extract significantly (p ≤ 0.05) decreased the above parameters. There were not remarkable changes (p ≤ 0.05) between all doses of A. eriophyllum and control group in level of creatinine. Also, administration of AE80 and AE160 significantly (p ≤ 0.05) ameliorated the concentrations of ALT and GGT, similar to the control group. No significant changes (p ≤ 0.05) were found between AE80 and AE160 groups in the levels of biochemical parameters (Figs. 2, 3, 4, 5, and 6).
ALP, AST, ALT, and GGT levels in tested groups. C control, UT untreated, AE Allium eriophyllum Boiss, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyl transferase. Different letter represent statistical difference between tested groups of animals (p ≤ 0.05)
Effect of A. eriophyllum aqueous extract on the levels of hematological parameters
The numbers of white blood cell (WBC), platelet, and red blood cell (RBC), the percentages of lymphocyte, neutrophils, and monocytes, and the levels of hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were significantly (p ≤ 0.05) reduced in the untreated group as compared to the control group. The treatment with A. eriophyllum aqueous extract significantly (p ≤ 0.05) enhanced the above parameters. There were not significant differences (p ≤ 0.05) in percentages of lymphocytes, neutrophils, monocytes, eosinophils, and basophils between several doses of A. eriophyllum and control group. No significant differences (p ≤ 0.05) were found among all groups in the percentages of eosinophils and basophils (Figs. 7, 8, 9, 10, 11, 12, 13, and 14).
Discussion
Phenylhydrazine (C6H8N2) is a chemical compound with low PubChem ID: 7516 and molar mass of 10,814 g/mol. It is toxic for the body and impairs different tissues while entering the body (Giffin and Allen 1993; Mansuy et al. 1982). Studies have reported that phenylhydrazine causes oxidative stress, production of free radicals, lipid peroxidation, oxidative degradation of spectrum cell membrane, and lysis of red blood cells (Ferrali et al. 1997; Giffin and Allen 1993; Mansuy et al. 1982). It was shown that phenylhydrazine caused the conversion of hemoglobin to methemoglobin; therefore it plays a main role in forming the Hains bodies (Rifkind 1965; Rifkind and Danon 1965; Horn et al. 1990). Phenylhydrazine causes deficiency in the glucose–6–phosphate dehydrogenase to cause fauvism (Hochstein 1971; Horn et al. 1990). Studies have shown phenylhydrazine, by increasing hemolysis (increased the levels of ferrous, ferritin, and erythropoietin), causes spleen enlargement and chronic failure (through hypertrophy of spleen cells), liver enlargement and chronic failure (through hypertrophy of liver cells), chronic and acute renal failure (by destruction of structures such as proximal and distal renal cells), and reduction of mean number of testicular sperms (through atrophy of testicular structure) (Ferrali et al. 1997; Beaven and White 1954; Goldberg and Stern 1975). Also it indicated that phenylhydrazine reduced body weight and increased the weight and volume of the adrenal glands, kidneys, liver, and spleen. In previous study revealed that phenylhydrazine with degenerating of proximal convoluted tubules, distal convoluted tubules, glomeruli, and hepatocytes increased the biochemical parameters of the kidneys and liver in the blood (Pham-Quang-Chi 1979; Witchett 1975). Considering to the above studies, our study showed that phenylhydrazine significantly (p ≤ 0.05) decreased the levels of body weight, WBC, lymphocyte, neutrophils, monocytes, platelet, RBC, Hb, PCV, MCV, MCH, and MCHC and enhanced the levels of ALP, AST, ALT, GGT, urea, creatinine, ferrous, ferritin, and erythropoietin. In spite of hematotoxicity, hepatotoxicity, and nephrotoxicity properties of phenylhydrazine, the treatment with several doses of aqueous extract of A. eriophyllum significantly (p ≤ 0.05) improved the levels of above parameters. In a study, Allium genus reduced the raised levels of hepatic biochemical parameters (ALP, ALT, and AST) and also the volume of the liver, hepatocytes, and sinusoids as compared to the CCl4-treated group (Goodarzi et al. 2017). In other study revealed that Allium genus had strong nephroprotective property against CCl4, so that it decreased the volume of kidneys, and their subcomponents include proximal convoluted tubules, vessels, and interstitial tissues in comparison with the untreated group. Also, the previous study indicated that Allium genus with increasing the clearance rate of the kidney reduced the concentration of urea and creatinine (Sherkatolabbasieh et al. 2017).
