Abstract
Sub-Saharan Africa with anemia prevalence of 68% could qualify as the anemia capital of the world. Anemia in this region affects mainly pregnant women and children. The high incidence of malaria in sub-Saharan Africa makes anemia incidence worse as malaria parasites cause hemolytic anemia. Available treatments seem unpalatable due to side effects. The populace seeks medical attention via their traditional therapies like herbs. This study is aimed at evaluating the use of Mucuna pruriens herb in the treatment of hemolytic anemia secondary to malaria. Thirty-six adult male rats weighing between 150 and 200 g were divided into six groups (n = 6). Group I served as the negative control and received syrup formulation without the extract (placebo) but allowed access to food and water ad libitum. Groups II to VI received phenyl hydrazine 60 mg/kg for 3 days to induce anemia. Additionally, groups IV to VI received 50 mg/kg, 100 mg/kg, and 200 mg/kg of Mucuna pruriens syrup formulation respectively, while group III received a standard drug. Feed and water consumption were measured weekly while PCV, Hb, RBC, platelet, ESR, MCV, MCHC, MCH, WBC, lymphocytes, neutrophils, monocytes, eosinophils, and basophils were measured before anemia induction and at days 0, 7, 14, and 21. Phytochemical analysis of the extract was also carried out. Administration of phenylhydrazine significantly decreased (p < 0.05) the levels of PCV, Hb, and RBC, respectively. Also, there is a significant increase in monocytes. Phytochemical analysis shows the presence of phenols, flavonoids, saponins, tannins, glycosides. Mucuna pruriens is effective in the treatment of PHZ induced hemolytic anemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The disease burden in Sub-Saharan Africa is alarming, accounting for up to 69% of the continent’s deaths (Aikins et al. 2010). Among this burden is the catastrophic incidence of malaria disease which has assumed a public health dimension. A malariometric survey of Sub-Saharan Africa shows that 90% of all deaths related to malaria globally occur in this region with children ages 1–5 years contributing a whopping 78% of this share. The economic burden of malaria to this region is estimated to be 12 billion dollars in direct cost, and this burden is primarily on the shoulders of the poor and rural dwellers that live below the poverty line (Alegana et al. 2020). Closely associated with the incidence of malaria in this region is the incidence of hemolytic anemia, that had prompted some researchers to recommend anemia status as an additional indicator for monitoring community malaria burden (Korenromp et al. 2004). The prevalence of anemia in the world is about 25% with Sub-Saharan Africa having prevalence as high as 68% in pre-school children and 57% in pregnant women (Magalhães and Clements 2011). Hemolytic anemia, secondary to malaria, is characterized by hemolysis of both infected and uninfected erythrocytes and dyserythropoiesis of the bone marrow since erythropoietic activity cannot adequately compensate for the loss (Jakeman et al. 1999; Price et al. 2001). Clinical manifestation of hemolytic anemia which is mainly as a result of decreased tissue oxygen supply leading to organ dysfunction and adaptive changes includes exhaustion and fatigue. Diagnosis of this anemic condition may record normochromic normocytic anemia, increased serum lactate dehydrogenase (LDH), elevated reticulocyte count, bilirubin and hemoglobin, decreased haptoglobin, etc (Barcellini and Fattizzo 2015). White (2018) showed that children in malaria-endemic regions of Sub-Saharan Africa suffer from reduced hemoglobin (Hb), reduced ability to compensate for Hb changes with the situation sometimes becoming critical and requiring hospitalization and or blood transfusion. Children aged 1–5 years and pregnant women in this region bear the bulk of the burden which is usually worse in rainy seasons (Accrombessi et al. 2015; Antony 2008; Crawley 2004; Kahigwa et al. 2002; Moraleda et al. 2017; Mulenga et al. 2005). The medical consequences of anemia in children range from poor cognitive development and growth to decreased immunity and a higher risk of death. Adding to the incidence and burden of anemia in pregnant mothers is the fact that the newborn usually have lower hemoglobin levels thereby reducing their survival. While anemia treatment worldwide has standard therapies, Sub-Saharan Africa relies more on decoction, fresh leaves, barks, and herbs for most of their healthcare needs (Innih et al. 2020; Suzanne et al. 2020). This is because most standard treatments are believed to have unwanted effects making it highly undesirable. They include side effects associated directly with the therapies like nausea, vomiting, and dark stool, which are iatrogenic in nature (Resmi et al. 2017). Since the use of plants in healthcare dates back to ancient times, development of a local herb used in the management of a disease could lead to discovery, contributing to evidence-based translational medicine (Goorani et al. 2019). This will give a lot of room for improvement in healthcare towards development of more modern therapies that will be acceptable, available, and affordable in Sub-Saharan Africa.
