Abstract
The co-firing of various ultra-low volatile coal-based solid wastes (CSWs) under oxy-fuel condition is a promising approach to elevate the energy conversion efficiency in coal chemistry industry. However, the kinetics and thermal transformation mechanisms during oxy-fuel co-combustion of various CSWs blends are still vague, which need to be further elucidated. Here, the physical–chemical features of CSWs were firstly revealed. Moreover, the oxy-fuel co-combustion characteristics and kinetics of various CSWs were investigated. The results illustrate that four chemical forms of fuel nitrogen could be obtained in pyrolyzed semi-coke (PS), while only two occurrence forms of fuel nitrogen were observed in two residual carbons, including fluidized bed gasification residual carbon (FRC) and coal-water-slurry gasification residual carbon (CRC). The reactivities of various samples could be promoted by the increasing temperature during gasification process. The combustion of volatile matter and fixed carbon could be promoted by an increase in oxygen content in oxy-fuel atmosphere. The combustion characteristic index (S) of 50% PS/50% CRC is raised by 59.3% and that of 50% PS/50% FRC is increased by only 19.0% with the oxygen concentration elevated from 30 to 40%. The effects of rising residual carbon proportion on PS/CRC blend are more obvious than those on PS/FRC blend. The additive of PS in blend could promote the combustion behaviors of residual carbon, especially on CRC. The mass loss rate of 50% PS/50% CRC blend changes significantly with the heating rate, which is greater than that of 50% PS/50% FRC blend. The average apparent activation energy (Eam) of 50% PS/50% CRC blend is lower than that of linear calculation result. The Eam of CRC could be reduced by blending PS, and the poor combustion behaviors of CRC are improved by addition of PS.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal still occupies over 50% of the energy consumption in China [1], and the reserve of lignite is quite huge [2]. The technology of coal chemistry is applied to increase the energy conversion efficiency of lignite [3]. The production of pyrolyzed semi-coke and gasification residual carbon is rising with the development of the coal chemical industry [4]. Part of semi-coke could be used as the raw material in the chemical industry, while the powdery semi-coke cannot be effectively consumed and is sometimes regarded as a kind of coal-based solid waste [5]. The volatile contents of semi-coke and gasification residual carbons are extremely low due to the pyrolysis and gasification processes of coal chemical industry [6]. The gasification residual carbon which is the by-product of the coal gasification process is usually regarded as the solid waste [7], and the usual treatment of gasification residual carbon is burial [8]. However, the carbon contents in these coal-based solid wastes are higher than that of conventional solid wastes. Thus, the efficient utilization of these coal-based solid wastes could raise the energy conversion efficiency of lignite in coal chemical industry. Moreover, the efficient utilization of these solid wastes rather than burial or burning could promote the environment protection and reduce the carbon emission. The combustion of these solid wastes in power plant under air condition is inefficient owing to their ultra-low volatile contents [9]. The technology of preheating combustion could enhance the firing efficiency of these kinds of fuels [10], while some instrument needs to be updated. In addition, the production of coal-based solid waste is increasing at present, and a large amount of semi-coke and residual carbons need to be dealt urgently. Hence, the combustion of these coal-based solid wastes with ultra-low volatile matters in utility boilers with advanced combustion technology is still feasible and effective.
The oxy-fuel combustion technology has been studied by lots of scholars to reduce the NOx emission and elevate the combustion efficiency in power plant [11]. The N2 in traditional air combustion is replaced by CO2 in oxy-fuel combustion, and the oxygen concentration in oxy-fuel combustion could be raised to increase the combustion efficiency of fuel [12,13,14]. In addition, the oxy-fuel combustion is beneficial for CO2 capture, which could reduce the greenhouse gas emission [15]. The decrease of CO2 emission also meets the requirements to achieve carbon peak by 2030 and carbon neutrality by 2060 in China [16]. Semi-coke and residual carbons both feature poor ignition characteristics and the NOx emission is high during combustion process. However, many scholars proved that the utilization of oxy-fuel combustion on coal-based solid fuels could reduce NOx formation amount. Therefore, the oxy-fuel combustion is beneficial to disposal these coal-based solid wastes. While, the volatile contents of residual carbons are much lower than that of semi-coke. Moreover, the carbon fraction of semi-coke is also higher than those of residual carbons. Thus, the semi-coke could be blended with residual carbon to raise the ignition features and calorific value of blends. Some research has been conducted on the co-combustion of coal and low volatile matter fuels [17], while few scholars pay attention to the co-firing of various coal-based solid wastes. Furthermore, the co-firing features of coal-based fuels in air and O2/CO2 atmospheres are quite different, which could be contributed to the high CO2 concentration. The co-firing characteristics of these coal-based solid wastes in O2/CO2 atmosphere are still unclear, and the kinetics during oxy-fuel co-combustion process are necessary to be further studied. The co-firing of various coal-based solid wastes under oxy-fuel condition in utility boiler could enhance the utilization of low-rank coal, and be beneficial for reducing the greenhouse gas and NOx emissions.
