Abstract
This work reports the combustion characteristics of two high ash Indian coal, petroleum coke, rice husk, and their blends to assess the effect of rice husk and petroleum coke blending on combustion characteristics of high ash Indian coals. Based on the combustion data obtained from the thermogravimetric analysis, various burning profile parameters such as ignition temperature, burnout temperature, activation energy, and combustion efficiency were analyzed to identify the impacts of petroleum coke and rice husk blending on coal combustion. Results show that petroleum coke having 8602 kcal/kg gross calorific value but inferior combustion properties compared to coal and rice husk. For coal A blends with 10% petroleum coke, with the increase in rice husk content in blends from 10 to 40%, ignition temperature decreased from 409 to 270OC, burnout temperature dropped from 506 to 502OC, inferring significant improvements in combustion properties compared to coal and petroleum coke. For coal B blends with 10% petroleum coke, at 300OC with the increase in rice husk in blends from 10 to 40%, combustion efficiency increased from 4.84 to 19.21%. For 20% petroleum coke of coal B blends, with the increase in rice husk in blends from 10 to 30%, activation energy decreased from 82.54 to 57.69 kJ/mol. Synergistic analysis infers that blending of higher biomass promotes synergistic due to catalytic effect. Analysis of changes in enthalpy, Gibbs free energy and entropy implied that individual combustion of both coal and petroleum coke is difficult compared to rice husk, whereas blending of rice husk makes the combustion process significantly favorable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Indian thermal power plants contribute about 60–65% of national energy needs by burning high ash coal [1]. Jharkhand, West Bengal, Chhattisgarh, Odisha and Madhya Pradesh are the major coal-producing states in India. Due to the complex distribution of combustibles and mineral matter in coal, ash content of coal varied widely across the mines. In many mines, coal ash varied significantly across different seams. As a result, thermal power plants faced difficulties during combustion of coal, in particular high ash coal. Combustion of high ash coal in power plant boilers causes many operational difficulties such as furnace slagging, fouling and low system output [2]. After studying the combustion behavior of high ash coal, Sahu et al. [3] and Deng et al. [4] found that such coal having ignition and burnout difficulty due to low volatile matter (VM) and higher mineral matter (MM). Jyanti et al. [5] reported that high ash coal having a lower O2 diffusion rate and reactivity due to high ash thickness. Behera et al. [1] observed that high ash coal has inferior burning profile and GCV compare to low VM coal. To reduce the impact of ash layer formed during combustion, improving VM and gross calorific value (GCV), scientists recommended blending high ash coal with high VM coal, biomass or similar feedstock [6,7,8]. Sung et al. [9] and Singh et al. [10] reported that biomass has better ignition and combustion properties than coal due to the presence of higher VM and highly reactive hydrocarbons. Aich et al. [7] conveyed that blending of high VM coal improved the combustion of low VM coal. Konwar et al. [11] and Vamvuka et al. [12] informed that the addition of biomass with coal improves the reactivity and activation energy (AE) of coal blends. Aich et al. [13] reported that sal leaves improved the burning profile, combustion efficiency and AE of high ash Indian coal. Recently petroleum coke (PC) has appeared to be an efficient alternative fuel to improve the GCV of coal blends. PC is a refinery waste material having GCV of about 8000–9000 kcal/kg [14]. In the Indian context, high ash coal, PC and biomass in a suitable blend ratio may be a promising option to solve the specific drawbacks of individual fuels. As per a recent study, about 750 million metric tonnes of biomass are available per year in India [15]. Rice straw, sugar cane, fallen tree leaves, rice husk, etc. are the major biomass used as energy sources in India. Large quantities of RH are generated in India as agricultural waste material and are burned in agricultural land. These RH can be blended with high ash coal and PC to reduce environmental pollution and meet the national energy needs [6]. In this context, the present work investigates the effect of RH and PC blending on the combustion characteristics of high ash Indian coals. Based on combustion studies using thermogravimetric analysis (TGA), burning profile parameters, performance indices, improvements in combustion efficiency, AE and thermodynamic parameters were analyzed to identify RH and PC blending impact on coal combustion. The results obtained in this work shall be helpful to get the pathways towards the combustion of ternary blends of coal, PC and RH for thermal utilities.
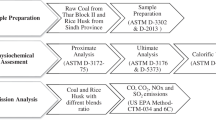
2 Experimental methods
Two different coal (namely coal A and coal B) of about 80 kg each were collected from different seams of the Bhatgaon coal mine, Chattisgarh, India. Individual coal samples were crushed to -50 mm size, -3 mm size and later pulverized to -75 μm and -212 μm following standard coal sample preparation method as required for various characterizations [7]. 50 kg of powder PC (-75 μm) was collected from the crude oil refinery. About 30 kg of RH was collected from the nearby rice mill of Dhanbad. RH was dried at 60OC for 24 h, shredded to -1 mm and pulverized to -212 μm. All the fuels were characterized by proximate analysis (ASTM D3173), ultimate analysis (EL-III, Vario) and GCV determination (AC-350; LECO). Functional groups present in different fuels were determined by FTIR analysis (Spectra 2; Perkin Elmer). The surface morphology of fuels was assessed using FESEM (Mono CL4; Supra’55). The surface characterization was carried out using the BET gas sorption analyzer (3FLEX 3500; Micromeritics Instrument Corporation). The ash composition of individual fuels was analyzed using X-ray fluorescence (XRF) (S8 Tiger; Burker). Combustion experiments of individual fuel and blended fuels were carried out using TGA (STA 449 F3 Jupiter; NETZCH) by heating the fuel from 30 to 700OC with a heating rate of 10 OC/min with the oxygen (O2) flow rate of 60 ml/min. Each experiment was repeated three times for the reliability of data. For combustion experiments, a uniform fuel particle size of -75 μm was used. To assess the impact of blending of PC and RH on coal combustion, experiments were carried out with varying weight fractions of individual coal, PC and RH named as CA8-PC1-RH1 (80% coal A, 10% PC, 10% RH), CA5-PC3-RH2 (50% coal A, 30% PC, 20% RH), CB8-PC1-RH1 (80% coal B, 10% PC, 10% RH), CB5-PC3-RH2 (50% coal B, 30% PC, 20% RH), etc. Characteristics temperatures were determined based on the differential thermogravimetric analysis (DTG) profile. Ignition temperature (Ti), where the weight loss rate is just more than 1%/min. peak temperature (Tmax), where weight loss rate is maximum (\({{DTG}}_{{max}}\)). Burnout temperature (TF), where the weight loss rate reaches below 1%/min indicating completion of combustion [1, 7]. Details of all such burning profile parameters were obtained from MS Excel datasheet generated from TGA instrument software. Further ignition index (\({{I}}_{{IX}}\)), combustion characteristics index (\({{C}}_{{IX}}\)), heat intensity index (\({{H}}_{{IX}}\)), and combustion efficiency \(\left({\upeta }_{{c}}\right)\) were estimated using Eq. (1–4) [6, 16].