Phenylhydrazine (PHZ) is a model of hemolytic anemia usually used in experimental therapies to assess new regimen. It was formally used as an antipyretic drug and later for the treatment of polycythemia vera but had been discontinued following high incidence of toxicity. The toxicity is wild spread affecting the various level of the mammalian system which qualifies PHZ to be used in models of anemia (Gheith and El-Mahmoudy 2018), Jaundice (Zhang et al. 2015), vascular dysfunction (Luangaram et al. 2007), pulmonary thrombosis (Sato et al. 2013), and enhancement of parasitemia levels in animal models (Zhu et al. 2015) depending on dosage and timing (Roque et al. 2008). In the hematological system, PHZ lead to a decrease in pack cell volume (PCV), hemoglobin (Hb), and red blood cells (RBC). Also, it could result in reticulocytosis, anisocytosis, poikilocytosis, and leukocytosis which manifests mainly in lymphocytes and monocytes and increase in Heinz body and free plasma Hb. There is also increase in mean corpuscular volume (MCV), distortion of osmotic resistance, and erythropoietin levels possibly as an adaptive response. These toxicological manifestations follow the ability of PHZ to oxidatively form phenyldiazinyl and phenyldiazene radicals that could break up to give phenyl radical (Ph•) and nitrogen. Ph• has the ability to bind DNA, proteins, and lipids leading to inhibition of protein synthesis, enzyme damage, membrane peroxidation etc. (Magnani et al. 1988; Pandey et al. 2017). It is believed that since Ph• is involved in the perturbations of the system at different levels, the body’s antioxidant system which includes both the enzymatic and the non-enzymatic types may protect the body from such effect(s) (Madhikarmi and Murthy 2015).
Sub-Saharan Africans rely a lot on natural remedies in the management of diseases in the region and as much as 80% of the population depends on the herbal medicines for their health needs. The development of a herbal therapy to combat hemolytic anemia secondary to malaria will not only reduce the regions’ burden of anemia but certainly will increase survivability (Kumar and Saha 2013). One of such herbal remedies in folk use is Mucuna pruriens (Velvet beans). It is of the family Fabaceae, an annual climbing legume that is known for its anti-Parkinson, neuro-protective, and fertility-enhancing properties (Deokar et al. 2016; Kumar and Saha 2013). It is known to contain several vitamins (A, C, E) and minerals (copper, zinc) which may partly account for its folklore usage in the management of anemia and other hematological diseases (Nweze et al. 2017).
This study evaluates the potential of Mucuna pruriens–based formulation in the management of PHZ induced hemolytic anemia and hematological and metabolic distortions. This is a step towards achieving plant-based therapy in a ready-to-administer form as a way of advancing local herbal usage in disease management and translational medicine.
Materials and methods
Harvesting of the Mucuna pruriens
Fresh leaves of Mucuna pruriens were collected from a forest in the Enugu State of Nigeria and scientifically identified by a plant taxonomist at the International Center for Ethno-medicine and Drug Development (InterCEDD) Nsukka, Nigeria.
Processing and extraction of Mucuna pruriens
The fresh leaves were thoroughly washed and air-dried at room temperature for 5 days until constant weight. The dry leaves were then pulverized and stored in an airtight container. Thirty grams of the powder were macerated in 100 ml of 80% ethanol in water (v/v) for 48 h and filtered using Whitman paper no 1. The filtrate was then concentrated to 10% of its original volume using a rotary evaporator. The sample was then dried over a water heater at 35 °C until constant weight.
Phytochemical screening
Phytochemical screening of the extract was performed to ascertain the different classes of phytochemicals present based on standard methods (Harborne, 1984).
Formulation of Mucuna pruriens–based syrup
The syrup formulation was based on the following:
Mucuna pruriens extract 20 g.
Citric acid1.2 g.
Benzoic acid0.1 g.
Sorbitol solution35 mL.
Glycerin5 mL.