Of the herbs that are antioxidant, Allium genus has strong antioxidant property. In a study indicated that Allium genus collected in Iran were rich in antioxidant compounds includes linolenic acid, methyl ester, phytol, neophytadiene 2-phenyl-5-methylindole, hexadecanoic acid, vitamin E, ethanol, 2-tetradecyloxy, n-tetracosane, hexatriacontane, γ-tocopherol, eicosane, n-ethyl-1,3-dithioisoindoline, 2-hexadecene, 3,7,11,15-tetramethyl, hexanedioic acid, and 1,4,8,11-tetraazacyclotetradecane (Goodarzi et al. 2018). Antioxidants can play a key role in destruction of free radicals and toxic materials and maintenance of hemostasis because free radicals interfere with biological cell membrane such as red blood cells through peroxidation of unsaturated fatty acids and bring about pathological changes (Omenn et al. 1996; Bjelakovic et al. 2004). Hence, it can be assumed that Allium genus plays a pivotal role in the prevention and treatment of several disorders such as hemolytic anemia by the destruction of free radicals.
Conclusion
According to the results, aqueous extract of A. eriophyllum ameliorated the levels of biochemical and hematological parameters and increase the reduced level of body weight in phenylhydrazine-induced anemic rats. It is suggested that clinical trials be conducted to achieve this therapeutic effect in human.
References
Beaven GH, White JC (1954) Oxidation of phenylhydrazines in the presence of oxyhaemoglobin and the origin of Heinz bodies in erythrocytes. Nature 27:389–391
Bjelakovic G, Nikolova D, Simonetti RG, Gluud C (2004) Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364:1219–1228
Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, Maggiorini M, Schoedon G, Alayash AI, Schaer DJ (2009) Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119(8):2271–2280
Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J (1998) Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80(2):173–195
Cheraghi M, Asadi-Samani M (2016) Hematopoietic medicinal plants based on ethnobotanical documents of Iran: a strategy to develop nature-based drugs effective on anemia. Pharm Lett 8(5):393–399
Delaquis PJ, Mazza G (1995) Antimicrobial properties of isothiocyanates in food preservation. J Food Technol 49(11):73–84
Farzaei MH, Zangeneh MM, Goodarzi N, Zangeneh A (2018) Stereological assessment of nephroprotective effects of Trachyspermum ammi essential oil against carbon tetrachloride-induced nephrotoxicity in mice. Int J Morphol 36(2):750–757
Ferrali M, Signorini C, Sugherini L, Pompella A, Maura L, Caciotti B, Ciccoli L, Comporti M (1997) Release of free redoxactive iron in the liver and DNA oxidative damage following phenylhydarzine intoxication. Biochem Pharmacol 53(11):1743–1751
Foroughi A, Zangeneh MM, Zangeneh A, Kazemi N (2016) A survey on antibacterial activities of Allium eriophyllum alcoholic extract: an ethnomedicinal plant. Iran J Public Health 45(2):32–32
Giffin H, Allen E (1993) The control and complete remission of polycythemia vera following the prolonged administration of phenylhydrazine hydrochloride. Am J Med Sci 185:1–13
Goldberg B, Stern A (1975) The generation of O2-by the interaction of the hemolytic agent, phenylhydrazine, with human hemoglobin. J Biol Chem 25(6):2401–2403
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4- induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Goodarzi N, Zangeneh MM, Zangeneh A (2018) The effect of ethanolic extract of Allium saralicum R.M. Fritsch on diabetic hepatopathy in male mice. Sci Res J Shahed Univ 25:21–30
Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H (2018) Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organometal Chem 32. https://doi.org/10.1002/aoc.4458
Han JH (2000) Antimicrobial food packaging. J Food Technol 54(3):56–65