Some studies were performed on the firing behaviors of semi-coke or residual carbon. Zhang et al. [18] studied the burning features of raw coal and semi-coke under air condition. The results showed that the semi-coke burning features were deteriorated with the rising pyrolysis duration time and temperature, and the addition of O2 and CO2 under pyrolysis condition could improve the reactivity of semi-coke. Wang et al. [19] probed the firing behaviors of semi-coke and residual carbon in O2/CO2 atmosphere. The burnout ratio increased with the volatile matter of fuel, which could also affect the ignition and burnout temperatures. These already-existing studies both emphasized on the burning behaviors of individual solid waste. While the co-combustion features of blends could be affected by the synergies between various fuels, and the kinetics of blends cannot be the superposition of those in two fuels. In addition, the synergies between various coal-based solid wastes could be affected by the physical–chemical characteristics of fuels. Therefore, the co-combustion behaviors of coal-based solid wastes and other fuels were studied by some scholars [20, 21]. Wang et al. [22] employed the antibiotic filter residual to be mixed with semi-coke to enhance the burning characteristics of blends in oxy-fuel atmosphere. The synergies of two solid wastes were different with the rising oxygen content. Moreover, the interactions between two fuels were also different with the increasing blending ratio of semi-coke. Meanwhile, the studies of Yao et al. [23] indicated that the ignition features of semi-coke and bituminous coal blends in air were better than those under oxy-fuel condition. Wang et al. [24] also focused on the combustion behaviors of semi-coke and coal. The results presented that the combustion indexes were not the linear calculation of those in two fuels, which was consistent with the research of Zheng et al. [25]. The previous studies implied that the synergies between different fuels could affect the co-firing behaviors, which was associated with the physical and chemical characteristics of fuels in blend. However, the existing studies mainly emphasize on the co-combustion of coal-based solid wastes with flammable fuel, and few scholars have investigated the co-firing behaviors of these coal-based solid wastes blends. The physical–chemical characteristics and combustion reactivity of various solid wastes are different, and the synergies between various solid wastes could affect the co-combustion features. These physical–chemical features and synergies of blends are unclear, which needs be further investigated. The high oxygen content in oxy-fuel atmosphere could improve the combustion behaviors of solid wastes, and the mechanisms of various coal-based solid wastes blends in oxy-fuel atmosphere are also indistinct at present. The high CO2 content atmosphere could affect the combustion and fuel-nitrogen migration behaviors of blends, and the synergies of various blends are different due to the differences between physical–chemical features of various samples. While, few studies were conducted to reveal the oxy-fuel combustion behaviors of various coal-based solid wastes. Therefore, the mechanisms of thermal transformation and kinetics of various coal-based solid wastes blends need to be further studied, which are still imprecise at present.
Here, the co-firing behaviors and kinetics of various coal-based solid wastes in oxy-fuel atmosphere were investigated via thermogravimetric experiments. The effects of oxygen concentration, blending ratio, heating rate and atmosphere on oxy-fuel co-combustion features were both studied. The kinetics of blends were further elucidated with the Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods. The present study can provide a guidance for enhancing the utilization of coal-based solid wastes, together with the reducing of the carbon and NOx emissions.
Experimental
Samples and experimental system
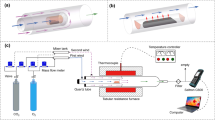
The thermogravimetric analyzer was always employed to study the combustion features and kinetics of blends by many researchers [26, 27]. In the present, the Shenmu coal pyrolyzed semi-coke (abbreviated as PS, hereafter), fluidized bed gasification residual carbon (FRC) and coal-water-slurry gasification residual carbon (CRC) were employed in the experiment to investigate the oxy-fuel co-firing behaviors of various coal-based solid wastes. These three samples could be both classified into the coal-based solid wastes (CSW) with ultra-low volatile matters. As shown in Tables 1 and 2, the chemical proportions and ash compositions of three CSWs are illustrated. The volatile matters of residual carbons are lower than that of PS. The carbon and fixed carbon contents of residual carbons are also lower than those of PS. Moreover, the ash fraction of PS is lower than those of FRC and CRC significantly. Therefore, the firing features of PS are better than those of residual carbons. The Fe-compounds and Al-compounds contents of residual carbons are much higher than those of PS. Otherwise, the content of CaO in PS is twice or more than those of FRC and CRC. The chemical properties and ash compositions are extremely different due to the different production processes of semi-coke and residual carbons, which might affect the thermal transformation during co-combustion process in O2/CO2 atmosphere. Furthermore, the synergies between various coal-based solid fuels might occur due to the differences of physical–chemical features between these samples. The nitrogen-containing functional groups of these CSWs are also different, which could be contributed to the differences in coal-chemistry production processes. Additionally, the X-ray photoelectron spectroscopy (XPS) technique was used to acquire the chemical distribution of nitrogen-containing functional groups of samples.