Distilled water (q.s)100 mL.
Animal husbandry
Thirty-six, 10-week-old Sprague Dawley rats weighing between 150 and 200 g were purchased from the animal house of Enugu State University of Science and Technology, Agbani Enugu state. They were housed in propylene cages embedded with husk padding which was changed every day to maintain experimental hygiene. Acclimatization was done for 1 week in a facility maintained at 12-h dark–light cycle, humidity of 50 ± 5%, and 25 °C ± 15% temperature. The animals had free access to food and water ad libitum during the acclimatization. The protocols adhered to the guidelines for the safe care and use of animals of Enugu State University of Science and Technology, Agbani Enugu state.
Chemicals
Analytical grade of phenylhydrazine and ethanol was purchased from Sigma-Aldrich, Missouri USA, through a local representative in Nigeria.
Standard drug
Astyfer syrup (Fidson Healthcare PLC Nigeria) was used as a standard therapy.
Acute oral toxicity studies
The rats received Mucuna pruriens hydro-ethanolic extract per oral at different doses ranging from 50 to 5000 mg/kg body weight (b.w.). The animals were examined for signs of toxicological manifestations by observing changes in their behavior, neural changes, and changes in their feeding behavior etc. There were no manifestations of toxicological signs or death even at the highest concentration of 5000 mg/kg. The extract, therefore, is assumed safe up to the maximum concentration.
Induction of anemia
Baseline hematological values were determined before the commencement of anemia induction. Anemia was induced in the rats by the administration of 60 mg/kg phenylhydrazine intraperitoneal (i.p.) injection for 3 days (Pandey et al., 2016). The rats were considered anemic when their red blood cell (RBC) and hemoglobin (Hb) levels fall to at least 30% of their baseline value. The anemic rats were used for groups II to VI.
Experimental design
The rats were divided into six (6) groups (n = 6) and were treated as follows:
Group I: normal/negative control.
Group II: positive control (received placebo or syrup formulation without the extract).
Group III: standard treatment (Astyfer syrup).
Group IV: formulation (50 mg/kg).
Group V: formulation (100 mg/kg).
Group VI: formulation (200 mg/kg).
Measurement of FI
A known quantity of feed was measured into the feeding container, and the animals were allowed access to the feed. The remnants were collected back and weighed to accurately quantify the actual feed consumed on weekly basis.
Measurement of WI
Weekly water intake was measured similar to feed intake.
Hematological analysis
About 2 ml of blood were collected from each animal into sodium ethylene diamine tetraacetic acid bottle (EDTA) by retro-orbital bleeding technique before anemia induction (BI), after anemia induction/day 0 (AI), and days 7, 14, and 21. The parameters measured were the pack cell volume (PCV) based on the method of Thrall and Weiser (2002), red blood cell ((Thrall and Weiser 2002), and hemoglobin (Hb) based on cynomet Hb method (Higgins et al. 2008). The white blood cell (WBC) measurement was done based on the hemocytometer method while the differential counts were done on dried thin blood smears that had been stained using the Leishman technique and counted using the meander method (Thrall and Weiser 2002). The mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV) were calculated using the standard formula (Thrall and Weiser 2002). The platelet count was based on the wright stain method (Bell and Neely 1980) while the erythrocyte sedimentation rate was based on the westergreen method (Thrall and Weiser 2002).
Data analysis
The data obtained were subjected to appropriate statistical analysis using GraphPad Prism version 8.0.1 (www.graphpad.com, San Diego, CA). The data were analyzed using one- or two-way analysis of variance as appropriate and differences among the groups were spotted out using Turkey’s post hoc test and presented as mean ± standard deviation (SD). Significance was set at p < 0.05.
Results
Phytochemical analysis
The extract of Mucuna pruriens was found to be rich in proteins, phenols, flavonoids, saponins, tannins, and glycosides (Table 1).
Key: ( +) = slightly present, (+ +) = moderately present, (+ + +) = highly present.
Effect of Mucuna pruriens formulation treatment on feed intake of animals made anemic using phenylhydrazine
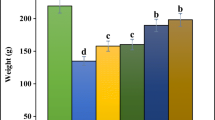
Treatment of the animals with phenylhydrazine significantly decreased (p < 0.001) feed intake from 293.16 g in week 1 to 254.16 g in week 3 when compared to control group (290.5 g in week 1 to 276.16 at week 3) representing a decrease of 13.3% and 9.5%, respectively. Treatment with Mucuna pruriens formulation at different doses ameliorated this metabolic alteration (Fig. 1).
