Abstract
Rising global population accelerates food waste generation, thereby creating a crisis in food waste management. A solution involves deriving value-added products like cellulose biopolymer from food waste. Chilli stalk wastes are one such food waste which are generated in large quantities and are unsuitable for field use or incineration due to health and environmental challenges. A greener alternative is extracting cellulose biopolymer from chilli stalk waste. The extraction of cellulose biopolymer from chilli stalk results in a renewable, biodegradable and economically efficient biomaterial with a broad range of applications. The extraction process involving alkali treatment (NaOH) and bleaching (alkaline H2O2), resulted in a yield of 29.85% cellulose biopolymer. The extracted cellulose was subjected to quantification and functional property analysis followed by characterization (FTIR, XRD, TGA, DSC and SEM) to analyse functional groups, crystallinity, thermal properties and surface morphology. Functional property analysis resulted in higher values when compared with commercial cellulose. The characterization techniques confirmed the effective removal of impurities such as lignin, hemicellulose and pectin by the chemical treatments. Cellulose sheets, fabricated using solvent casting, exhibited exceptional biodegradability (85.36%) within 20 days, surpassing conventional food packaging materials, commercial food packaging paper (15.95 ± 0.12% [%w/w]) and plastic sheets (7.89 ± 0.33% [%w/w]) over the same time period. The novelty of this research lies in the innovative valorization of chilli stalk waste, which often remains unused in large quantities globally. This study introduces a cost-effective method to convert it into a value-added, highly biodegradable biopolymer. The resulting cellulose sheets provide an eco-friendly substitute for traditional food packaging materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The rapid increase in the global population is generating a substantial need for elevated food production. The increased food consumption brings forth the challenges posed by food processing waste and post-consumption waste. Based on the data released by the Food and Agriculture Organization (FAO), 1.3 billion tons of food waste is being generated per year approximately, where half can be attributed to agro-wastes. The majority of the generated food waste is being dumped in landfills, leading to various environmental concerns [1]. The remaining portion is either incinerated for energy or utilised in composting processes. The global population is projected to spike to 25% of the current population by the year 2050, which demands a substantial increase in agricultural production to fulfil the food and nutrition requirements [2]. The increased food production will lead to the rise in food waste generation. The food wastage index from UNEP’s (United Nations Environment Programme) states that India is the second largest food waste producer followed by China [3]. About one-third of the food generated in India is being wasted or spoiled. The annual food waste generated in India from households alone amounts to 68.7 million tonnes, which accounts for 50 kg per person [4]. A solution to mitigate the problems associated with the overproduction of food waste is to convert them into value-added products, such as biodiesel, biopolymers and other biochemical products [5]. Biopolymers can be derived either by extracting them from food waste sources, such as cellulose, or through fermentation processes using microbes to produce biopolymers like chitin from fungi and polyhydroxyalkanoates from bacteria [6]. The cellulose biopolymer can be extracted from food waste and agro-waste through treatments with primary chemicals.
Cellulose is the most abundant organic compound on earth, it is synthesised as the primary cell wall component in plants and algae, it is also produced by certain bacteria [7]. It is a long-chain polysaccharide composed of a linear chain of repeating glucose units ([C6H10O5]n) linked through β (1 → 4) glycosidic linkages. Cellulose in pure form is odourless, tasteless and hydrophobic in nature, with a melting temperature of 467 °C [8]. Cellulose polymer has distinctive features such as biodegradability, biocompatibility and high thermal stability which makes it an ideal candidate for the application in various fields such as paper production, biomedicine and food packaging industries. Cellulose can also be employed as the substrate for fuel production through fermentation [9].
Before the extraction of cellulose from the waste substrate, it should be segregated, cleaned, dried and powdered. This will maximise the surface area of the substrate and enhance the results of the chemical treatments [10]. The cellulose extraction follows a delignification step and bleaching which will remove the cementing material such as pectin, lignin and hemicellulose leaving behind the cellulosic fibres [11]. The alkali treatment using KOH/NaOH is the most common method to remove lignin and other alkali soluble components present in the substrate. The bleaching step further eliminates hemicellulosic material and residual lignin components [12]. The common bleaching agents utilised are alkaline peroxide, sodium hypochlorite, sodium chlorate, etc. Alkali treatment and bleaching not only eliminate the lignin and hemicellulose but also break down fibres into microfibrillar forms [13]. This removal of hemicellulose and other non-cellulosic materials including pigments, creates voids within the fibres, leading to swelling fine structures that alter physical structures, morphology, dimensions and mechanical properties [14]. The chemical treatments tend to increase the fibre properties including crystallinity, surface properties and mechanical properties [15].
In the current study, the cellulose extraction by utilising chilli (Capsicum annum) stalk waste was investigated. The chilli processing industry generates a substantial amount of waste, with approximately 22.87% of chilli weight being discarded as waste material [16]. The chilli stalk waste cannot be used in fields as manure because of the production of allelochemicals during their decomposition [17]. The allelochemicals generated can hinder the growth and development of crops, leading to a decrease in yield. Incineration of the chilli waste is also not advisable as it releases fumes that can cause eye irritation and lung irritation, and contribute to air pollution [18]. Hence, chilli stalk waste can be utilised to extract cellulose biopolymer as a greener approach. The current work was aimed to extract the cellulose biopolymer from chilli stalk waste and to fabricate a cellulose sheet using the extracted cellulose. It is significant to mention that the studies related to the extraction of cellulose from chilli stalk waste have not received much attention, as per the available literature. Thus, the novelty of this research is developing a simple and sustainable protocol for the extraction of commercially important cellulose biopolymer from discarded chilli stalks and its application for the fabrication of biodegradable sheets.
2 Methodology
2.1 Substrate collection and processing
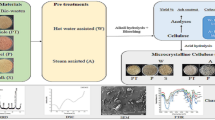
The waste stalks (calyx and pedicle) of dried red chilli (Byadagi variety: Capsicum annuum) were procured from KR Market, Bangalore, India. The debris from the substrate was removed manually, followed by a thorough washing to remove the adhered dirt and dust. The washed substrate was then dried overnight at 60 °C and powdered (Fig. 1) [19]. The chilli stalk powder was stored for further use.