Here, a synchronous thermogravimetric analyzer Labsys Evo was used to study the oxy-fuel co-firing features of various CWSs blends. All samples were dried at 105 °C for 24 h before tests. Moreover, the sample preparation method was corresponded to the standard [ISO 18283:2006(E)]. The mass of each experimental sample was 20 ± 0.1 mg with a particle size of 0–100 µm. The gas flow rate was set as 40 mL min−1 in each test. The temperature was ranged from 30 to 1200 °C with different heating rates (10, 20, 30 and 40 °C min−1). The different oxygen concentrations of 10%, 21%, 30% and 40% were focused in O2/CO2 atmosphere, and the condition of 21% O2/79% N2 was also studied in the experiment. In addition, the atmospheres of N2, CO2 and 50% N2/50% CO2 were also investigated in the test. The residual carbon blending ratios of 0, 25%, 50%, 75% and 100% were set in the test. More detailed experimental parameters and case conditions could be found in Table 3. Before each test, the temperature of instrument was set as 30 °C more than 20 min, and the temperature reading and buoyancy effect were employed to calibrate the thermogravimetric analyzer.
Combustion parameters and kinetics analysis
The combustion parameters are necessary for illustrating the oxy-fuel co-combustion situation. The definitions of combustion parameters such as ignition temperature (Ti), burnout temperature (Tb), and peak combustion temperature (Tp) et al. could be referred to previous researches [28,29,30]. In addition, the Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS) methods were both employed to conduct the kinetics analysis of samples, and the calculation methods of two kinetics analysis were expressed by previous studies [31,32,33]. The kinetic parameters of samples were calculated by FWO method in previous research [28]. The KAS method is more reliable than FWO method due to that the temperature integration of KAS method is enhanced.
Results and discussion
Reactivity comparison between various coal-based solid fuels
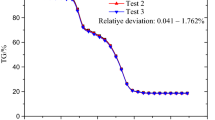
Figure 1 shows the repeated tests of Shenmu coal pyrolyzed semi-coke combustion in 30% O2/70% CO2 atmosphere, and the reproducibility of experiment is acceptable. Here, the physical–chemical features of experimental samples are revealed, and some of them are shown in Fig. 2. The reactivities of various coal-based solid wastes are different due to the differences of production processes, therefore the physical–chemical features of different CSWs need to be further elucidated. The X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) techniques were both employed to study the physical–chemical properties of CSWs. The low-temperature plasma ash of CSW was investigated through XRD in the present study, which could obtain the crystalline phase distribution of samples excepting the effects of organic compounds on XRD measurements [34, 35]. The SiO2 could be found in both low-temperature plasma ash of CSWs, and the CaCO3 could also be observed in that of PS and CRC. The decomposition of CaCO3 could affect the thermal transformation of samples during combustion test. When the residual carbons are blended with PS, the combustion characteristic parameters of blends are not the linear calculation results. According to the results of Table 2, the SiO2 fraction of CRC is over 50%, which is much higher than those of PS and FRC. The fuel-nitrogen occurrence forms of CSWs are also different. Four fuel-nitrogen occurrence forms could only be observed in PS, while just two fuel-nitrogen occurrence forms could be obtained in residual carbons. The proportions of pyrrolic nitrogen (N-5) in three samples are both over 50%, which are higher than other fuel-nitrogen occurrence forms fractions significantly. Consequently, the fuel-nitrogen of PS and FRC could be converted to NOx easier than CRC according to the previous study [36]. Figure 2d shows the micro-morphologies of three CSWs with the magnification times of 1000 and 2000. PS particles usually present smooth surface and some of PS particles show clear and sharp edges. The spherical particles could be observed in PS. While the micro-morphologies of residual carbons are different from those of PS considerably. The edges and corners of residual carbons particles are fuzzy. Moreover, the shapes of residual carbons particles are irregular, and a wide range of particle sizes could be obtained in FRC and CRC particles.