IIX represents ignition characteristics of fuel, how rapidly or slowly any fuel gets ignited. Its value should be higher for better ignition performance [13]. CIX indicates how fuels behave during combustion with higher value reflects better combustion [6]. HIX signifies reaction stability between fuel and O2 gas with lower HIX values expressing better reaction stability [13]. Combustion efficiency (ηc) infers the percentage of material combusted from the total combustible matter presence in fuel at a given temperature. Its value should be higher for better combustibility [6]. AE of combustion was determined using Coats Redfern (CR) method using Eq. (5) widely used in coal combustion [17, 18].
Thermodynamic parameters such as enthalpy (\(\Delta {H})\), Gibbs free energy (ΔG) and entropy (ΔS) were assessed using the following Eq. (6–8) [6].
ΔH signifies reaction nature (endothermic or exothermic) during combustion. A higher value of ΔH indicates more endothermic energy is required for combustion [19]. ΔG infers decomposition complexity of fuel during combustion [20]. A higher value of ΔG signifies that higher decomposition energy is needed for complete combustion. ΔS indicates the combustion reactivity of fuel. The higher value of ΔS infers combustion reactivity is favorable [6].
3 Results and discussions
3.1 Characterization of individual fuel
Results obtained from the proximate analysis, ultimate analysis and GCV determinations on as received basis of coal A, coal B, PC and RH are shown in Table 1. It is observed from Table 1 that coal A has 79.64% ash, 10.35% VM and 1024 kcal/kg GCV compared to 49.41% ash, 19.58% VM and 3761 kcal/kg GCV for coal B. In this context PC has 0.35% ash, 12.33% VM and very high GCV of 8602 kcal/kg compared to coals and RH. Typically coal similar to coal B is used as feed coal in Indian pulverized coal fired thermal power plants and coal A is used in fluidized bed combustor units. RH has a significantly higher VM (68.26%) and intermediate GCV (4304 kcal/kg) compare to other individual fuels. Among all the fuels, PC has highest sulfur (7.66%) and fixed carbon (FC), while coal A has the lowest FC (7.70%). Hence, a smaller quantity of PC may be blended with coal and RH to improve the lower GCV of coal. Higher VM present in RH shall be helpful to improve the overall VM of blended fuel and combustion. Table 2 shows the ash composition of all the fuels obtained from XRF analysis. It is observed from Table 2 that, SiO2 is the major compound present in coal A (63.01%), coal B (60.96%) and RH (74.64%). Both coals have a higher quantity of Al2O3 compared to PC and RH. CaO and SO3 are present in higher amounts in PC compared to coals and RH. Fe2O3, TiO2, Na2O, K2O, MgO, P2O5, and MnO are present in smaller amounts in all the fuels. The presence of different oxides significantly affects the combustion characteristics of individual fuel by modifying the ash layer properties, slagging and fouling tendency. Alkali oxides such as Fe2O3, CaO, MgO, Na2O, K2O present in such fuels have catalytic effects on combustion and improve the heat transfer across the fuel [21]. As a result, the presence of alkali oxides improves the thermal devolatilization of hydrocarbons increases and thus, fuel ignites easily [21]. Based on the ash composition, base acid fraction (\({BAF}),\) slagging index (SGI), slagging viscosity (Sv), fouling index (FI) and ash fusion temperature index (AFTI) were estimated using the following Eq. (9–13) [22, 23].
BAF represents the ash deposition characteristics of any fuel with a higher BAF value signify a higher slagging tendency. Generally, the BAF value < 0.5 implies low slag formation, 0.5–1.0 medium slag formation and BAF > 1 implies high slagging characteristics [22]. SGI values < 0.6 infers less slagging, 0.6–2.0 represents medium and > 2 represents high slagging [22].\({{S}}_{{v}}\) value > 78 infers low deposition tendency, 66–78 represents medium deposition tendency and \({{S}}_{{v}}\) < 66 indicate high deposition [22]. \({{F}}_{{I}}\) ≤ 0.6 shows low fouling and 0.6–40 infers high fouling tendency [23]. AFTI infers ash fusion characteristics of fuel and its value should be on the lower side [22]. Based on the calculated values, it was observed from Table 3 that PC has higher BAF (0.823), SGI (6.30),\({{S}}_{{v}}\) (43.70), FI (3.836) inferring high slag deposition tendency compared to coal A [BAF (0.081), SGI (0.039),\({{S}}_{{v}}\) (93.40), FI (0.237)], coal B [BAF (0.0.036), SGI (0.0152), \({{S}}_{{v}}\) (94.8), FI (0.0642)] and RH [BAF (0.0.147), SGI (0.047), \({{S}}_{{v}}\) (92.31), FI (0.719)]. AFTI of coal B is higher (62.80), intermediate for RH (22.91) and lower for PC (1.27). BAF, SGI, \({{S}}_{{v}}\) and FI values for all the blends are near to values of coal. While AFTI values of blends are on the lower side. With the increase in PC in blended fuel, BAF, SGI, \({{S}}_{{v}}\) and FI moves away from desirable values of coal. PC's weight in the blended fuel should be on the lower side to keep slagging and fouling within permissible limits. With the increase in RH weight in the blend, all the indices value reaches their permissible limits. Hence, RH can be blended with coal and PC to overcome the deposition characteristics in the boiler wall.