2.2 Cellulose extraction from chilli stalk
The cellulose extraction was carried out by following alkali treatment and bleaching procedure. The chilli stalk powder (10 g) was added into 150 mL of 4% (%w/v) NaOH for alkali treatment and the mixture was then stirred on a magnetic stirrer at 500 rpm for 2 h at 60 °C [20]. This treatment was repeated thrice with intermediate washing to neutralise pH. The alkali treatment was followed by bleaching using alkaline peroxide treatment. The biomass was added to 150 mL of 4% (%v/v) H2O2 and 4% (%w/v) NaOH; the mixture was stirred on a magnetic stirrer at 500 rpm for 2 h at 60 °C [21]. Bleaching step was repeated twice with intermediate washing using distilled water to neutralise the pH. The extracted cellulose from chilli stalk waste was dried overnight in a hot air oven at 60 °C and stored for further studies.
2.3 Quantitative estimation of extracted cellulose
To assess the purity of the extracted cellulose, a quantitative estimation using standard anthrone test was conducted [22]. Before the estimation of cellulose from extracted chilli stalk cellulose, the sample was subjected to acid pretreatments to remove the non-cellulosic components. Extracted cellulose (1 g) was added to 3 mL of acetic/nitric reagent (150 mL 80% (%v/v) acetic acid and 15 mL of conc. nitric acid) and was incubated at 100 °C for 30 min in a water bath. Subsequently, the solution was centrifuged at 5000 rpm for 10 min and the resulting pellet was recovered. The pellet underwent thorough washing to eliminate any traces of the acetic/nitric reagent. To the washed pellet, 10 mL of 67% (%v/v) sulphuric acid (precooled) was added and thoroughly mixed. This solution was then incubated at 4 °C for 1 h for ensuring complete dissolution of the pellet, 1 mL of this solution was diluted to 100 mL using 67% (%v/v) sulphuric acid (precooled). From the resulting solution, 1 mL was used as a test solution for the determination of cellulose content and 1 mL of 67% (%v/v) sulphuric acid was maintained as blank. The absorbance was measured at 630 nm using the Shimadzu UV-1800 spectrophotometer [23].
2.4 Functional property analysis of extracted cellulose
The functional properties of a biopolymer help to understand the range of applicability of the biopolymer in various fields. The analysed functional properties for the extracted cellulose include bulk density, packed density, hydrated density, water retention capacity, oil retention capacity, emulsifying activity and settling volume [24]. Bulk density and packed density aids in comprehending the structural and volumetric aspects of the biopolymer, which is important for the applicability and storage considerations of the biopolymer [25]. The hydrated density and water retention capacity of a biopolymer relate to its ability to retain water. This property is significant in various fields, including structural engineering and biomedicine [26]. Oil retention capacity is the ability of any material to hold oil and emulsifying activity is the maximum amount of oil that can be emulsified by the biopolymer. Both these properties are significantly exploited in industries, particularly in the food industry and pharmacology [27]. Settling volume can be employed to understand the ability of a biopolymer to form colloidal solutions for their applicability in producing sol, emulsion, foam and aerosol [28]. The methodology followed for the determination of functional properties are listed in Table 1.
2.5 Characterization of extracted cellulose
Characterization of the extracted cellulose was performed to analyse and understand the functional groups present, as well as various physicochemical properties. Various characterization techniques for the biopolymer include Fourier transform infrared (FTIR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and scanning electron microscopy (SEM).
2.5.1 FTIR
FTIR investigates the functional groups present in the sample by analysing the characteristic infrared bands obtained [29]. The FTIR analysis was performed by scanning the sample between 4000 and 400 cm−1 IR frequency using the Shimadzu IR Spirit-L FTIR spectrophotometer at the Common Instrumentation Lab, Department of Life Sciences, CHRIST (Deemed to be University). The scanning was performed at a resolution of 4 cm−1.
2.5.2 XRD
XRD analyses the angular diffraction (2θ) pattern of the biopolymer between 5° to 90° to understand its crystal phase and structure [30]. MiniFlex 600 XRD instrument at Central Instrumentation Facilities, CHRIST (Deemed to be University), Bangalore, was used for XRD analysis of the extracted cellulose.
2.5.3 TGA
TGA is employed to understand the thermal stability and thermal degradation of the extracted biopolymer [31]. The biopolymer was exposed to temperatures ranging from 40 to 800 °C at a heating rate of 20 °C/min, using SDT Q600 V20.9 Build 20 (Thermal Gravimetric Analyzer) at School of Advanced Science, Vellore Institute Technology, Vellore.
2.5.4 DSC
The material properties like glass transition range, crystallisation and melting point of the extracted biopolymer can be understood through the DSC spectrum [32]. The sample was subjected to a temperature range from 20 to 200 °C at a heating rate of 10 °C/min, using NETZSCH DSC 204F1 at Sophisticated Test and Instrumentation Centre (STIC), Cochin University of Science & Technology Campus, Kochi.
2.5.5 SEM
The detailed surface morphology of the biopolymer can be obtained through SEM imaging [33]. The images were obtained using Thermo Scientific Apreo 2 SEM, Central Instrumentation Facilities, CHRIST (Deemed to be University), Bangalore.
2.6 Fabrication of cellulose sheet
For cellulose sheet fabrication 1.5 g of the extracted chilli stalk cellulose was mixed with 100 mL of distilled water by stirring on a magnetic stirrer for 6 h at room temperature. In succession, the mixture was homogenised at 7500 rpm for 20 min and was subjected to incubation at 4 °C overnight for the removal of confined gas from the sample. Subsequently, 50 mL cellulose mixture was homogenised again at 7500 rpm for 20 min and the homogenised mixture was transferred into a casting container [34]. The container was placed in a hot air oven at 60 °C until the sheets were dried.