Effect of Mucuna pruriens formulation treatment on feed intake of animals exposed to phenylhydrazine-induced anemia. A Effect of Mucuna pruriens–based formulation on feed intake of animals subjected to phenylhydrazine induced anemia (n = 6). B AUC of feed intake subjected to one-way ANOVA. *** significantly different from the control at p < 0.001, ### significantly different from the positive control group at p < 0.001, respectively
Effect of Mucuna pruriens formulation treatment on water intake of animals made anemic using phenylhydrazine
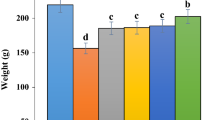
Administration of phenylhydrazine to animals decreased water intake from 728 ml in week 1 in the untreated group to 435 ml by week 3 when compared to the negative control group (490 to 488 ml). There is no significant difference in water intake between the groups (Fig. 2).
Effect of Mucuna pruriens formulation treatment on PCV, Hb, RBC, platelets, ESR, MCV, MCHC, and MCH of animals made anemic using phenylhydrazine
Table 2 shows the effect of Mucuna pruriens formulation treatment on PCV, Hb, RBC, platelets, ESR, MCV, MCHC, and MCH of animals made anemic using phenylhydrazine. The PCV of all the animals were comparable before the start of the experiment and were between 38.00 ± 4.28% and 43.50 ± 1.50%. Hb content was between 12.75 ± 1.15 g/dl and 14.36 ± 0.96 g/dl, RBC level between 4.69 ± 0.72 × 106/µl and 6.90 ± 2.96 × 106/µl, platelets between 233.33 ± 57.55% and 270.33 ± 18.11%, ESR between 0.33 ± 0.15 mm/h and 1.10 ± 0.37 mm/h, MCV between 55.60 ± 8.65 fl and 74.23 ± 11.54 fl, MCHC between 30.00 ± 2.66 g/dl and 34.89 ± 2.98 g/dl, and MCH between 20.81 ± 3.08 pg and 27.19 ± 2.23 pg, respectively. Treatment of the animals with phenylhydrazine caused a significant decrease (p < 0.05) in PCV of the positive control value of 24.50 ± 1.89% when compared with value of negative control group, 42.55 ± 1.59%. Also, Hb levels of the positive control group significantly decreased (p < 0.05) to 8.70 ± 0.70 g/dl as against the negative control group value of 14.00 ± 0.26 g/dl. Administration of phenylhydrazine also affected the levels of RBC and platelets with the positive control values significantly decreasing (p < 0.05) to 3.03 ± 0.78 × 106/µl and 170.66 ± 87.68% as against that of the negative control values (6.86 ± 1.53 × 106/µl and 234.83 ± 74.20%), respectively. Also, phenylhydrazine increases significantly the level of MCV from 60.74 ± 10.55 fl in the negative control group as against 80.86 ± 15.86 fl in the positive control group. Treatment with Mucuna pruriens–based formulation ameliorated these hematological alterations to different degrees.
Results are presented as mean ± standard deviation, n = 6. a,b,c: significantly different from the control at p < 0.001, 0.01, and 0.05, respectively, while d andf: significantly different from the positive control at p < 0.001 and 0.05, respectively.
Table 3 shows the effect of Mucuna pruriens formulation treatment on WBC, lymphocytes, neutrophils, monocytes, eosinophils, and basophils of animals made anemic using phenylhydrazine. The result shows that the values for different parameters measured were comparable before the induction of anemia using phenylhydrazine. WBC is between 11.92 ± 3.15 103/µL and 20.00 ± 3.67 103/µl, lymphocytes between 72.2 ± 2.22% and 79.70 ± 3.45%, neutrophils between 12.4 ± 0.88% and 16.6 ± 1.12%, monocytes between 3.8 ± 0.34% and 8.0 ± 0.86%, eosinophils between 2.0 ± 0.33% and 3.4 ± 0.45% and basophils between 0.2 ± 0.03% and 1.0 ± 0.20%, respectively. Administration of phenylhydrazine only mildly and non-significantly increased WBC and significantly increased (p < 0.05) the levels of monocytes, eosinophils, and basophils. Monocytes, eosinophils, and bosophils increased significantly (p < 0.05) from 9.2 ± 0.98%, 4.0 ± 0.67%, and 0.2 ± 0.03% in the negative control group 15.6 ± 1.34%, 9.1 ± 1.86%, and 4.0 ± 0.50%, respectively, in the positive control group. Treatment with Mucuna pruriens formulation reversed these immunological responses sometimes to the base value (Table 3).