Figure 1 shows the surface morphology of coal A, coal B, PC and RH. It is observed from Fig. 1 that coal A is almost non-porous, while coal B and PC have some porosity and RH surface is highly porous. RH shows clear fibrous and more macropore structure resulting in more longitudinal cracks appearing on the surface and thinner skeleton wall. As the porous structure is helpful for higher gas adsorption and better combustion reaction, it is expected that coal A will be problematic to burn while RH will be easy to burn. Results obtained from BET-analysis (Table 1) also indicate a similar pattern. Table 1 shows that coal A has inferior surface area (6.92 m2/g) and RH has higher surface area (29.16 m2/g), while PC and coal B have intermediate surface area.
FTIR analysis provides insight of different chemical bonds such as -CH, C = C, C-O, Si–O present in fuels. All such bonds indirectly represent different types of hydrocarbons and their relative weight percentage through peak intensity. As combustion of aliphatic, aromatic, cyclic etc. functional group present in fuel are different, fuel's overall combustion characteristics also depend on such bonds. It is observed from Fig. 2 that, FTIR-spectra of both coal samples is almost similar. Peak between 3900 and 3400 cm−1 for coals is due to –OH functional groups such as crystalliferous water, alcohols, phenols and carboxylic acid [17]. Peak at 3650–3350 cm−1 present in coal, PC and RH corresponds to –OH functional groups such as alcohols, phenols and carboxylic acid [24]. Broad peak at 3412 cm−1 present in RH corresponds to –OH group infers higher amounts of oxygen and hydrogen compared to other fuels. Peak at 2925–2850 cm−1 corresponds to aliphatic –CH2, -CH3, and –CH stretch are present in all the fuels [1]. However, for RH peak is broader, signifying the presence of more volatile hydrocarbons compounds. Peak at 1610–1630 cm−1 in coals and PC corresponds to aromatic C = C bond, indicating the higher amount of aromatic hydrocarbons in coal and PC [25]. Peak at 1420 cm−1 in PC is assigned to aliphatic –CH bending. Peak around 1030–1060 cm−1 corresponds to C–O–C groups are present in coals and RH, signifying the presence of hemicellulose and cellulose in them [25]. Peaks below 1000 cm−1 for all the fuels are assigned to clay minerals such as quartz, kaolinite, montmorillonite, and illite groups [25].
3.2 TGA-DTG of coal A, coal B, PC and RH
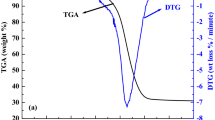
Figure 3a–b shows distinct weight-loss trends representing individual combustion characteristics of coal A, coal B, PC and RH obtained from TGA and DTG. Individual TGA curves infer that with an increase in temperature ranges up to 200OC, about 1–5% weight loss occurs for different fuels, attributed to the release of inherent moisture. Minor weight gain was observed in 200–380OC temperature range for PC due to the adsorption of oxygen gas in porous structures [17]. For RH, rapid weight loss started after 240OC, indicating the initiation of the combustion process. For coal A and coal B, such rapid weight loss started after 310OC and after 380OC for PC. Theoretically, this temperature is considered ignition temperature (\({{T}}_{{i}}\)), where the rate of weight loss is above 1%/min [17, 26]. Later, rate of weight loss reaches peak value (~ 300OC for RH and 450-460OC for coal A, coal B and PC) corresponding to \({{T}}_{{max}}\) and \({{DTG}}_{{max}}\). After 520OC, weight loss reduces to below 1%/min, signifying combustible completion and achieving TF [26]. Details of all such burning profile parameters were obtained from MS Excel datasheet generated from TGA instrument software are summarized in Table 4. It is observed from Table 4 that RH has a lower \({{T}}_{{i}}\) of 242OC, compared to coal B (383OC), PC (383OC) and coal A (427OC). Coal A and PC shows higher \({{T}}_{{max}}\) compared to coal B and RH as they have lower VM. Similarly, PC shows highest \({{DTG}}_{{max}}\) (16.31 wt%/min) compared to coal A (1.67 wt%/min), coal B (4.29 wt%/min), and RH (8.47 and 2.53 wt%/min). It can be observed from DTG profile that only one wide and main peak was found in 450-470OC for coal A, coal B and PC compared to multiple peaks for RH. The presence of two peaks in RH was due to two-step combustion. The first peak at 299OC is due to the combustion of cellulose, hemicellulose, and lignin; while the second peak at 418OC is due to the combustion of solid carbon and remaining lignin. It is also seen from Table 4 that, IIX and CIX for RH (14.83 wt/min OC2, 38.63 wt2/min2 OC3) is higher compared to coal A (0.92 wt/min OC2, 0.61 wt2/min2 OC3), coal B (3.10 wt/min OC2, 4.57 wt2/min2 OC3) and PC (11.12 wt/minOC2, 25.44 wt2/min2 OC3). Higher values of IIX and CIX for RH infers that the combustion performance of RH is better than other fuels due to higher porosity and the presence of easily combustible cellulose, hemicellulose and lignin in RH. Lower HIX value for RH (0.31OC) compared to coal A (1.84OC), coal B (1.33OC) and PC (0.38OC) indicate the rate of heat release is better for RH. Overall, combustion indices analysis infers that biomass having good combustion performance compared to poor performance for coal A and coal B while PC having intermediate combustion performance.