2.7 Biodegradation study of fabricated cellulose sheet
The dried chilli stalk cellulose sheets were carefully removed and were cut into 2 cm × 2 cm dimensions and then weighed. The cut sheets were enclosed in a non-biodegradable plastic mesh and were buried with 1 kg of garden soil in a plastic container (Fig. 2). In a similar manner commercial brown food packing paper (100 GSM) and food packing plastic sheets of 28 Micron were also cut, enclosed and buried in 1 kg garden soil in a plastic container [35]. The setup was incubated at room temperature by maintaining a moisture content of 60%. The percentage degradation was recorded every 10 days. Before weighing the degraded sheets, they were washed with distilled water to remove soil particles and then dried at 60 °C [24]. The degradation percentage of the biopolymer was calculated by using the equation:
3 Results and discussion
3.1 Cellulose extraction from chilli stalk
Cellulose, the complex carbohydrate which forms the structural framework of plant cell walls, will be ever present alongside with other components such as lignin, pectin and hemicellulose. Hence, for cellulose extraction, it is important to treat the selected substrates with chemicals which can solubilise or degrade these components, ultimately leaving behind valuable cellulose fibres. The cellulose extraction from chilli stalk powder was carried out through alkali treatment and bleaching. The alkali treatment using 4% NaOH solubilised the hemicellulose impurities and improved the cellulose fibre properties. The bleaching using alkaline peroxide (4% H2O2 and 4% NaOH) proved effective in delignifying and removing residual hemicellulose (Fig. 3). The chemical reaction involved in the bleaching occurs through the degradation by peroxide radicals. The yield of extracted chilli stalk cellulose was measured to be 29.850 ± 0.240% (%w/w), which was in line with the reported cellulose content in chilli stem of 27.4% (%w/w). Ma et al. extracted cellulose from chilli stem nitric acid–ethanol method. The study reported yield between 15% (%w/w) and 34.5% (%w/w) [17]. Industrial pepper bio-waste was utilised as substrate by Holilah et al. for the production of microcrystalline cellulose. Cellulose extraction was performed using alkali treatment using 5% (%w/v) NaOH and a bleaching method similar to the current study (alkaline peroxide). The fibres of industrial pepper bio-waste were reported to be 39.8 ± 0.9% (%w/w) and the treatment methods increased the cellulose content to 77.9% (%w/w) [36]. The extracted cellulose aligned a similar yield with these available literatures. In another similar study investigating the extraction of cellulose from the stalk of grapes, Araújo et al. (2023) obtained a yield of 21.98% (%w/w). The study followed an alkali treatment using 4 mol/L NaOH and bleaching using alkaline peroxide [37].
3.2 Quantitative estimation of extracted cellulose
The Anthrone method was employed for determining the overall cellulose content of the extracted cellulose from chilli stalk. The overall cellulose content in the sample will help to identify the purity of the extracted fibres. Using the standard graph of commercial cellulose, the amount of cellulose was quantified as 71.23 ± 0.34% (%w/w). In a similar work focusing on the extraction of cellulose nanocrystals from chilli leftover by Nagalakshmaiah et al., a cellulose content of 67.3% (%w/w) was obtained from the yielded cellulose nanocrystals [38]. Razali and Kasim isolated cellulose from Pepper Pericarp Waste using alkali treatment with different NaOH concentration and bleaching using acetic chlorite. The study reported the extraction of biomass with 65.97% of cellulose [39]. In a study exploring the extraction of cellulose fibres from Agro-waste Capsicum annum stem, Vinod et al. reported a cellulose content of 63.46 ± 3.42% in oxalic acid treated fibres and 54.27 ± 4.74% in NaOH-treated fibres [40]. The cellulose obtained in the current study surpasses the reported purity levels in the available literature. Hence, it can be concluded that the extraction method used in the current study removed the impurities efficiently.
3.3 Functional property analysis of extracted cellulose
The comparison of functional properties of cellulose extracted from chilli stalk and commercial cellulose was noted as Table 2. The functional properties measured are bulk density (BD), particle density (PD), hydration density (HD), water retention capacity (WRC), oil retention capacity (ORC), emulsifying activity (EA) and settling volume (SV). It was observed that the values for these functional properties were higher for the cellulose extracted from chilli stalk than that of the commercial cellulose powder.
The BD of the extracted cellulose (0.091 ± 0.001 g/mL) was found to be higher in comparison to commercial cellulose (0.039 ± 0.003 g/mL). There exists a complex interplay between the factors influencing powder bulk density, surface activity and cohesion [41]. Higher PD (0.407 ± 0.010 g/mL) of the extracted cellulose than the commercial cellulose (0.248 ± 0.032 g/mL) suggest the higher low properties and its compressibility. The HD of the extracted cellulose was measured at 1.047 ± 0.011 g/mL, while that of commercial cellulose was found to be 0.433 ± 0.037 g/mL. The higher HD suggests the potential utilisation of the extracted cellulose in tablet-making applications. Swelling, commonly recognized as an indicator of tablet disintegration ability, can be assessed through the determination of hydration capacity, swelling capacity and moisture sorption profile [42]. WRC of the extracted cellulose 9.843 ± 0.041 g water/g which was higher than the results obtained by hence can be used as creaming agent or thickener in food items such as ice cream, shredded cheese, powdered drink mixes and fast food. WRC holds significant importance as it influences the texture, juiciness and taste of food formulations, especially impacting the shelf life of bakery products such as cakes, biscuits and cookies [43]. A higher ORC suggests potential applications as emulsifiers for high-fat food products such as mayonnaise, pound cake, ice cream, whipped topping and similar items [44]. The ORC obtained for the extracted cellulose 2.883 ± 0.094 g oil/g which was much higher than commercial cellulose, 1.782 ± 0.043 g oil/g. The elevated EA of the extracted cellulose (62.03 ± 0.933 g oil/g) compared to commercial cellulose (48.90 ± 0.341 g oil/g) enhances its suitability for such applications. The high SV of 0.407 ± 0.011 m/g3, when compared to that of commercial cellulose (0.289 ± 0.031 m/g3), can be attributed to the larger size of extracted cellulose particles compared to the finer particles of commercial cellulose [23]. The analysis of functional properties indicates that the extracted cellulose is a promising candidate for the use in food additives and pharmaceutical applications, such as tablet formation. These potential applications for extracted chilli cellulose will be considered in future studies.