The burning features of various CSWs blends could be affected by the synergies between these solid wastes, and the reactivities of 50% PS/50% FRC and 50% PS/50% CRC blends were both investigated in this section. The TG and DTG curves of various CSWs and blends during pyrolysis/gasification processes are depicted in Figs. 3 and 4. For CSWs and blends, the mass losses in N2 atmosphere are much lower than those in CO2 and 50% CO2/50% N2 atmospheres. It could be concluded that the C-CO2 reaction might appear in high CO2 content atmosphere. The reactivity of CSW in high CO2 concentration atmosphere is more active than that under N2 condition obviously. When the temperature is less than 800 °C, the mass losses in three atmospheres are both small. When the temperature is raised over 1100 °C, a significant peak could be observed in DTG curve under CO2 or 50% CO2/50% N2 condition for all samples. Moreover, the absolute value of peak in CO2 atmosphere is greater than that under 50% CO2/50% N2 condition. It could be explained that the CO2-C reaction is more violent in CO2 atmosphere. In addition, the temperature at peak in 50% CO2/50% N2 atmosphere is greater than that in CO2 atmosphere slightly, especially for blends of PS and residual carbons. It could be explained that the 50% CO2/50% N2 condition has lower CO2 content than that in CO2 atmosphere, which needs higher temperature to promote the reaction of CO2 and C.
In CO2 atmosphere, the mass losses of PS and FRC are over 80% and that of CRC is less than 40%. For blend of 50% PS/50% CRC, the mass loss is over 60%. In addition, the mass loss of 50% PS/50% FRC is nearly 90%. It could be concluded that the reactivity of residual carbon could be enhanced by co-firing with semi-coke. While the mass loss rates under some conditions are still high, and the reactions are still under unstable status, especially in the atmosphere with CO2. The temperature set in the experiment is between 30 and 1200 °C, which means the temperature could be set higher to promote the gasification process. Hence, the temperature should be set over 1200 °C under condition with high CO2 content to promote the gasification process, and the high temperature could also elevate the reactivities of samples.
Influences of oxygen content on oxy-fuel co-firing features
Here, the various oxygen concentrations of 10–40% were probed to reveal the influences of oxygen content on oxy-fuel co-firing behaviors clearly. In addition, the atmosphere of 21% O2/79% N2 was also investigated in the experiment to state the difference between co-combustion characteristics of O2/N2 and oxy-fuel conditions at 21% O2. The co-firing features on experimental samples of PS, FRC and CRC were both studied in the experiment. Moreover, the blends of 50% PS/50% FRC and 50% PS/50% CRC were also focused. With the oxygen contents changed, the flow rate of gas was always kept as 40 mL min−1. As shown in Figs. 6 and 7, the TG and DTG curves of experimental samples (PS, FRC, CRC, 50% PS/50% FRC and 50% PS/50% CRC) in different atmospheres with various oxygen contents were depicted. Table 4 illustrates the combustion parameters for experimental samples in different atmospheres with various oxygen contents. The burnout ratios of various coal-based solid wastes are different due to the differences in physical–chemical properties. The Ti of PS is much lower than those of residual carbons. It could be concluded that the ignition and burnout behaviors of PS are both better than those of residual carbons. Ti and burnout ratio of blends are situated between those of individual CSW, which means that the firing features of residual carbons could be promoted by semi-coke. The four peaks could be observed in the DTG curves of different samples. Here, the DTG curves of PS are employed to state the physical meanings of four peaks and clarify the changes in the combustion process. As shown in Fig. 5, four peaks could be observed in DTG curves. The first peaks ranged from 0 to 100 °C reflect the dehydration process. The second peaks at around 600 °C could be related to the combustion of volatile matter and fixed carbon. The third peaks of DTG curves are between 800 and 950 °C, which could be contributed to the decomposition of CaCO3. In oxy-fuel atmosphere with different oxygen contents, the fourth peak could also be observed in DTG curves due to the violent C–CO2 reaction. In addition, the temperature at the fourth peak decreases with the oxygen concentration raised, which could be explained that the CO2 content reduces with the oxygen content raised. With the oxygen content elevated to 40%, the fourth peak could not be observed in DTG curve, therefore the high CO2 concentration is necessary for occurrence of C–CO2 reaction. For different experimental samples, the absolute values of second peaks at DTG curves rise with the oxygen content, which means that the increase of oxygen concentration in O2/CO2 atmosphere could enhance the combustion of volatile matter and fixed carbon. Moreover, for different samples and blends, the absolute values of second peaks in DTG curves of air combustion are always between those of 21% O2/79% CO2 and 30% O2/70% CO2 atmospheres. As depicted in Table 4, Ti and Tb are reduced with the rising oxygen content for all samples. Ti and Tb of air condition are both lower than those of 21% O2/79% CO2 atmosphere, which could prove that the combustion behaviors of O2/N2 atmosphere are better than those of O2/CO2 at 21% oxygen content. When the oxygen content is elevated to 30%, the Ti and Tb are both lower than those of air condition except the blend of 50% PS/50% CRC. While the Ti and Tb of PS and CRC in 30% O2/70% CO2 atmosphere are both lower than those of air combustion, therefore the interactions between PS and CRC could affect the co-firing behaviors of PS and CRC blend in O2/CO2 atmosphere (Table 4).