3.3 TGA-DTG of coal B–PC and coal B-RH blends
Results obtained from combustion studies of coal B –PC and coal B-RH blends are shown in Fig. 4a–b. Associated burning profile parameters are summarized in Table 5. It can be observed from Fig. 4a that coal-PC blended fuel exhibits single-stage weight loss profile. With the increase in PC from 10 to 20%, DTG curves shift towards the right side and the intensity of peak decreases, \({{T}}_{{i}}\) and \({{T}}_{{F}}\) increased suggesting inferior combustion of the blend. In contrast, RH blended fuel exhibits two-stage weight loss profile. With the increase in RH from 10 to 20%, DTG curves shifted towards the left side and the intensity of the first peak increased, indicating a reduction in \({{T}}_{{i}}\) and \({{T}}_{{F}}\) as RH content increased. From Table 5, \({{T}}_{{i}}\) decreased from 286 to 255OC as RH increased from 10 to 20% compared to individual \({{T}}_{{i}}\) of coal B (384OC). For PC blend, Ti and TF increased from 384 and 515 to 385OC and 517OC, respectively, compared to coal B (384OC and 512OC). IIX and CIX values of RH blends improved from 4.27 to 4.70 wt/min OC2 and 6.02 to 7.23 wt2/ min2 OC3, respectively, compared to IIX and CIX values for PC blends. Such improvements in \({{T}}_{{i}}\), IIX and CIX of RH blends infer a significant improvement in ignition characteristics of coal due to easy decomposition of biomass, presence of abundant VM and alkali rich oxides such as Fe2O3, CaO, K2O, Na2O, and MgO (Table 2). Whereas ignition and combustion properties of PC blends reduces due to low VM content, inferior surface area and porosity and huge content of solid carbon. Therefore, blending of PC and RH with coal can improve the combustion behavior of PC and low GCV problem of RH and coal.
3.4 TGA-DTG of coal-RH-PC blends
Results obtained from combustion studies of different fuel blends are shown in Fig. 5a–d. Corresponding DTG curves are shown in insets of individual TGA curves and burning profile parameters are summarized in Tables 6 and 7. It can be observed from Fig. 5a that all the blended fuel exhibits two-stage weight loss profile irrespective of increase in RH content from 10 to 40%. DTG curves shifts towards the left side and the intensity of first peak increased, inferring a decrease in \({{T}}_{{i}}\), \({{T}}_{{max}}\) and \({{T}}_{{F}}\) as RH content increased from 10 to 40%. \({{T}}_{{i}}\) decreased from 409 to 270OC as RH increased from 10 to 40% compared to individual \({{T}}_{{i}}\) of coal A (427OC) and PC (383OC). IIX and CIX values also improved from 1.81 to 4.01 wt/min OC2 and 1.76 to 5.93 wt2/ min2 OC3, respectively, compared to IIX and CIX values for coals. Such improvements in \({{T}}_{{i}}\), IIX and CIX signify a significant improvement in ignition characteristics of coal due to increase in alkali rich oxides such as Fe2O3, K2O, Na2O and MgO from 8.16 to 9.05% and easy decomposition of biomass, presence of abundant VM along with increase in FC from PC. Light and easily decomposable hydrocarbons present in RH ignites at lower temperature and provides necessary heat and AE for coal and PC. As a result, entire blend ignites and burned at lower temperature compared to individual coal and PC. Insignificant changes in the second peak, TF and HIX values inferred no major changes in burnout characteristics of coal in spite of 10% PC blending. Similar phenomena were observed for TGA-DTG plots shown in Fig. 5b for 20% PC and 30% PC blends with coal A. As observed from Table 6 that with an increase in PC from 10% (CA7-PC1-RH2) to 30% (CA5-PC3-RH2) with constant RH (20%), burning profile parameters does not vary significantly. Such insignificant variations in burning profile parameters inferred that coal A could be replaced with PC without altering the combustion profile. However, with the increase in RH from 10 to 30%, \({{T}}_{{i}}\) decreased from 273 to 249OC with a constant 20% PC. It is observed from Table 7 that for 10% PC, with the increase in RH from 10 to 40%, \({{T}}_{{i}}\) decreased from 356 to 252OC, TF decreased from 513 to 510OC, IIX improved from 3.89 to 7.07 wt/min OC2, CIX enhanced from 6.67 to 13.52 wt2/min2 OC3, compared to individual values of coal B. However, with the increase in PC from 10% (CB7-PC1-RH2) to 30% (CB5-PC3-RH2) with constant RH (20%), burning profile parameters does not vary significantly. Such observation inferred that RH mainly contributes towards alternation of combustion profile while PC improved the heat release rate.