3.4 Characterization of extracted cellulose
3.4.1 FTIR
The FTIR spectra provide insight into the functional groups present in the analysed sample. Figure 4 illustrates the FTIR spectrum and Table 3 details the functional groups present in the extracted chilli stalk cellulose. The broad peak at 3337.29 cm−1 can be attributed to the stretching of O–H bonds from the intramolecular and intermolecular hydrogen bonds [45]. The broad peak also indicates the hydrophilic nature of the extracted cellulose. The symmetrical and asymmetrical stretching vibrations of the C-H group were represented by the band at 2928.87 cm−1 [46]. These two peaks (3337.29 cm−1 and 2928.87 cm−1) are the characteristic peaks of cellulose material [47]. The peaks at 1602.24 cm−1, 1099.58 cm−1 and 899.65 cm−1 can be attributed to the rocking bands of C-O, C-H and CH2 respectively. The sharp peak at 1602.24 cm−1 corresponds to the O–H bendings observed in water molecules and the peak at 1019.61 cm−1 denotes the vibrations of C–O–C pyranose ring stretching [48]. The β-glycosidic bonds between the glucose molecules of cellulose are represented by the peak at 899.65 cm−1. The peak at 1339.49 cm−1 can be attributed to the skeletal vibrations of C–C and C-O functional groups. The above discussed peaks from the spectra indicate the distinctive characteristics of cellulose extracted from chilli stalk. The peaks at 1745.04 cm−1 and 1510 cm−1 with low intensity represent hemicellulose and lignin, respectively. The low intensity of these impurities in the FTIR spectra suggests effective removal of these compounds from the extracted cellulose [49].
3.4.2 XRD
Figure 5 illustrates the XRD spectrum of the cellulose extracted from the chilli stalk. The high intensity peak at 2θ = 22.62° and the low intensity peak at 2θ = 16.8° can be attributed to the characteristic peaks of cellulose type I allomorph [56]. Cellulose I allomorph is widely present in plant cell walls [57]. The peaks at 2θ values of 22.62°, 16.8° and 29.48° correspond to the crystallographic planes of (110), (200) and (040) convincing the presence of cellulose type I allomorph. The crystallinity index (CI) for the extracted chilli stalk cellulose was determined to be 41.707%. The obtained result was similar to the CI reported by Jabli et al. (2018) and Jeyabalaji et al. (2022). The fibres extracted by Jabli et al. from Nerium oleander reported a CI of 43.4% [58]. A CI of 46.62% was reported by Jeyabalaji et al. (2022), from the extracted Acalypha indica root cellulose [55]. The high intensity peak at 2θ = 22.62°, with no other major peaks, suggests the absence of impurities like lignin and hemicellulose from the extracted chilli stalk cellulose [59].
3.4.3 TGA
Figure 6 illustrates the TGA spectrum of the extracted chilli stalk cellulose. Initially, a weight loss of 8.042% was observed from 32.05 to 149.71 °C, which can be concluded as the loss of water trapped with the extracted biomass. From 149.71 to 400.33 °C, a weight loss of 46.84% was observed; this can be associated with fast pyrolysis of the cellulose biopolymer. The fast pyrolysis at higher temperature indicates the higher thermal stability of the extracted cellulose [60]. During fast pyrolysis, the cellulose biopolymers undergo decarboxylation, depolymerization and decomposition [61]. Subsequently, slow pyrolysis commenced at 400.33 °C and concluded at 780.86 °C with a polymer degradation of 10.05%. The remaining 35.08% may be associated with the extremely heat-resistant lignin impurities and the formation of biochar during the analytical process [62].
3.4.4 DSC
The DSC thermogram of the extracted chilli stalk cellulose is illustrated as Fig. 7. The obtained single endothermic peak started at 61.4 °C and ended at 141.0 °C. The highest intensity point of the peak was captured at 97.2 °C which can be related to the liquid to gas transition of water molecules attached to the biopolymer. This peak also represents the vaporisation of volatile substances from the fibres [63]. This result suggests that the extracted cellulose exhibits a reduced affinity for water molecules, as evidenced by the lower energy required for water content loss. This characteristic is highly desirable for composite production [64]. The temperature range of the endothermic peak of the extracted chilli cellulose, ranging from 97.6 to 139.9 °C, aligns with the glass transition temperature of cellulose.
3.4.5 SEM
Figure 8a and b portray the SEM image of the extracted chilli stalk cellulose at 500 × and 2500 × magnification respectively. The surface morphology of the extracted cellulose appeared as an abrasive fibrillar pattern of individualised fibres. The obtained result regarding the morphology of the extracted cellulose fibres after chemical treatments was similar to the results obtained by Balasubramani et al. (2024) [65]. This texture assures the successful removal of non-cellulosic impurities, including hemicellulose, lignin, pectin and wax materials, through chemical treatments. The presence of the mentioned impurities would have resulted in a smooth texture for the cellulose fibres [66]. The fibrous structure also confirms the separation of fibril structures which binds the biomass. Moreover, the reduction in the size of cellulose fibres can lead to an increased fibre aspect ratio, ultimately improving the fibres’ reinforcing capacity for composite applications [67].
3.5 Sheet fabrication using extracted cellulose
The homogenisation process transformed the fibrous cellulose from chilli stalks into a powdery form, resulting in a white colloidal solution. The fabricated sheet from the colloidal solution appeared white with a light-yellow tinge and appeared as (Fig. 9). The fabricated sheet highly resembles the sheets fabricated by Sankar et al. [23] and Umesh et al. [24] in texture and colour. The sheet’s texture and material resembled that of paper, presenting a sturdy structure that maintained its shape after bending. This characteristic feature makes it a promising candidate for biodegradable scaffolds, biomedical applications [68], biodegradable food packaging [69] and biodegradable electronic materials [70].