The combustion characteristic index (S) which could reflect the combustion features rises with the oxygen content. For residual carbon, the S under air condition is always between those of 21% O2/79% CO2 and 30% O2/70% CO2 atmospheres. While for PS, the S in air atmosphere is lower than that in oxy-fuel atmosphere at 21% oxygen content. As illustrated in Fig. 8, the comparisons of S between experimental results and linear additives for blends of 50% PS/50% FRC and 50% PS/50% CRC are shown. The linear additive results of S are always higher than those of experimental results for blends. The differences between S of experimental results and linear additives are also presented in Fig. 8, and those of 50% PS/50% FRC blend are much lower than those of 50% PS/50% CRC blend. For 50% PS/50% FRC blend, the maximum value of difference between S of experimental result and linear additive is 21.03%. However, the differences between S of experimental results and linear additive for 50% PS/50% CRC blend are ranged from 22.5 to 67.5%, and the maximum value is observed under 30% O2/70% CO2 condition. As depicted in Fig. 6c, the combustion behaviors of CRC are much poorer than those of other CSWs. The co-firing behaviors of blends could be deteriorated by residual carbons with poor firing features, especially CRC. While, the rising oxygen concentration could enhance the co-firing behaviors of blends. The S of 50% PS/50%FRC and 50% PS/50% CRC are elevated by 88.9% and 87.3% with the oxygen content increased from 10 to 40%. When the oxygen content is elevated from 30 to 40%, the S of 50% PS/50% CRC is increased by 59.3% and that of 50% PS/50% FRC is just 19.0%, which could prove that the increasing oxygen content under oxy-fuel condition could enhance the co-combustion behaviors of blends in oxy-fuel atmosphere, especially for blend of PS and CRC.
Effects of residual carbon blending ratio on oxy-fuel co-combustion characteristics
The technology of co-firing various CSWs in O2/CO2 atmosphere is a useful way to consume the large amount of CSWs. While, the interactions between different CSWs could affect the co-firing behaviors according to the results of “Reactivity comparison between various coal‑based solid fuels” and “Influences of oxygen content on oxy‑fuel co‑firing features” sections. Therefore, the effects of blending ratios in residual carbons need to be further investigated, which are helpful to understand the synergy between different CSWs during co-combustion process in O2/CO2 atmosphere. Here, the residual carbon blending ratios of 0, 25%, 50%, 75% and 100% were studied in the experiment at a 40 mL min−1 gas flow rate in 30% O2/70% CO2 atmosphere. The TG and DTG curves of semi-coke and residual carbons are shown in Fig. 9. The burnout ratios of blends reduce with the rising proportions of residual carbons in both PS/FRC and PS/CRC blends. With the proportion of residual carbon raised from 0 to 100%, the burnout ratio of PS/FRC blend is reduced by 77.6% and that of PS/CRC blend is decreased by 94.2%. The effects of residual carbon proportion on PS/CRC blend are more obvious than those on PS/FRC blend. In DTG curves, the peak at around 900 °C which could be related to the CaCO3 decomposition is decreased with the residual carbon mass fraction raised, especially for PS and FRC blend. It could be contributed to the absent of CaCO3 in FRC according to the low-temperature plasma ash crystalline phase distribution in “Reactivity comparison between various coal‑based solid fuels” section.
Table 5 shows the combustion parameters for various CSWs blends. The Ti of blend is raised with the mass percentage of residual carbon due to its poor combustion features. The Tb of PS/FRC blend increases with the proportion of FRC, while that of PS/CRC blend decreases with the CRC mass percentage raised. The burnout ratio and Tb of CRC are low. When the PS is blended with CRC, the combustion features of PS/CRC blend are poorer than those of PS. The heat from PS combustion might be transported to CRC, therefore the Tb and burnout ratio of blend are close to those of CRC. Hence, the Tb of PS/CRC blend is decreased with the CRC proportion elevated. The maximum mass loss of PS is related to the CaCO3 decomposition at around 900 °C, while that of residual carbon is related to the burning of volatile matter and fixed carbon at around 600 °C. Therefore, the Tp of PS is much higher than those of blends and residual carbons.