3.5 Effect of blending on combustion efficiency
Based on the TGA data, combustion efficiency or amount of combustible material burned at any time was estimated using Eq. 4. Figure 6a and b shows that variations of \({\upeta }_{{c}}\) at 300OC, 350OC, 400OC, 450OC and 500OC for coal A and coal B blends. It is observed from Fig. 6a that RH has better \({\upeta }_{{c}}\) at all temperatures compared to coal A, coal B and PC due to the availability of more VM and lower ash compared to PC and coal. With the increase in temperature from 300 to 500OC, \({\upeta }_{{c}}\) surged to 97.06% for PC, 100% for coal A, 96.76% for coal B and 100% for RH. For coal A blends, \({\upeta }_{{c}}\) of CA8-PC1-RH1 is 26.13% at 400OC compared to coal A (17.80%) and PC (3.14%). At 450OC, \({\upeta }_{{c}}\) improves from 50.69 to 70.79% as RH increase from 10 to 40% with a constant PC of 10%. For 20% PC, at 450OC \({\upeta }_{{c}}\) increase from 41.27 to 53.54% as RH increased from 10 to 30%. Such an increase in \({\upeta }_{{c}}\) with RH is due to an increase of hydrogen and VM [6, 13]. For constant RH of 10%, \({\upeta }_{{c}}\) at 350OC decrease from 12.32% to 7.92% with an increase PC from 10 to 30%. For constant RH of 20%, \({\upeta }_{{c}}\) at 400OC reduces from 31.16 to 28.16% as PC increase from 10 to 30%. Such reduction in \({\upeta }_{{c}}\) with increased PC was due to reduction of VM and overall porosity blends. Such results inferred that RH improves the \({\upeta }_{{c}}\) of blends significantly while increasing PC creates obstacles in proper combustion. Similar phenomena were observed for coal B blends. At 450OC, \({\upeta }_{{c}}\) improves from 52.74% to 63.04% as RH increase from 10 to 40% with a constant 10% PC. Overall, \({\upeta }_{{c}}\) at any temperature is higher for coal A blends due to higher ash in coal B. Based on the present study, it can be concluded that desirable \({\upeta }_{{c}}\) may be achieved by varying the PC and RH based on the coal properties.
3.6 Synergistic interaction during combustion
Synergic influences between coal A/ coal B, PC and RH during the combustion were investigated by comparing the experimental weight loss profile and calculated weight loss profile for blends using Eqs. (14–15) [21].
\({\Delta M}\) value close to zero (~ ± 1%) infers negligible interaction between raw fuels in the blend. Negative \({\Delta M}\) signifies the profitable interaction, while positive \({\Delta M}\) indicates non-beneficial interaction among the fuels [21]. Figure 7a–b presents the fluctuations of \({\Delta M}\) for coal A blends and coal B blends. It can be observed from Fig. 7a–b that the synergic effect deviated with temperature in three zones. In zone I, negligible deviations was observed, signifying no active interaction between raw fuels in the blend. Larger deviations were seen in zone II, which is the primary combustion range, indicating a major synergistic effect among fuels. At zone III, \({\Delta M}\) remains constant. \({\Delta M}\) in zone I fluctuated between -1.0% and 1.0% for coal A blends (upto 300OC) and coal B blends (upto 290OC). Such inferior synergy effect may be due to moisture evaporation and release of lighter hydrocarbon, raw fuel properties dominant the weight loss profile. For coal A blends, zone II varied from 300 to 520OC. For coal A blends, \({\Delta M}\) values are mainly positive, signifying non-profitable interaction between individual fuels. Such a positive deviation infers that the non-catalytic reaction happens in blends and higher ash thickness of coal A reduces the beneficial combustion interaction among individual fuels [21]. For coal B blends, higher weight content of RH show negative \({\Delta M}\) values indicating that higher RH weight and low ash resistance of coal B in blends improves the interaction between fuels. Such results infer that synergistic effect at higher RH content improved due to reduction in ash thickness and increase in cellulose, hemicellulose, lignin and alkali earth metal in blends. In zone III, all the blends show constant \({\Delta M}\) values due to the complete combustion of combustible matter. Hence, synergistic investigation infers that blending of higher RH with lower ash coal is profitable during co-combustion of coal, PC and RH.
3.7 Kinetic analysis of raw fuels and tri fuel blends
Table 8 summarizes the calculated values of AE and frequency factor (f) estimated using CR method (Eq. 5). It can be observed from Table 8 that AE for coal A, coal B, PC and RH were 81.42, 87.59, 182.11, and 60.09 kJ/mol, respectively. RH shows the lowest AE due to highest VM (68.26%) and lower ash compared to VM lean PC (12.35%), coal A and coal B. Overall, AE of both coals are lower than the typical high ash Indian coal AE of 90–150 kJ/mol [17], probably due to lower FC present in coals. PC shows the highest AE values due to very high carbon (87.28%) and insufficient VM (12.37%). Also, the presence of basic oxides in RH lowers the ignition temperature and AE [21]. It can also be observed from Table 8 that AE for blends are lower than for individual coal. For coal A blends with 10% PC, AE decrease from 72.99 to 56.58 kJ/mol as RH increase from 10 to 40%. Such reduction in AE with the increase in RH is due to the lighter hydrocarbon presence in biomass. Similar phenomena were also seen for coal B blends. For 10% PC, AE reduces from 78.59 kJ/mol to 54.27 kJ/mol when RH increases 10% to 40%. It can also be seen from Table 8 that, with the increase in PC in blends, AE increases. For coal A blends with 10% RH, AE increase from 72.99 to 89.11 kJ/mol as PC increase from 10 to 30%. For coal B blends with 20% RH, AE increase from 70.12 to 70.63 kJ/mol as PC increase from 10 to 20%. An increase in AE with PC indicates difficulty in the combustion of blends, which may be due to scarcity of VM, non-porous structure and high ignition temperature of PC. Overall, experimental results showed that, addition of RH reduces the AE of blends while addition of PC increased AE. Hence RH and PC weight percentage may be selected based on desirable AE for good combustion and with GCV enhancement by PC in blended fuel. From Table 8 it can be observed that f of RH is the highest (2.38 × 1015 s−1) among individual fuels signifying the fastest combustion reactivity due to the presence of abundant lighter hydrocarbon, higher alkali earth metal and porous skeleton compared to PC, coal A and coal B. Overall, it can be seen that RH improves the combustion performance of blends while PC reduces it. Hence, a suitable blend ratio must be chosen by altering PC and RH with coal to get the blended fuel with desirable AE and combustion characteristics.