3.6 Biodegradation study of fabricated cellulose sheet
The percentage of biodegradation for the cellulose sheet (CS), food packaging plastic sheet and food packaging brown paper was calculated after every 10 days (Fig. 10). The biodegradation percentage data was calculated as mean ± SD performed in triplicate which is significant at p < 0.05. After 20 days of soil burial, the plastic sheet and packaging paper exhibited a biodegradation percentage of 7.89 ± 0.33% (%w/w) and 15.95 ± 0.12% (%w/w) respectively, whereas the fabricated CS displayed 85.360 ± 0.407% degradation percentage after 20 days (Table 4). The data of biodegradation of CS was further supported by comparing the FTIR data of CS before burial and after 20 days of burial (Fig. 11). The characteristic peaks for cellulosic material at 3337.29 cm−1, 2928.87 cm−1, 1602.24 cm−1, 1099.58 cm−1 and 899.65 cm−1 exhibited a rapid decrease in the transmittance percentage which confirms the biodegradation of the polymer. The result obtained is supported by the reducing bands for cellulose in the study conducted by Ołdak et al. [71]. The study reported a decrease in peak values at 1000 cm−1–1200 cm−1 and 1500 cm−1–1800 cmx`−1 regions of cellulose for cellulose/polyethylene sheets. A similar pattern was observed by Doh et al. 2020 for the biodegradation of cellulose nanocomposite sheet with alginate. The study reported a decrease in the intensities of all major peaks which are characteristic to cellulose nanocrystals. Mainly the peaks at 1133 cm−1 and 1248 cm−1 which are attributed to C-O stretching and peak of C = C bending at 996 cm−1 were notably reduced [72].
The exceptionally rapid biodegradation rate of the biopolymer sheet establishes it as an excellent choice for biodegradable food packaging material. The rapid biodegradation rate also alleviates the burden on landfills caused by food packaging materials.
The degradation process for polymeric sheets primarily occurs on the surface due to lack of penetration ability by the enzymes produced by soil microbes to the inner layers of these sheets [73]. The deterioration of the polymer will commence after the microbial adhesion and colonisation on the polymer surface [74]. Surface deterioration will be succeeded by enzyme penetration into the interior parts polymer, facilitating microbial colonisation and thereby enhancing the degradation rate. Biodeterioration progresses through the fragmentation of the polymer, followed by the degradation of the polymer into monomers with lower molecular weights [75]. The monomers will either diffuse inside the microbes or will be taken up as energy sources for catabolism or anabolism. The remaining portion of the polymers undergoes mineralisation through the activities of exoenzymes and free radicals [76]. A complete degradation of the polymer will release CO2, H2O and CH4, along with other byproducts including microbial biomass, salts and organic compounds, which differ from polymer to polymer [77].
Ai et al. (2021) reported that the cellulose prepared by delignified banana stem cellulose with ionic liquid 1-Allyl-3-methylimidazolium chloride degraded to less than ~ 95% within 4 weeks of soil burial [78]. In the study conducted by Wang et al., a cellulose nanocrystal-zinc oxide film was fabricated with polylactic acid. The biodegradation study of the film reported 28% biodegradation after 110 days of soil burial [79]. Dong et al. crafted straws using lignose cellulose and reported complete biodegradation within 30 days in a natural environment setup [80]. Jaiswal et al. developed an electrocardiograph (ECG) device using a fabricated cellulose sheet. The complete device was buried in soil for both biodegradation and the recovery of machine parts. The research indicated a 78% (%w/w) biodegradation within 128 days [81]. Upon comparison with these literatures, it is clear that the biopolymer sheet produced in this research with cellulose extracted from chilli stalk demonstrates significantly higher biodegradation. This expands the potential applications for the fabricated sheet.
4 Conclusion
The cellulose extraction utilising the chilli stalk waste was carried out through alkali treatment using NaOH and subsequent bleaching using alkaline peroxide. The extraction process yielded 29.850 ± 0.240% (%w/w) with 71.23 ± 0.34% (%w/w) purity. The purity of extracted cellulose was analysed using the anthrone method. For determining the applicability of the extracted cellulose in various fields different functional properties were analysed and compared with commercially available cellulose powder. Later the extracted cellulose was subjected to various characterization techniques (TIR, XRD, TGA, DSC and SEM) to determine the functional groups present, crystallinity, thermal properties and surface morphology. The FTIR spectra exhibited characteristic peaks which were shown by cellulosic material. XRD analysis of the extracted cellulose revealed a CI of 41.707% and the 2θ values indicated the presence of a cellulose type I. The TGA and DSC thermograms confirmed the elimination of lignin, pectin and hemicellulose impurities and the TGA curve also revealed the high thermal stability of the extracted biopolymer. The cellulose sheet fabricated using the extracted cellulose showcased a biodegradability of 85.360 ± 0.407% (%w/w) within 20 days of soil burial. This research highlights the viability of chilli stalk waste for cost-effective cellulose biopolymer extraction, followed by the fabrication of biodegradable cellulose sheets. Future studies on the extracted cellulose should delve into the potential applications of these sheets in fields such as biomedical implants, pharmaceuticals, drug delivery and cosmetics, offering eco-friendly alternatives to existing products in these domains.
Data availability
The original data related to this research work will be available on special request to the authors.