Results are presented as mean ± standard deviation, n = 6a,b,c: significantly different from the control at p < 0.001, 0.01, and 0.05, respectively, while d,e and f: significantly different from the positive control at p < 0.001, 0.01, and 0.05, respectively.
Effect of Mucuna pruriens formulation on rate of recovery of PCV, Hb, and RBC (%)
Administration of phenylhydrazine to animals caused a decrease in PCV, Hb, and RBC. Treatment with Mucuna pruriens formulation caused the aforementioned parameters to recover relative to the baseline at different rates. The positive control recovered by 48% on day 7, 72% on day 14, and 91% on day 21 which is significantly (p < 0.05) slower when compared to the treatment groups with recoveries that range from 144 to 224%. Also, the rate of recovery of Hb levels in the positive control group; 38%, 61%, and 79% for days 7, 14, and 21, respectively, are significantly (p < 0.05) different from the groups that received Mucuna pruriens formulation which ranges from 130 to 228%. Similarly, recovery of RBC from baseline in the positive control group is 28% on day 7, 45% on day 14, and 54% on day 21 which is significantly different from the rate of the treatment groups that range from 22 to 271% (Fig. 3).
Discussion
The infectious disease burden in Sub-Saharan Africa is calamitous with malaria contributing significantly towards this problem (Aikins et al. 2010; Alegana et al. 2020). Closely associated with malaria burden in Sub-Saharan Africa is the problem of hemolytic anemia secondary to erythrocyte membrane damage associated with both infected and uninfected cells (White 2018). This situation is worse in children age 1–5 years and pregnant women especially during rainy seasons (Accrombessi et al. 2015; Antony 2008; Crawley 2004; Kahigwa et al. 2002; Kenangalem et al. 2016; Moraleda et al. 2017; Mulenga et al. 2005). With an anemia burden of 68% in children and over 50% among pregnant women, development of a therapy that can reverse this trend will be a welcome development (Magalhães and Clements 2011). Since the consequences of anemia are usually disastrous; poor immunity, poor cognitive development, retarded growth, and sometimes death, prevention, and or effective management of this situation will improve life span, productivity, and intelligent quotient of tomorrow leaders in this region. Most Africans rely on herbal treatment for healthcare needs because of the age-long belief that orthodox medicines are synthetic, unaffordable, and bedeviled with numerous side effects (Innih et al. 2020; Suzanne et al. 2020). Alternative therapies had become a major source of healthcare delivery system in Sub-Saharan Africa with as many as 80% of the populace depending on it (Kumar and Saha 2013). This study validated the folklore claims that Mucuna pruriens leaf extract could be used in the management of hemolytic anemia. Formulation of syrup, based on Mucuna pruriens as the active, will contribute towards evidence-based translational medicine research especially in Sub-Saharan Africa.