3.8 Thermodynamic analysis
Calculated values of various thermodynamic parameters such as ΔH, ΔG and ΔS for coal A, coal B, PC, RH and their blends are summarized in Table 9. It can be observed from Table 9 that PC has the highest ΔH (176.01 kJ/mol) followed by coal A (75.24 kJ/mol), coal B (79.86 kJ/mol) and RH (54.34 kJ/mol). Lowest ΔH for RH signifies better decomposition characteristics due to easy decomposable aliphatic hydrocarbons in RH compared to aromatic, rich hydrocarbons in coal and PC [27]. For coal A blends with 10% PC, with an increase in RH from 10 to 40%, ΔH reduce from 66.73 to 50.24 kJ/mol. Similarly, for coal B, for 20% PC with an increase in RH from 10 to 30% ΔH reduce from 77.31 to 53.17 kJ/mol. A similar observation was seen for other blends with 20% and 30% PC. Reduction in enthalpy with the increase in RH is due to the combustion of VM, which are rich in hemicelluloses and cellulose present in RH. It is also seen from Table 9 that, for coal A blends with 10% RH, with an increase in PC from 10 to 30%, ΔH increased from 66.73 to 82.86 kJ/mol. For coal B blends with 10% RH, with an increase in PC from 10 to 30%, ΔH increased from 73.36 to 79.44 kJ/mol. Such results indicate that the addition of PC increases the difficulty for combustion due to reduction in active pores, aromatic, rich hydrocarbons and scarcity of VM in the blend. Analysis of ΔG for different fuels inferred that, PC had the highest ΔG (226.92 kJ/mol) followed by coal A (137.19 kJ/mol), coal B (136.48 kJ/mol) and RH (30.74 kJ/mol). Higher ΔG values for PC indicate the difficulty of combustion. For coal A blends with 10% PC with an increase in RH from 10 to 40%, ΔG reduce from 98.68 to 55.18 kJ/mol. Similar observation was seen for 20% and 30% PC and coal B blends. Such reduction in ΔG with increase in RH inferred that addition of RH in blends eases the combustion process. For coal A blends with 10% RH, with an increase in PC from 10 to 30%, ΔG increased from 98.68 to 133.36 kJ/mol. For coal B blends with 10% RH with an increase in PC from 10 to 30%, ΔG increased from 95.42 to 116.28 kJ/mol. Such increase in ΔG with PC indicates difficulty during combustion. Among the individual fuels, coal B having minimum ΔS (-86.21 J/mol.K) followed by coal A (-83.36 J/mol.K), PC (-69.35 J/mol.K) and RH (39.07 J/mol.K). Lower ΔS values for PC and coals were due to an increase in ash layer thickness over the unburned fuel particle as combustion proceeds and inferred difficulty in combustion. For coal A blends with 10% PC with an increase in RH from 10 to 40%, ΔS increase from -42.43 to -6.47 J/mol.K. Improvement in entropy with the increase in RH was due to the availability of higher VM, hemicelluloses, cellulose and lignin in blends inferring enhancement of reactivity of blends. For coal B blends at 20% RH with an increase in PC from 10 to 30%, ΔS decreased from -30.24 to -36.23 J/mol.K. Reduction in entropy with the increase in PC indicates the reduction in reactivity of blends.
3.9 Comparison of present work with a literature
Table 10 summarizes the comparison of the present work with similar work on co-combustion of coal and biomass blends. It can be observed from Table 10 that RH, pine sawdust (PS), hardwood (HW), corncob (CC), cornstalk (CS), sugarcane bagasse (SB), wood sawdust (WS) and petroleum coke (PC) are the major alternative fuel or that has been blended with coal with a weight ratio of 70–80% coal and remaining alternative fuel. However, most of the work was reported with lower ash (9.36–37.50%) coal. Aich et al. [26] have only used reject coal (57% ash) with eucalyptus leaves. Yuzbasi et al. [28] reported that PC has inferior burning profile (Ti: 469 and TF: 867OC) along with higher GCV (7935 kcal/kg) compared to coal. Typically, combustion of low ash coal is easy due to less ash layer resistance and availability of higher combustible matter. In this context, the present work reports the utilization of high ash coal (49.41% and 79.64%). It is observed from Table 10 that most of the work reports coal-biomass blends with a significant reduction in \({{T}}_{{i}}\) and other combustion-related parameters. Similar improvement in Ti and other combustion parameters has also been observed for coal B-RH blends. While combustion properties for coal B-PC blend is inferior. AE for coal-PC-RH blend is similar to the values reported in coal-biomass blends as reported in the literature. In this context, the present work suggests that PC also can be blended in a suitable weight ratio along with coal and RH to improve the GCV of the blend without affecting the natural reductions of \({{T}}_{{i}}\) and other combustion properties. Thus, co-combustion of coal, PC and RH may be used in thermal power generation to reduce the dependency on low ash coal.
Overall based on the experimental studies, it can be observed that addition of PC with coal improves the GCV of blend and addition of RH improves the combustion performance of blends. For power plant application, optimum coal-PC-RH blend ratio is identified by compatibility analysis of blends with the existing design of boiler. Any boiler is designed for particular types of coal with some variations in (about ± 2%) in proximate analysis, ultimate analysis and other additive coal properties. As combustion characteristics are non-additive, typically ± 10 OC variation in ignition temperature and burnout temperature from designed/ideal coal and blended fuel is permitted. Any higher deviations from designed values lead to combustion issues such as delayed or early ignition, unburned carbon in ash, and hydrocarbons in the flue gas. For example, if any power plant is designed for coal reported by Behera et al. [1] (49.1% ash; GCV: 3741 kcal/kg; Ti: 377, Tmax: 473 OC, TF: 516 OC) may be replaced by CA6-PC3-RH1 fuel blend (Ash: 49.58, GCV: 3625 kcal/kg; Ti: 391 OC, Tmax: 478, TF: 507 OC). However, in actual plant practice, other non-additive parameters such as ash fusion temperature, grindability index, swilling index etc., also should be matched apart from combustion parameters compatibility. The present study inferred that suitable blend ratio of coal-PC-RH may be selected based on power plant design to reduce ignition problems, unburned carbon in ash and hydrocarbons in flue gas.