Figure 10 shows the effects of residual carbons proportions on combustion parameters for blends. The rising CRC or FRC proportion could change the co-firing features, therefore there are some differences between burning parameters of experimental and linear calculation results for various CSWs blends. The differences between burning parameters of experimental and linear calculation results of PS/FRC blends are smaller than those of PS/CRC blends, especially Tb. It could be explained that the physical–chemical features of FRC are closer to those of PS than those of CRC. According to the Table 1, the volatile, fixed carbon contents of CRC are much lower than those of PS and FRC. In addition, the ash content of CRC is higher than those of PS and FRC significantly. The Tb of PS/FRC blends between experimental results and linear additives are close. It could be related to the small difference between fixed carbon contents of PS and FRC. When the mass percentage of CRC is elevated from 0 to 80%, the decreasing trend of Tb is steady. While when the CRC proportion is increased from 80 to 100%, the Tb is reduced by 17.3%. It could be contributed to the low Tb of CRC sample, and the low Tb of CRC could be related to the high ash content and low fixed carbon content of CRC according to the results in Tables 1 and 2. The additive of PS could rise the Tb of blend significantly, and the burnout ratio could also be raised by PS additive according to the TG curves in Fig. 9. Meanwhile, as shown in Fig. 10a, the rise of Ti with CRC proportion increased from 80 to 100% is more obvious than those of other conditions. The S of PS/CRC blend is lower than that of linear additive, which could be explained that the heat from PS firing could not promote the ignition of CRC effectively. It could be contributed to the ultra-low volatile and fixed carbon contents of CRC. The heat from PS burning is not enough for CRC effective combustion. Hence, the combustion characteristics of residual carbon could be enhanced by co-firing with PS under oxy-fuel condition, and the effects of PS additive on PS/CRC blends are more significant than those on PS/FRC blends.
Kinetics analysis of semi-coke and residual carbon blends
Here, PS, CRC and PS/CRC blend were both employed to obtain the kinetics of coal-based solid wastes. The differences between reactivities of PS and CRC are great, therefore the blend of 50% PS and 50% CRC is focused in this section. The three kinds of experimental samples were tested in 30% O2/70% CO2 atmosphere at heating rates of 10, 20, 30 and 40 °C min−1, and the flow rate of gas was always kept as 40 mL min−1. The Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) methods were both employed to analyze the kinetics of samples. Table 6 illustrates the combustion parameters for PS, CRC and 50% PS/50% CRC blend under 30% O2/70% CO2 condition. The Ti of PS reduces with the heating rate raised, while those of CRC and 50% PS/50% CRC blend change slightly with the heating rate increased. The Tb of three samples both rise obviously with the heating rate. The (dm/dt)max and (dm/dt)mean are both increased with the heating rate and the rising trends are significant, which are shown in Fig. 11. As the heating rate elevated, the mass loss rates of PS are more significant than those of CRC. For 50% PS/50% CRC blend, as the heating rate elevated from 10 to 40 °C min−1, the (dm/dt)max and (dm/dt)mean are increased by 72.3% and 229.0%, respectively. It could be concluded that the effects of heating rate on mass loss rates of 50% PS/50% CRC blend are significant, especially on (dm/dt)mean. While the increase ratio of (dm/dt)mean are greater than (dm/dt)max, therefore the kinetics analysis needs to be further discussed.
The apparent activation energy (Ea) of experimental samples is depicted in Table 7. The conversion ratios (α) of 0.2–0.8 are selected to show the main combustion stage due to that the ignition and burnout stages of combustion are instability and could not reflect the main combustion stage of samples. The Ea of KAS and FWO methods both reduce with the rising α. The average apparent activation energy (Eam) of KAS method for PS and CRC is 29.46 and 59.27 kJ mol−1, respectively. It could be concluded that CRC is more difficult to be ignited and burnout than PS. The PS has developed pore structure, which could be useful for reducing the Eam. According to Table 1, the volatile content of CRC is just a quarter of PS and the ash content of CRC exceeds 50%, which could be related to the production process of CRC and it deteriorates the combustion features of CRC. The Eam of 50% PS/50% CRC is between that of PS and CRC, and is also lower than the linear additive results. Thus, the interactions between PS and CRC could lessen the Eam of blend, and the poor combustion behaviors of CRC could be promoted by co-firing with PS. Hence, the technology of co-firing various CSWs under oxy-fuel condition could enhance the poor combustion characteristics of residual carbons, and be beneficial for utilization of CSWs with ultra-low volatile contents.