In this study, administration of phenylhydrazine to animals led to a decrease in feed intake and animal weight. This is believed to be associated with increased erythrocyte destruction which will ultimately lead to low oxygen supply and dysfunction of organs like the liver and spleen. It is known that the “liver–brain” link could control appetite and amount of fat stored in the adipose tissue since the brain can communicate directly to the liver to regulate glucose production via the hypothalamic glucose/fatty acid sensing (Fam et al. 2012). Also, phenylhydrazine is a known disruptor of glucose-6-phosphate dehydrogenase, a key enzyme in pentose phosphate pathway that supplies energy to cells, and deficiency of it creates energy crisis. The groups that were treated with the formulation showed improved feeding when compared to the untreated. Mucuna pruriens is known to have antioxidant properties which might have ameliorated the toxic effects of PHZ on erythrocyte membrane thereby reducing hemolysis and improve oxygen supply to the organs and restoring normalcy in feeding. Also, phenylhydrazine administration caused a significant decrease (p < 0.05) in RBC, Hb, PCV, and platelets which agrees with the work of other researchers that had worked with other natural products on PHZ model (Ashour 2014; Shukla and Singh 2015). This is probably because of PHZ ability to cause lipid peroxidation and oxidative degradation of RBC proteins and membrane skeleton leading to RBC damage. Part of the effect will be an increase in free plasma Hb and a decrease in cell Hb (Table 2). This can mediate the activation of the innate immune system leading to other cascades of events like activation of inflammatory and thrombotic pathways (Rapido 2017). Also, a significant decrease in RBC will lead to substantial decrease in PCV since RBC make up the bulk of PVC. There is also a significant increase (P < 0.05) in MCV which agreed with the work of Agbor et al. (2005). When the change in animal weight (not shown) is put together with the results of RBC, Hb, PCV, MCH, and MCV, there is an impression of macrocytic anemia. It is known that PHZ has the ability to induce macrocytic anemia which is common in vitamin B12 and folate deficiencies (Chauhan et al. 2015). Treatment with Mucuna pruriens formulation improved the rate of return of the values of PCV, RBC, and Hb to baseline when compared to the untreated (Fig. 3). Mucuna pruriens is known to be rich in vitamins (A-, C-, B-, complex), mineral (iron, copper, zinc), and other beneficial nutrients (Nweze et al. 2017). Iron and vitamin B12 are the main therapies in the management of anemia because iron is the limiting step in the synthesis of Hb which is the oxygen carrier and B12 serves as a co-factor in the synthesis of RBC. PHZ as a model of anemia is known to cause alteration of Iron metabolism in addition to the anemia (Pandey et al. 2014). Zinc is believed to improved Hb production and RBC formation via increase in erythropoiesis in an animal study of zinc supplementation (Chen et al. 2018).
Administration of PHZ in this study only provoked a mild immunological response as there is no significant difference (p < 0.05) in the level of WBC observed. This part of the result disagrees with most other works in PHZ model that had observed significant immunological responses (Chourasiya et al. 2019; Roque et al. 2008; Zangeneh et al. 2019). There are a significant increase in the levels of monocytes, eosinophils, and basophils but not lymphocytes and neutrophils. This lack of significant change in WBC among different experimental groups can be attributed to the percentage contributions of different WBC differentials since lymphocytes and neutrophils account for over 80% of WBC. Changes in monocytes, eosinophils, and basophils may not significantly affect the level of WBC. Also, it is known that different responses are possible following PHZ administration since dose and time are major variables in this model. Some researchers had used 60 mg/kg once in anemia induction while others used lower doses for longer duration to induce anemia. This and species differences could account for different immunological response observed (Roque et al. 2008).
Phytochemical analysis of Mucuna pruriens showed the presence of alkaloids, phenol, carbohydrates, flavonoids, saponins, tannins, and glycosides. Phenolics, flavonoids, and tannins which are known for their antioxidant properties (Muniyandi et al. 2019). Antioxidants could mop up the free radicals that damage the erythrocyte membrane thereby protecting the cell membrane from hemolysis and extending the life span of the erythrocyte. This may in part be the mechanism of Mucuna pruriens in combating hemolytic anemia.
Conclusion
This study support the folk use of Mucuna pruriens leaf in the treatment of anemia.
References
Accrombessi M, Ouédraogo S, Agbota GC, Gonzalez R, Massougbodji A, Menéndez C and Cot M (2015) Malaria in pregnancy is a predictor of infant haemoglobin concentrations during the first year of life in Benin, West Africa. PLoS One 10(6):e0129510
Agbor GA, Oben JE, Ngogang JY (2005) Haematinic activity of Hibiscus cannabinus. Afr J Biotech 4(8):833–837
Aikins ADG, Unwin N, Agyemang C, Allotey P, Campbell C and Arhinful D (2010) Commentary Tackling Africa's chronic disease burden: from the local to the global.