4 Conclusion
In this work, the effect of PC and RH blending on combustion characteristics of two high ash Indian coal was studied. Overall characterization studies inferred that PC has the highest GCV (8602 kcal/kg) compared to coal and RH. RH contains higher VM (68.26%) and acceptable slagging characteristics over PC. The presence of higher alkali-rich minerals/oxides such as K2O, Na2O, MgO and Fe2O3 improved the combustion characteristics of RH. Analysis of burning profile parameters inferred that PC having inferior combustion properties compared to coal and RH. At 10% PC of coal B blends, with the increase in RH content in blends from 10 to 40%, \({{T}}_{{i}}\) reduced from 356 to 252OC, TF decreased from 513 to 510OC, inferring significant improvements in combustion properties compared to coal and PC. Synergistic analysis infers that ash thickness creates hurdles in combustion interaction, while higher biomass promotes synergistic due to catalytic effect. For coal A blends with 20% PC, at 400OC, with the increase in RH content in blends from 10 to 30%, combustion efficiency increased from 16.29 to 31.56%. The kinetic analysis shows that PC having higher AE (182.11 kJ/mol) over both coal and RH (60.09 kJ/mol). For coal A blends with 20% PC, with the increase in RH content in blends from 10 to 30%, AE decreased from 82.53 to 63.29 kJ/mol. For coal B blends with 30% PC, AE decreased from 84.70 to 74.89 kJ/mol, with the increase in RH content in blends from 10 to 20%. Analysis of \(\Delta {H}\), ΔG and ΔS inferred that individual combustion of both coal and PC is difficult compared to RH. The blending of RH with coal and PC makes the overall combustion process significantly favorable. Overall current work implied that the addition of PC with coal is helpful to improve GCV values significantly, whereas blending of RH improved the slagging, ignition, and combustion behavior of blends.
Data availability
Data supporting the findings of this study will be available upon request.
Abbreviations
- \({{M}}_{{Exp}}^{{Blend}}\) :
-
Experimental weight loss profile for blend
- \({{M}}_{{Cal}}^{{Blend}}\) :
-
Calculated weight loss profile for blend
- \({\eta }_{c}\) :
-
Combustion efficiency (%)
- AE:
-
Activation energy (kJ/mol)
- BAF:
-
Base acid fraction
- CIX :
-
Combustion characteristics index (wt2/min2 OC3)
- CR:
-
Coats-Redfern method
- DTG:
-
Differential thermogravimetry
- DTGmax :
-
Peak combustion rate (weight%/min) corresponding to Tmax.
- DTGmean :
-
Mean combustion rate (weight%/min)
- f :
-
Frequency factor (s−1)
- FI :
-
Fouling index
- G(α):
-
Mechanism function
- GCV:
-
Gross calorific value (kcal/kg)
- HIX :
-
Heat intensity index (OC)
- IIX :
-
Ignition index (wt/min OC2)
- Mi :
-
Weight loss profile of raw fuel
- MS:
-
Microsoft
- PC:
-
Petroleum coke
- R:
-
Universal gas constant (8.314 J/mol. K)
- RH:
-
Rice husk
- SGI:
-
Slagging index
- Sv:
-
Slagging viscosity
- TF :
-
Burnout temperature (OC)
- TGA:
-
Thermogravimetric analysis
- Ti :
-
Ignition temperature (OC)
- Tmax :
-
Peak temperature (OC)
- W110 :
-
Weight (%) at 110OC
- WEN :
-
Weight (%) at burnout temperature
- WIT :
-
Weight (%) at any temperature
- Xi :
-
Weight fraction in the blend
- α:
-
Conversion
- β:
-
Heating rate (OC/min)
- γ:
-
Boltzmann constant (1.3806 × 10–23 m2 kg/ s2 K)
- ΔG:
-
Gibbs free energy (kJ/mol)
- ΔH:
-
Enthalpy (kJ/mol)
- \({\Delta M}\) :
-
Deviation (%) in synergy
- ΔS:
-
Entropy (J/mol. K)
- ΔT1 /2 :
-
Temperature interval of DTG/DTGmax = 0.5
- ρ:
-
Planck constant (6.626 × 10–34 m2 kg/ s
References
Behera D, Nandi BK (2021) Effect of coal particle density on coal properties and combustion characteristics. Powder Technol 382:594–604
Saljnikov A, Vučićević B, Komatina M, Gojak M, Goričanec D, Stevanović Z (2009) Spectroscopic research on infrared emittance of coal ash deposits. Exp Thermal Fluid Sci 33:1133–1141
Sahu SG, Sarkar P, Chakraborty N, Adak AK (2010) Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process Technol 91:369–378
Deng J, Li B, Xiao Y, Ma L, Wang CP, Wang BL, Shu CM (2017) Combustion properties of coal gangue using thermogravimetry-Fourier transform infrared spectroscopy. Appl Therm Eng 116:244–252
Jayanti S, Maheswaran K, Saravanan V (2007) Assessment of the effect of high ash content in pulverized coal combustion. Appl Math Model 31:934–953
Kumar P, Nandi BK (2021) Combustion characteristics of high ash Indian coal, wheat straw, wheat husk and their blends. Mater Sci Energ Technol 4:274–281
Aich S, Nandi BK, Bhattacharya S (2021) Combustion characteristics of high ash Indian thermal, heat affected coal and their blends. Int J Coal Sci Technol. https://doi.org/10.1007/s40789-021-00419-3
Thanapal SS, Annamalai K, Ansley RJ, Ranjan D (2016) Co-firing carbon dioxide-torrefied woody biomass with coal on emission characteristics. Biomass Convers Biorefin 6:91–104
Sung Y, Lee S, Kim C, Jun D, Moon C, Choi G, Kim D (2016) Synergistic effect of co-firing woody biomass with coal on NOx reduction and burnout during air-staged combustion. Exp Thermal Fluid Sci 71:114–125
Singh M, Gupta A, Yadav K, Karishma J, Shrivastava P, Seth RK, Kulshreshtha A, Dhakate SR (2021) Co-combustion properties of torrefied rice straw-sub-bituminous coal blend and its Hardgrove Grindability Index. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01696-3
Konwar K, Nath HP, Bhuyan N, Saikia BK, Borah RC, Kalita AC, Saikia N (2019) Effect of biomass addition on the devolatilization kinetics, mechanisms and thermodynamic of a northeast Indian low rank sub-bituminous coal. Fuel 256:115926
Vamvuka D, Panagopoulos G, Sfakiotakis S (2020) Investigation potential co-firing of corn cobs with lignite for energy production. Thermal analysis and behavior of ashes. Int J Coal Prep Util. https://doi.org/10.1080/19392699.2020.1856099.