Conclusions
Here, the thermogravimetric experiments were employed to study the co-firing characteristics of various ultra-low volatile coal-based solid wastes (CSW) blends under oxy-fuel condition. The influences of atmosphere, heating rate and blending ratio were focused on the combustion behaviors of blends and the kinetics of blends were further analyzed. Meanwhile, the reactivity and physical–chemical features of CSWs were both revealed. The main conclusions are as follows:
The CaCO3 observed in both PS and CRC could affect the thermal transformation of samples. Four fuel-nitrogen occurrence forms could be observed in PS, while just two fuel-nitrogen occurrence forms could be obtained in residual carbons. The C–CO2 reaction could be related to the peak over 1000 °C during gasification process. The absolute values of second peaks at DTG curves increase with the oxygen content, which means that the rising oxygen concentration in oxy-fuel atmosphere could promote the combustion of volatile matter and fixed carbon. As the oxygen content elevated from 30 to 40%, the S of 50% PS/50% CRC is increased by 59.3% and that of 50% PS/50% FRC is raised by just 19.0%, which could be determined that the rising oxygen content could promote the co-combustion behaviors of blends, especially for PS/CRC blends.
The influences of residual carbon mass fraction on PS/CRC blend are more significant than those on PS/FRC blend. Tb of PS/FRC blend elevates with the FRC proportion, while that of PS/CRC blend is reduced with the proportion of CRC increased. It could be related to the low ash content of CRC. The burning features of residual carbons could be enhanced by blending with PS in oxy-fuel atmosphere, and the effects of PS additive on PS/CRC blends are more significant than those on PS/FRC blends. The influences of heating rate on mass loss rates of 50% PS/50% CRC blend are obvious, especially on (dm/dt)mean. The average apparent activation energy (Eam) of 50% PS/50% CRC is between that of PS and CRC, and is also lower than the linear calculation results. The synergies between PS and CRC could lessen the Eam of blend, and the combustion of CRC could be promoted by co-firing with PS.
References
Hou J, Wang Z, Liu P. Current practice and future projection for coal-to-SNG in China. Resour Policy. 2022;75:102376.
Yu X, Luo Z. Effect of 0–1 mm pulverized coal on desulfurization and upgrading of low grade lignite in dry cascade separation bed with compound force field. Fuel. 2022;309:122116.
Zhang Y, Xu Z, Liu D, Chen Y, Zhao W, Ren G. The influence of water occurrences in CWSs made of lignite and bituminous coal on slurrying performances. Powder Technol. 2022;398:117150.
Wang C, Wang C, Jia X, Zhao L, Wang P, Che D. NO formation characteristics and fuel-nitrogen transformation mechanism during co-firing of low-volatile carbon-based solid fuels with bituminous coal. Fuel. 2021;291:120134.
Wang C, Wang C, Tang G, Zhang J, Gao X, Che D. Co-combustion behaviors and NO formation characteristics of semi-coke and antibiotic filter residue under oxy-fuel condition. Fuel. 2022;319:123779.
Peng Z, Ning X, Wang G, Zhang J, Li Y, Huang C. Structural characteristics and flammability of low-order coal pyrolysis semi-coke. J Energy Inst. 2020;93(4):1341–53.
Yu M, Sun C, Wang L, Zang K, Li M, Zhou L, Zheng Y. Semi-coke activated persulfate promotes simultaneous degradation of sulfadiazine and tetracycline in a binary mixture. Chem Eng J. 2021;416:129122.
Wang C, Wang C, Feng Q, Mao Q, Gao X, Du Y, Li G, Che D. Experimental evaluation on NOx formation and burnout characteristics of oxy-fuel co-combustion of ultra-low volatile carbon-based solid fuels and bituminous coal. Energy. 2022;248:123578.
Zhao R, Qin J, Chen T, Wu J. TG-FTIR study on co-combustion of bituminous coal semicoke and lignite. J Therm Anal Calorim. 2020;147:1849–58.
Ding H, Ouyang Z, Zhang X, Zhu S. The effects of particle size on flameless combustion characteristics and NOx emissions of semi-coke with coal preheating technology. Fuel. 2021;297:120758.
Shaddix CR, Molina A. Fundamental investigation of NOx formation during oxy-fuel combustion of pulverized coal. Proc Combust Inst. 2011;33:1723–30.
Li S, Xu Y, Gao Q. Measurements and modelling of oxy-fuel coal combustion. Proc Combust Inst. 2019;37:2643–61.
Raheem DG, Yılmaz B, Özdoğan S. Comparison of oxygen enriched and oxy-combustion characteristics of lignite in a CFB: Modelling and experimental verification. Powder Technol. 2021;389:355–67.
Liu Q, Zhong W, Yu A. Oxy-fuel combustion behaviors in a fluidized bed: a combined experimental and numerical study. Powder Technol. 2019;349:40–51.
Qiu Y, Zhong W, Shao Y, Yu A. Reactive force field molecular dynamics (ReaxFF MD) simulation of coal oxy-fuel combustion. Powder Technol. 2020;361:337–48.
Mallapaty S. How China could be carbon neutral by mid-century. Nature. 2020;586(7830):482–4.