Hochstein P (1971) Glucose – 6 – phosphate dehydrogenase deficiency: mechanism of drug – induced hemolysis. Exp Eye Res 11(3):389–395
Holley RA, Patel D (2005) Improvement in shelf life and safety of perishable foods by plant essential oils and smoke antimicrobials. J Food Microbiol 22(4):273–292
Horn S, Gopas J, Bashan N (1990) A lectin like receptor on murine macrophage is involved in the recognition and phagocytosis of human red cells oxidized by phenylhydrazine. Biochem Pharmacol 39(4):775–780
Janahmadi Z, Nekooeian AA, Mozafari M (2015) Hydroalcoholic extract of Allium eriophyllum leaves attenuates cardiac impairment in rats with simultaneous type 2 diabetes and renal hypertension. Res Pharm Sci 10(2):125–133
Kim J, Marshal M, Wei C (1995) Antibacterial activity of some essential oil components against five food borne pathogens. J Agric Food Chem 43(11):2839–2845
Lee HW, Kim H, Ryuk JA, Kil KJ, Ko BS (2014) Hemopoietic effect of extracts from constituent herbal medicines of Samul-tang on phenylhydrazine-induced hemolytic anemia in rats. Int J Clin Exp Pathol 7(9):6179–6185
Lippi G, Schena F, Salvagno GL, Aloe R, Banfi G, Guidi GC (2012) Foot-strike haemolysis after a 60-km ultramarathon. Blood Transfus 10(3):377–383
Mansuy D, Battioni P, Mahy JP, Gillet G (1982) Comparison of the hemoglobin reactions with methyl-and phenyl-hydrazine; intermediate formation of a hemoglobin Fe-(II)-methyldiazene complex. Biochem Biophys Res Commun 106(1):30–36
Mozafari M, Nekooeian AA, Janahmadi Z (2015) The antihypertensive effects of hydroalcoholic extract of allium eriophyllum in leaves in rats with simultaneous type 2 diabetes and renal hypertension. Int Cardiovasc Res J 9(1):34–40
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S (1996) Effects of a combination of beta carotine and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334(18):1150–1155
Pham-Quang-Chi (1979) Toxicity of inhaled phenylhydrazine (Russian). Gig Tr Prof Zabol 3:45–47
Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P (2013) Clinical Guidelines Committee of the American College of Physicians. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 159(11):770–779
Rifkind RA (1965) Heinz body anaemia–an ultrastructural study II red cell sequestration and destruction. Blood 26(4):433–448
Rifkind RA, Danon D (1965) Heinz body anaemia–an ultra structural study I Heinz body formation. Blood 25(6):885–895
Sayyedrostami T, Pournaghi P, Ebrahimi Vosta-Kalaeea S, Zangeneh MM (2018) Evaluation of the wound healing activity of Chenopodium botrys leaves essential oil in rats (a short-term study). J Essent Oil Bear Plants 21(1):164–174
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA (2003) Footstrike is the major cause of hemolysis during running. J Appl Physiol 94(1):38–42
Witchett CE (1975) Exposure of dog erythrocytes in vivo to phenylhydrazine and monomethylhydrazine. A freeze-etch study of erythrocyte damage. Aerospace medical research laboratory, Aeorospace medical division, AFSC, Wright-Patterson Air Force Base, report no: AMRL-TR-74-88. NTIS Publication No AD-A011 555
Zhaleh M, Sohrabi N, Zangeneh MM, Zangeneh A, Moradi R, Zhaleh H (2018) Chemical composition and antibacterial effects of essential oil of Rhus coriaria fruits in the west of Iran (Kermanshah). J Essent Oil Bear Plants 21(2):493–501
Funding
The study was financially supported by the Kermanshah University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Zangeneh, M.M., Zhaleh, M., Salmani, S. et al. Assessment of the anti-anemic effect of aqueous extract of Allium eriophyllum Boiss leaf in phenylhydrazine-treated Wistar male rats. Comp Clin Pathol 28, 427–434 (2019). https://doi.org/10.1007/s00580-018-2851-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2851-x