Aich S, Nandi BK, Bhattacharya S (2019) Utilization of sal leaves and sal leaves char to improve the combustion performance of reject coal. Energy Sources Part A Recovery Util Environ Eff 41:2299–2312
Kumari N, Saha S, Sahu G, Chauhan V, Roy R, Datta S, Chavan PD (2020) Comparison of CO2 gasification reactivity and kinetics: petcoke, biomass and high ash coal. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00882-z
Minsiry of New and Renewable Energy, Government of Inida, Bioenergy overview 2021. https://mnre.gov.in/bio-energy/current-status. Accessed 27 Sept 2021
Paniagua S, García-Pérez AI, Calvo LF (2019) Biofuel consisting of wheat straw–poplar wood blends: thermogravimetric studies and combustion characteristic indexes estimation. Biomass Convers Biorefin 9:433–443
Behera D, Nandi BK, Bhattacharya S (2020) Variations in combustion properties of coal with average relative density and functional groups identified by FTIR analysis. Int J Coal Prep Util 7:766–777
Açıkalın K (2021) Evaluation of orange and potato peels as an energy source: a comprehensive study on their pyrolysis characteristics and kinetics. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01387-z
Mishra RK, Mohnaty K (2020) Kinetic analysis and pyrolysis behaviour of waste biomass towards its bioenergy potential. Bioresour Technol 311:123480
Dhyani V, Kumar J, Bhaskar T (2017) Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Biores Technol 245:1122–1129
Xinjie L, Singh S, Yang H, Wu C, Zhang S (2021) A thermogravimetric assessment of the tri-combustion process for coal, biomass and polyethylene. Fuel. 287:119355
García R, Pizarro C, Álvarez A, Lavín AG, Bueno JL (2015) Study of biomass combustion wastes. Fuel 148:152–159
Vamvuka D, Pitharoulis M, Alevizos G, Repouskou E, Pentari D (2009) Ash effects during combustion of lignite/biomass blends in fluidized bed. Renew Energy 34:2662–2671
Okolo GN, Neomagus HWJP, Everson RC, Roberts MJ, Bunt JR, Sakurovs R, Mathews JP (2015) Chemical-structural properties of South African bituminous coals: Insights from wide angle XRD-carbon fraction analysis, ATR-FTIR, solid state 13C NMR, and HRTEM techniques. Fuel 158:779–792
Wang G, Zhang J, Shao J, Liu Z, Zhang G, Xu T, Guo J, Wang H, Xu R, Lin H (2016) Thermal behavior and kinetic analysis of co-combustion of waste biomass/low rank coal blends. Energy Convers Manage 124:414–426
Aich S, Nandi BK, Bhattacharya S (2019) Effects of eucalyptus leaves blending on combustion characteristics of an Indian reject coal. Energy Sources Part A Recovery UtilEnviron Eff. https://doi.org/10.1080/15567036.2019.1644397
Artham T, Doble M (2008) Biodegradation of aliphatic and aromatic polycarbonates. Macromol Biosci 8:14–24
Yuzbasi NS, Selcuk N (2012) Air and oxy-fuel combustion behaviour of petcoke/lignite blends. Fuel 92:137–144
Liu X, Chen M, Wei Y (2015) Combustion behavior of corncob/bituminous coal and hardwood/bituminous coal. Renew Energy 81:355–365
Luo R, Zhou Q (2017) Combustion kinetic behavior of different ash contents coals co-firing with biomass and the interaction analysis. J Therm Anal Calorim 128:567–580
Galina NR, Luna CMR, Arce GLAF, Ávila I (2019) Comparative study on combustion and oxy-fuel combustion environments using mixtures of coal with sugarcane bagasse and biomass sorghum bagasse by the thermogravimetric analysis. J Energy Inst 82:741–754
Namkung H, Lee YJ, Park JH, Song GS, Choi JW, Choi YC, Park SJ, Kim JG (2018) Blending effect of sewage sludge and woody biomass into coal on combustion and ash agglomeration behavior. Fuel 225:266–276
Acknowledgements
The authors would like to acknowledge the Department of Fuel Minerals and Metallurgical Engineering, IIT (ISM) Dhanbad for TGA, CHNS, FESEM and proximate analysis and Central research Facility, IIT (ISM) Dhanbad for XRF analysis.
Author information
Authors and Affiliations
Contributions
Mr. Pritam Kumar has performed all the experimental work reported in this work. He also has prepared the manuscript draft.
Dr. Barun Kumar Nandi, is the Doctoral thesis supervisor for Mr. Pritam Kumar. He has instructed the sequence of experiments, analysis methods and significantly contributed in interpretation of experimental results, kinetic analysis. Finally manuscript has been prepared by both the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, P., Nandi, B.K. Effect of rice husk and petroleum coke blending on combustion characteristics of high ash Indian coals. Biomass Conv. Bioref. 13, 13065–13079 (2023). https://doi.org/10.1007/s13399-021-02090-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02090-9