Alegana VA, Okiro EA, Snow RW (2020) Routine data for malaria morbidity estimation in Africa: challenges and prospects. BMC Med 18:1–13
Antony AC (2008) Severe anemia in Malawian children. N Engl J Med 358(21):2291
Ashour TH (2014) Phenylhydrazine-induced hemolytic anemia in rats. Res J Med Sci 8:67–72
Barcellini W and Fattizzo B (2015) Clinical applications of hemolytic markers in the differential diagnosis and management of hemolytic anemia. Disease markers 2015
Bell A, Neely C (1980) Smear platelet counts. South Med J 73(7):899–901
Chauhan SP, Sheth NR and Suhagia BN (2015) Hematinic effect of fruits of Opuntia elatior Mill. on phenylhydrazine-induced anemia in rats. Ayu 36(2):208
Chen Y-H, Feng H-L, Jeng S-S (2018) Zinc supplementation stimulates red blood cell formation in rats. Int J Mol Sci 19(9):2824
Chourasiya A, Sahu RK and Khan MA (2019) Anti-Anemic and haemopoietic evaluation of Trigonella foenum-graecum (Fenugreek) in rodent model. J Drug Del Therap 9(4-s): 332–337
Crawley J (2004) Reducing the burden of anemia in infants and young children in malaria-enAn updated review on taxonomydemic countries of Africa: from evidence to action. Am J Trop Med Hyg 71(2_suppl):25–34
Deokar G, Kakulte H, Kshirsagar S (2016) Phytochemistry and pharmacological activity of Mucuna pruriens: a review. Pharmaceutical and Biological Evaluations 3(1):50–59
Fam BC, Joannides CN, Andrikopoulos S (2012) The liver: key in regulating appetite and body weight. Adipocyte 1(4):259–264
Gheith I and El-Mahmoudy A (2018) Laboratory evidence for the hematopoietic potential of Beta vulgaris leaf and stalk extract in a phenylhydrazine model of anemia. Braz J Med Biol Res 51(11)
Goorani S, Koohi MK, Zangeneh A, Zangeneh MM, Moradi R (2019) Pharmacological evaluation of anti-anemic property of aqueous extracts of Falcaria vulgaris leaf in rats. Comp Clin Pathol 28(5):1221–1227
Harborne J (1984) Phytochemical method: a guide to modern techniques of plant analysis. Chapman and Hall Publications, London and New York
Higgins T, Beutler E, Doumas B (2008) Measurement of haemoglobin in blood. In: Burtis C, Ashwood E, Bruns D (eds) Tietz Fundamentals of Clinical Chemistry, 6th edn. Saunders Elsevier, Missouri, USA, pp 524–525
Innih SO, Omage SO, Omage K (2020) Hematinic effects of Spondias mombin and its protective role against the spleenotoxic effect of phenylhydrazine. Clinical Phytoscience 6:1–9
Jakeman G, Saul A, Hogarth W, Collins W (1999) Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119(2):127–133
Kahigwa E, Schellenberg D, Sanz S, Aponte JJ, Wigayi J, Mshinda H, Menendez C (2002) Risk factors for presentation to hospital with severe anaemia in Tanzanian children: a case–control study. Tropical Med Int Health 7(10):823–830
Kenangalem E, Karyana M, Burdarm L, Yeung S, Simpson JA, Tjitra E, Douglas NM (2016) Plasmodium vivax infection: a major determinant of severe anaemia in infancy. Malar J 15(1):1–10
Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW (2004) Impact of malaria control on childhood anaemia in Africa–a quantitative review. Tropical Med Int Health 9(10):1050–1065
Kumar P, Saha S (2013) An updated review on taxonomy, phytochemistry, pharmacology and toxicology of Macuna pruriens. J Pharmacogn Phytochem 2(1)
Luangaram S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P (2007) Protective effects of quercetin against phenylhydrazine-induced vascular dysfunction and oxidative stress in rats. Food Chem Toxicol 45(3):448–455
Madhikarmi NL, Murthy KRS (2015) Biochemical studies on phenylhydrazine induced experimental anemic albino rats. Journal of Universal College of Medical Sciences 3(1):41–47
Magalhães RJS, Clements AC (2011) Spatial heterogeneity of haemoglobin concentration in preschool-age children in sub-Saharan Africa. Bull World Health Organ 89:459–468
Magnani M, Rossi L, Cucchiarini L, Stocchi V, Fornaini G (1988) Effect of phenylhydrazine on red blood cell metabolism. Cell Biochemistry and Function: Cellular Biochemistry and Its Modulation by Active Agents or Disease 6(3):175–182