References
Zhongming Z, Linong L, Xiaona Y et al (2021) UNEP food waste index report 2021. https://www.unep.org/resources/report/unep-food-waste-index-report-2021
van Dijk M, Morley T, Rau ML, Saghai Y (2021) A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat Food 2:494–501. https://doi.org/10.1038/s43016-021-00322-9
Environment UN (2021) UNEP Food Waste Index Report 2021. In: UNEP - UN Environment Programme. https://www.unep.org/resources/report/unep-food-waste-index-report-2021. Accessed 1 Dec 2023
Lahiri A, Daniel S, Kanthapazham R et al (2023) A critical review on food waste management for the production of materials and biofuel. J Hazard Mater Adv 10:100266. https://doi.org/10.1016/j.hazadv.2023.100266
Yukesh Kannah R, Merrylin J, Poornima Devi T et al (2020) Food waste valorization: biofuels and value added product recovery. Bioresource Technol Rep 11:100524. https://doi.org/10.1016/j.biteb.2020.100524
Ranganathan S, Dutta S, Moses JA, Anandharamakrishnan C (2020) Utilization of food waste streams for the production of biopolymers. Heliyon 6:e04891. https://doi.org/10.1016/j.heliyon.2020.e04891
Allen H, Wei D, Gu Y, Li S (2021) A historical perspective on the regulation of cellulose biosynthesis. Carbohydr Polym 252:117022. https://doi.org/10.1016/j.carbpol.2020.117022
Singh V, Indoria S, Jisha KJ, Gardas RL (2021) Structure and solubility of polysaccharides. Polysaccharides 325–336. https://doi.org/10.1002/9781119711414.ch15
Torgbo S, Sukyai P (2020) Biodegradation and thermal stability of bacterial cellulose as biomaterial: the relevance in biomedical applications. Polym Degrad Stab 179:109232. https://doi.org/10.1016/j.polymdegradstab.2020.109232
Kumar V, Pathak P, Bhardwaj NK (2020) Waste paper: an underutilized but promising source for nanocellulose mining. Waste Manag 102:281–303. https://doi.org/10.1016/j.wasman.2019.10.041
Midhun Dominic CD, Raj V, Neenu KV et al (2022) Chlorine-free extraction and structural characterization of cellulose nanofibers from waste husk of millet (Pennisetum glaucum). Int J Biol Macromol 206:92–104. https://doi.org/10.1016/j.ijbiomac.2022.02.078
Qazanfarzadeh Z, Ganesan AR, Mariniello L et al (2023) Valorization of brewer’s spent grain for sustainable food packaging. J Clean Prod 385:135726. https://doi.org/10.1016/j.jclepro.2022.135726
de Fonseca AS, Panthapulakkal S, Konar SK et al (2019) Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind Crops Prod 131:203–212. https://doi.org/10.1016/j.indcrop.2019.01.046
Samir A, Ashour FH, Hakim AAA, Bassyouni M (2022) Recent advances in biodegradable polymers for sustainable applications. npj Mater Degrad 6:1–28. https://doi.org/10.1038/s41529-022-00277-7
Nagarajan KJ, Ramanujam NR, Sanjay MR et al (2021) A comprehensive review on cellulose nanocrystals and cellulose nanofibers: pretreatment, preparation, and characterization. Polym Compos 42:1588–1630. https://doi.org/10.1002/pc.25929
Meena G, Pant D, Kumar S (2006) Economics of chilli processing in Rajasthan. Agric Sci Dig 26:83–86
Ma Y, Chai X, Bao H et al (2023) Study on nanocellulose isolated from waste chilli stems processing as dietary fiber in biscuits. PLoS One 18. https://doi.org/10.1371/journal.pone.0281142
Tiwari A (2021) A review study on chillies as food. IJIRCST 9:100–105
Sharma C, Sharma SN, Srivastava R (2022) Analysis of cellulose extracted from waste products. Colloid Polym Sci 300:1027–1036. https://doi.org/10.1007/s00396-022-05005-w
Tesema BD, Chamada TA (2023) Analysis of bagasse cellulose-based hydrogel for methylene blue removal from textile industry wastewater. Int J Chem Eng 2023. https://doi.org/10.1155/2023/2313874
Li H, Zhang H, Xiong L et al (2019) Isolation of cellulose from wheat straw and its utilization for the preparation of carboxymethyl cellulose. Fibers Polym 20:975–981. https://doi.org/10.1007/s12221-019-7717-6
Ganguly P, Sengupta S, Das P, Bhowal A (2020) Synthesis of cellulose from peanut shell waste and its use in bioethanol production. In: Ghosh SK, Sen R, Chanakya HN, Pariatamby A (eds) Bioresource utilization and bioprocess. Springer Singapore, Singapore, pp 81–91
Sankar Santhosh A, Umesh M, Kariyadan S et al (2023) Fabrication of biopolymeric sheets using cellulose extracted from water hyacinth and its application studies for reactive red dye removal. Environ Res 117466. https://doi.org/10.1016/j.envres.2023.117466
Umesh M, Santhosh AS, Shanmugam S et al (2022) Extraction, characterization, and fabrication of cellulose biopolymer sheets from Pistia stratiotes as a biodegradative coating material: an unique strategy for the conversion of invasive weeds into value-added products. J Polym Environ 30:5057–5068. https://doi.org/10.1007/s10924-022-02511-4
Verheijen FGA, Zhuravel A, Silva FC et al (2019) The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 347:194–202. https://doi.org/10.1016/j.geoderma.2019.03.044
Huang H, Reddy NG, Huang X et al (2021) Effects of pyrolysis temperature, feedstock type and compaction on water retention of biochar amended soil. Sci Rep 11:7419. https://doi.org/10.1038/s41598-021-86701-5
Chen F, Zhao J, Wang H et al (2023) Oil-retention and oil-bearing tribological properties of nanoporous copper prepared using a chemical dealloying method. Metals 13:1232. https://doi.org/10.3390/met13071232
Lin F, Zhu X, Li J et al (2019) Effect of extracellular polymeric substances (EPS) conditioned by combined lysozyme and cationic polyacrylamide on the dewatering performance of activated sludge. Chemosphere 235:679–689. https://doi.org/10.1016/j.chemosphere.2019.06.220
Asemani M, Rabbani AR (2020) Detailed FTIR spectroscopy characterization of crude oil extracted asphaltenes: curve resolve of overlapping bands. J Pet Sci Eng 185:106618. https://doi.org/10.1016/j.petrol.2019.106618
Swain Y, Badamali SK (2020) Microwave assisted synthesis and spectroscopic characterisation of diphenyl carbonate functionalised nanoporous starch. J Polym Res 27:315. https://doi.org/10.1007/s10965-020-02277-0
Ahuja D, Kumar L, Kaushik A (2021) Thermal stability of starch bionanocomposites films: exploring the role of esterified cellulose nanofibers isolated from crop residue. Carbohydr Polym 255:117466. https://doi.org/10.1016/j.carbpol.2020.117466