Yan Y, Sun R, Sun L, Zhu W, Chen D, Qi H, Wu J. Determining the optimum fuel concentration for ignition and combustion of semi-coke and bituminous coal blends with rich/lean burner. J Energy Inst. 2022;100:225–36.
Zhang J, Wang C, Jia X, Wang P, Che D. Experimental study on combustion and NO formation characteristics of semi-coke. Fuel. 2019;258:116108.
Wang C, Feng Q, Mao Q, Wang C, Li G, Che D. Oxy-fuel co-combustion performances and kinetics of bituminous coal and ultra-low volatile carbon-based fuels. Int J Energ Res. 2020;45(2):1892–907.
Burra KRG, Liu X, Wang Z, Li J, Che D, Gupta AK. Quantifying the sources of synergistic effects in co-pyrolysis of pinewood and polystyrene. Appl Energ. 2021;302:117562.
Li J, Burra KRG, Wang Z, Liu X, Gupta AK. Co-gasification of high-density polyethylene and pretreated pine wood. Appl Energ. 2021;285:116472.
Wang C, Gao X, Tang G, Zhao L, Mao Q, Du Y, Che D. Thermogravimetric study on oxy-fuel co-combustion characteristics of semi-coke and antibiotic filter residue. J Therm Anal Calorim. 2022;147:9505–22.
Yao H, He B, Ding G, Tong W, Kuang Y. Thermogravimetric analyses of oxy-fuel co-combustion of semi-coke and bituminous coal. Appl Therm Eng. 2019;156:708–21.
Wang C, Wang C, Jia X, Gao X, Wang P, Feng Q, Che D. Experimental investigation on combustion characteristics and kinetics during co-firing bituminous coal with ultra-low volatile carbon-based solid fuels. J Energy Inst. 2021;95:87–100.
Zheng S, Hu Y, Wang Z, Cheng X. Experimental investigation on ignition and burnout characteristics of semi-coke and bituminous coal blends. J Energy Inst. 2020;93:1373–81.
Jayaraman K, Kok MV, Gokalp I. Pyrolysis, combustion and gasification studies of different sized coal particles using TGA-MS. Appl Therm Eng. 2017;125:1446–55.
Jayaraman K, Kok MV, Gokalp I. Thermogravimetric and mass spectrometric (TG-MS) analysis and kinetics of coal-biomass blends. Renew Energy. 2017;101:293–300.
Jayaraman K, Kök MV, Gökalp I. Combustion mechanism and model free kinetics of different origin coal samples: thermal analysis approach. Energy. 2020;204:117905.
Qi H, Sun R, Peng J, Meng X, Cao Z, Wang Z, Ren X, Yuan M, Zhang L, Ding S. Experimental investigation on the ignition and combustion characteristics of pyrolyzed char and bituminous coal blends. Fuel. 2020;281:118732.
Zhao R, Dai R, Chen T, Qin J, Zhang J, Wu J. Investigation on combustion, gaseous pollutants emission and ash characteristics during co-combustion of semicoke and coal slime. J Environ Chem Eng. 2021;9:106249.
Kok MV, Varfolomeev MA, Nurgaliev DK, Kandasamy J. Application of TGA-MS technique for oil shale characterization and kinetics. J Therm Anal Calorim. 2022;147:10767–74.
Boumanchar I, Chhiti Y, M’Hamdi Alaoui FE, Elkhouakhi M, Sahibed-dine A, Bentiss F, Jama C, Bensitel M. Investigation of (co)-combustion kinetics of biomass, coal and municipal solid wastes. Waste Manage. 2019;97:10–8.
Kok MV, Ozgur E. Characterization of lignocellulose biomass and model compounds by thermogravimetry. Energ Source Part A. 2017;39:134–9.
Wang C, Zhao L, Yuan M, Liu C, Wang C, Zhao L, Wang P, Che D. Effects of ashing method and blending on ash characteristics of pyrolyzed and gasified semi-cokes. Fuel. 2020;271:117607.
Li L, Chen S, Wang S, Tao X, Zhu X, Cheng G, Gui D. Influence of pickling on the surface composition and flotability of Daliuta long-flame coal. Powder Technol. 2019;352:413–21.
Wang P, Wang C, Yuan M, Wang C, Zhang J, Du Y, Tao Z, Che D. Experimental evaluation on co-combustion characteristics of semi-coke and coal under enhanced high-temperature and strong-reducing atmosphere. Appl Energ. 2020;260:114203.
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (No. 52176129).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Wang, C., Zhao, L. et al. Oxy-fuel co-combustion characteristics and kinetics of various ultra-low volatile coal-based solid waste blends. J Therm Anal Calorim 147, 15017–15032 (2022). https://doi.org/10.1007/s10973-022-11692-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11692-y