Moraleda C, Aguilar R, Quintó L, Nhampossa T, Renom M, Nhabomba A, Achtman AH (2017) Anaemia in hospitalised preschool children from a rural area in mozambique: a case control study in search for aetiological agents. BMC Pediatr 17(1):1–10
Mulenga M, Malunga P, Bennett S, Thuma PE, Shulman C, Fielding K, Greenwood BM (2005) Factors associated with severe anaemia in Zambian children admitted with Plasmodium falciparum malarial anaemia. Ann Trop Paediatr 25(2):87–90
Muniyandi K, George E, Sathyanarayanan S, George BP, Abrahamse H, Thamburaj S, Thangaraj P (2019) Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats. India Food Science and Human Wellness 8(1):73–81
Nweze NE, Ezema C, Ezema AS, Eze JI, Ezema WS (2017) Toxicity and nutritional studies on Mucuna pruriens leaves from Nsukka. South-Eastern Nigeria Comparative Clinical Pathology 26(3):569–574
Pandey K, Meena AK, Jain A, Singh R (2014) Molecular mechanism of phenylhydrazine induced haematotoxicity: a review. Ame J Phytomed Clin Therapeut 2:390–394
Pandey P, Meena AK, Yadav S, Singh RK (2017) Eclipta Alba A herbal remedy for the prevention of hemolytic anemia. Eur J Biomed 4(9):359–363
Pandey S, Ganeshpurkar A, Bansal D, Dubey N (2016) Hematopoietic effect of Amaranthus cruentus extract on phenylhydrazine-induced toxicity in rats. Journal of Dietary Supplements 13(6):607–615
Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, White NJ (2001) Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg 65(5):614–622
Rapido F (2017) The potential adverse effects of haemolysis. Blood Transfus 15(3):218
Resmi S, Fathima L, Vijayaraghavan R (2017) Formulation of an herbal extract for anemia treatment and its effect on physical work and intelligence capacity in adolescent girls with iron deficiency in India. Afr J Pharm Pharmacol 11(24):284–248
Roque M, Danna C, Gatti C and Veuthey T (2008) Hematological and morphological analysis of the erythropoietic regenerative response in phenylhydrazine-induced hemolytic anemia in mice. Scand J Lab Anim Sci 35(3):181–190
Sato H, Sakairi T, Fujimura H, Sugimoto J, Kume E, Kitamura K, Takahashi K (2013) Hematological and morphological investigation of thrombogenic mechanisms in the lungs of phenylhydrazine-treated rats. Exp Toxicol Pathol 65(4):457–462
Shukla P, Singh R (2015) Toxicogenomics of phenylhydrazine induced hematotoxicity and its attenuation by plumbagin from Plumbago zeylanica. Pharmacogn Mag 11(Suppl 3):S380
Suzanne BB, Adeline FY, Theodora KK, Dairou H, Pradel KL, Aristide KM and Clerge T (2020)
Thrall M and Weiser MG (2002) Haematology. Laboratory Procedures for Veterinary Technicians. Fourth Edition, Mosby Incorporated, Missouri, USA. 29–74
White NJ (2018) Anaemia and malaria. Malar J 17(1):1–17
Zangeneh MM, Zangeneh A, Salmani S, Jamshidpour R, Kosari F (2019) Protection of phenylhydrazine-induced hematotoxicity by aqueous extract of Ocimum basilicum in Wistar male rats. Comp Clin Pathol 28(2):331–338
Zhang L, Yuan B, Wang H and Gao Y (2015) Therapeutic effect of Agaricus brasiliensis on phenylhydrazine-induced neonatal jaundice in rats. BioMed Res Int 2015
Zhu X, Liu J, Feng Y, Pang W, Qi Z, Jiang Y, Cao Y (2015) Phenylhydrazine administration accelerates the development of experimental cerebral malaria. Exp Parasitol 156:1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All the experimental procedures followed ethical approval for the care and use of animals in research. All the experimental procedures were performed according to the committee for the purpose of control and supervision of experiments on animals, norms, and approved by the Enugu State University of Science and Technology Animal Ethical Committee with an approval number ESUT/PHARM/0152.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okolo, K.O. Protective effects of hydro-ethanolic leaf extract based formulation of Mucuna pruriens (Fabaceae) on phenylhydrazine induced hemolytic anemia and metabo-hematological alterations in rats. Comp Clin Pathol 30, 765–774 (2021). https://doi.org/10.1007/s00580-021-03278-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03278-1