Tripathy S, Patra S, Parida C, Pradhan C (2023) Green biodegradable dielectric material made from PLA and electron beam irradiated luffa cylindrica fiber: devices for a sustainable future. Environ Sci Pollut Res Int 30:114078–114094. https://doi.org/10.1007/s11356-023-30477-w
Radoor S, Karayil J, Jayakumar A et al (2020) Structure and surface morphology techniques for biopolymers. In: Khan A, Mavinkere Rangappa S, Siengchin S, Asiri AM (eds) Biofibers and biopolymers for biocomposites: synthesis, characterization and properties. Springer International Publishing, Cham, pp 35–70
Reshmy R, Philip E, Paul SA et al (2021) A green biorefinery platform for cost-effective nanocellulose production: investigation of hydrodynamic properties and biodegradability of thin films. Biomass Convers Biorefin 11:861–870. https://doi.org/10.1007/s13399-020-00961-1
Chin K-M, Sam ST, Ong HL et al (2022) Biodegradation improvement of bioinspired crosslinked and noncrosslinked polyvinyl alcohol nanocomposites with cellulose nanocrystals extracted from rice straw through natural soil burial exposure. Polym Compos 43:6955–6965. https://doi.org/10.1002/pc.26757
Holilah H, Prasetyoko D, Ediati R et al (2021) Hydrothermal assisted isolation of microcrystalline cellulose from pepper (Piper nigrum L.) processing waste for making sustainable bio-composite. J Clean Prod 305:127229. https://doi.org/10.1016/j.jclepro.2021.127229
Araújo L, Machado AR, Pintado M et al (2023) Toward a circular bioeconomy: extracting cellulose from grape stalks. Eng Proc 37:86. https://doi.org/10.3390/ECP2023-14746
Nagalakshmaiah M, Kissi NE, Mortha G, Dufresne A (2016) Structural investigation of cellulose nanocrystals extracted from chili leftover and their reinforcement in cariflex-IR rubber latex. Carbohydr Polym 136:945–954. https://doi.org/10.1016/j.carbpol.2015.09.096
Razali SM, Kasim KF (2022) Assessment of natural cellulosic powder from pepper pericarp waste (Piper nigrum L.) after alkalization and bleaching treatment: effect of alkali concentration and treatment cycle. Sains Malays 51:1061–1074
Vinod RSM, Srisuk R et al (2023) Agro-waste Capsicum Annum stem: an alternative raw material for lightweight composites. Ind Crops Prod 193:116141. https://doi.org/10.1016/j.indcrop.2022.116141
Salish K, Ambrose RPK (2021) Predicting powder caking using cohesion energy density. Powder Technol 393:312–322. https://doi.org/10.1016/j.powtec.2021.07.079
Al-Sharabi M, Markl D, Mudley T et al (2020) Simultaneous investigation of the liquid transport and swelling performance during tablet disintegration. Int J Pharm 584:119380. https://doi.org/10.1016/j.ijpharm.2020.119380
Sundarraj AA, Ranganathan TV (2018) Extraction and functional properties of cellulose from jackfruit (Artocarpus integer) waste. Int J Life Sci Pharma Res 9:4309–4317. https://doi.org/10.13040/ijpsr.0975-8232.9(10).4309-17
Gao Y, Lin D, Peng H et al (2023) Low oil Pickering emulsion gels stabilized by bacterial cellulose nanofiber/soybean protein isolate: an excellent fat replacer for ice cream. Int J Biol Macromol 247:125623. https://doi.org/10.1016/j.ijbiomac.2023.125623
Kumari P, Pathak G, Gupta R et al (2019) Cellulose nanofibers from lignocellulosic biomass of lemongrass using enzymatic hydrolysis: characterization and cytotoxicity assessment. DARU J Pharm Sci 27:683–693. https://doi.org/10.1007/s40199-019-00303-1
Melikoğlu AY, Bilek SE, Cesur S (2019) Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr Polym 215:330–337. https://doi.org/10.1016/j.carbpol.2019.03.103
Ndwandwa N, Ayaa F, Iwarere SA et al (2023) Extraction and characterization of cellulose nanofibers from yellow thatching grass (Hyparrhenia filipendula) straws via acid hydrolysis. Waste Biomass Valorization 14:2599–2608. https://doi.org/10.1007/s12649-022-02014-2
Kassab Z, Kassem I, Hannache H et al (2020) Tomato plant residue as new renewable source for cellulose production: extraction of cellulose nanocrystals with different surface functionalities. Cellulose 27:4287–4303. https://doi.org/10.1007/s10570-020-03097-7
Sánchez-Gutiérrez M, Espinosa E, Bascón-Villegas I et al (2020) Production of cellulose nanofibers from olive tree harvest—a residue with wide applications. Agronomy 10:696. https://doi.org/10.3390/agronomy10050696
Md Salim R, Asik J, Sarjadi MS (2021) Chemical functional groups of extractives, cellulose and lignin extracted from native Leucaena leucocephala bark. Wood Sci Technol 55:295–313. https://doi.org/10.1007/s00226-020-01258-2
Sabarinathan P, Rajkumar K, Annamalai VE, Vishal K (2020) Characterization on chemical and mechanical properties of silane treated fish tail palm fibres. Int J Biol Macromol 163:2457–2464. https://doi.org/10.1016/j.ijbiomac.2020.09.159
Yoganandam K, Ganeshan P, NagarajaGanesh B, Raja K (2020) Characterization studies on Calotropis procera fibers and their performance as reinforcements in epoxy matrix. J Nat Fibers 17:1706–1718. https://doi.org/10.1080/15440478.2019.1588831
Kassab Z, El Achaby M, Tamraoui Y et al (2019) Sunflower oil cake-derived cellulose nanocrystals: extraction, physico-chemical characteristics and potential application. Int J Biol Macromol 136:241–252. https://doi.org/10.1016/j.ijbiomac.2019.06.049
Al Kamzari SMA, Nageswara Rao L, Lakavat M et al (2023) Extraction and characterization of cellulose from agricultural waste materials. Mater Today: Proc 80:2740–2743. https://doi.org/10.1016/j.matpr.2021.07.030
Jeyabalaji V, Kannan GR, Ganeshan P et al (2022) Extraction and characterization studies of cellulose derived from the roots of Acalypha indica L. J Nat Fibers 19:4544–4556. https://doi.org/10.1080/15440478.2020.1867942
Song K, Zhu X, Zhu W, Li X (2019) Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresources Bioprocess 6:1–8. https://doi.org/10.1186/s40643-019-0279-z
Rongpipi S, Ye D, Gomez ED, Gomez EW (2019) Progress and opportunities in the characterization of cellulose–an important regulator of cell wall growth and mechanics. Front Plant Sci 9:1–28. https://doi.org/10.3389/2Ffpls.2018.01894
Jabli M, Tka N, Ramzi K, Saleh TA (2018) Physicochemical characteristics and dyeing properties of lignin-cellulosic fibers derived from Nerium oleander. J Mol Liq 249:1138–1144. https://doi.org/10.1016/j.molliq.2017.11.126
Singh A, Ranawat B, Meena R (2019) Extraction and characterization of cellulose from halophytes: next generation source of cellulose fibre. SN Appl Sci 1:1311. https://doi.org/10.1007/s42452-019-1160-6
Hachaichi A, Kouini B, Kian LK et al (2021) Extraction and characterization of microcrystalline cellulose from date palm fibers using successive chemical treatments. J Polym Environ 29:1990–1999. https://doi.org/10.1007/s10924-020-02012-2
Rasheed M, Jawaid M, Karim Z, Abdullah LC (2020) Morphological, Physiochemical and thermal properties of microcrystalline cellulose (MCC) extracted from bamboo fiber. Molecules 25. https://doi.org/10.3390/molecules25122824
Kumar A, Singh Negi Y, Choudhary V, Kant Bhardwaj N (2020) Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as Agro-waste. J Mater Phys Chem 2:1–8. https://doi.org/10.12691/jmpc-2-1-1
Cichosz S, Masek A, Wolski K, Zaborski M (2019) Universal approach of cellulose fibres chemical modification result analysis via commonly used techniques. Polym Bull 76:2147–2162. https://doi.org/10.1007/s00289-018-2487-7
Rasheed M, Jawaid M, Parveez B et al (2020) Morphological, chemical and thermal analysis of cellulose nanocrystals extracted from bamboo fibre. Int J Biol Macromol 160:183–191. https://doi.org/10.1016/j.ijbiomac.2020.05.170
Balasubramani V, Nagarajan KJ, Karthic M, Pandiyarajan R (2024) Extraction of lignocellulosic fiber and cellulose microfibrils from agro waste-palmyra fruit peduncle: Water retting, chlorine-free chemical treatments, physio-chemical, morphological, and thermal characterization. Int J Biol Macromol 259:129273. https://doi.org/10.1016/j.ijbiomac.2024.129273
Singh JK, Rout AK (2022) Characterization of raw and alkali-treated cellulosic fibers extracted from Borassus flabellifer L. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-03238-x
Zhang Z, Cai S, Li Y et al (2020) High performances of plant fiber reinforced composites—a new insight from hierarchical microstructures. Compos Sci Technol 194:108151. https://doi.org/10.1016/j.compscitech.2020.108151
Seddiqi H, Oliaei E, Honarkar H et al (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28:1893–1931. https://doi.org/10.1007/s10570-020-03674-w
Rajeswari A, Christy EJS, Swathi E, Pius A (2020) Fabrication of improved cellulose acetate-based biodegradable films for food packaging applications. Environ Chem Ecotoxicol 2:107–114. https://doi.org/10.1016/j.enceco.2020.07.003
Cui X, Lee JJL, Chen WN (2019) Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: towards non-toxic flexible electronics. Sci Rep 9:18166. https://doi.org/10.1038/s41598-019-54638-5
Ołdak D, Kaczmarek H, Buffeteau T, Sourisseau C (2005) Photo- and bio-degradation processes in polyethylene, cellulose and their blends studied by ATR-FTIR and Raman spectroscopies. J Mater Sci 40:4189–4198. https://doi.org/10.1007/s10853-005-2821-y
Doh H, Dunno KD, Whiteside WS (2020) Cellulose nanocrystal effects on the biodegradability with alginate and crude seaweed extract nanocomposite films. Food Biosci 38:100795. https://doi.org/10.1016/j.fbio.2020.100795
Kushwaha A, Goswami L, Singhvi M, Kim BS (2023) Biodegradation of poly(ethylene terephthalate): mechanistic insights, advances, and future innovative strategies. Chem Eng J 457:141230. https://doi.org/10.1016/j.cej.2022.141230
Qin M, Chen C, Song B et al (2021) A review of biodegradable plastics to biodegradable microplastics: another ecological threat to soil environments? J Clean Prod 312:127816. https://doi.org/10.1016/j.jclepro.2021.127816
Khoshtinat S (2023) State-of-the-art review of aliphatic polyesters and polyolefins biodeterioration by microorganisms: from mechanism to characterization. Corros Mater Degrad 4:542–572. https://doi.org/10.3390/cmd4040029
Peng W, Wang Z, Shu Y et al (2022) Fate of a biobased polymer via high-solid anaerobic co-digestion with food waste and following aerobic treatment: insights on changes of polymer physicochemical properties and the role of microbial and fungal communities. Bioresour Technol 343:126079
Kawai F, Kawabata T, Oda M (2020) Current state and perspectives related to the polyethylene terephthalate hydrolases available for biorecycling. ACS Sustain Chem Eng 8:8894–8908. https://doi.org/10.1021/acssuschemeng.0c01638
Ai B, Zheng L, Li W et al (2021) Biodegradable cellulose film prepared from banana pseudo-stem using an ionic liquid for mango preservation. Front Plant Sci 12:625878. https://doi.org/10.3389/fpls.2021.625878
Wang YY, Yu H-Y, Yang L et al (2019) Enhancing long-term biodegradability and UV-shielding performances of transparent polylactic acid nanocomposite films by adding cellulose nanocrystal-zinc oxide hybrids. Int J Biol Macromol 141:893–905. https://doi.org/10.1016/j.ijbiomac.2019.09.062
Dong T, Chen W, Cai C et al (2023) Water-stable, strong, biodegradable lignocellulose straws replacement for plastic straws. Chem Eng J 451:138970. https://doi.org/10.1016/j.cej.2022.138970
Jaiswal AK, Kumar V, Jansson E et al (2023) Biodegradable cellulose nanocomposite substrate for recyclable flexible printed electronics. Adv Electron Mater 9. https://doi.org/10.1002/aelm.202201094
Acknowledgements
The authors would like to thank the School of Advanced Science, Vellore Institute Technology, Vellore and Sophisticated Test and Instrumentation Centre (STIC), Cochin University of Science and Technology, Kerala, for helping in TGA and DSC analysis.
Author information
Authors and Affiliations
Contributions
ASS: execution of lab works, manuscript preparation and editing; MU: conceptualization, research supervision and editing.
Corresponding author
Ethics declarations
Ethical approval
This research work does not involve the use of any human and/ or animal studies and thus ethical approval is not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santhosh, A.S., Umesh, M. Valorization of waste chilli stalks (Capsicum annuum) as a sustainable substrate for cellulose extraction: insights into its thermomechanical, film forming and biodegradation properties. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05370-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05370-2