Abstract
A number of industries currently produce many tons of agroindustrial wastes with significant consequences on the environment and human and animal health. In recent years, increasing emphasis has been placed on reducing this negative impact. This review article aims to investigate the use of pretreatment methods that can be applied as an alternative to the usage of residual biomass. In addition, we seek to highlight the efficiency of the processes as well as possible weaknesses, which are associated with high energy and reagent consumption, low yields, and possible secondary impacts. Generally, the waste chemical composition consists mainly of cellulose, hemicellulose, and lignin; these can be fractionated, extracted, and purified to produce different value-added products, such as biofuels, organic acids, enzymes, biopolymers, and chemical additives. Despite the multiple possibilities to produce different products from lignocellulosic biomass, further research is still required to enhance the efficiency of the methods used nowadays and find new procedures.

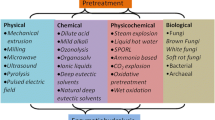
Flowchart of pretreatment methods for lignocellulosic residues and some of the possible groups of products obtained with their processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural activities can lead to multiple waste accumulation and environmental pollution. Both consequences derive from factors such as the practice of industrial activities and compound usage that are intended to optimize and improve agroindustrial outputs [1]. Agroindustrial wastes produced around the world constitute, annually, a large amount of waste material (approximately 37,522,440,479 kg in 2017) [2]. These should be fulfilled by an appropriate pretreatment process, in order to avoid adverse effects against the environment, human, and animal health. However, most of the agroindustrial wastes around the world do not receive adequate post-treatment and end up being incinerated or disposed in makeshift landfills [3].
Within the last decades, numerous promising studies have been made, aiming to develop and improve agroindustrial wastes disposal methods designed for the obtainment of value-added products [4]. One of the most prominent types of wastes is that of lignocellulosic nature [5, 6]. Lignocellulosic biomass is generally composed of a complex mixture of cellulose, hemicellulose, and lignin; therefore, for the efficient use of its components, it is necessary to carry out adequate separation processes. Due to the high amount of matter present within the lignocellulosic biomass, an agroindustrial separation process is not common. With the purpose of obtaining acceptable yields in the components, additional pretreatment steps are generally required [7,8,9,10,11]. During the pretreatment stage, the natural characteristics of lignocellulosic compound bonds are modified by altering the supramolecular structures. This makes the pretreatment stage essential to enhance the depolymerization of the structural components in subsequent treatments, increasing the degradability by using biological, physical, and chemical methods [12]. Once said step is performed, it is possible to obtain the constituent components [7]. Different subproducts can be obtained, which can have a wide application range, categorized in bioenergy, biomaterials, chemical precursors, enzymes, among others.
The different value-added products obtained after a pretreatment process offer several benefits in comparison with those that have not been pretreated, such as environmental, net energy gain, and economical aspects [13,14,15,16]. These indicators can be a growth opportunity for local farmer communities, employment opportunities, industry sector, and energy sectors [17]. These advantages account for a pretreatment process to be a very promising and feasible technology.
In order to provide a better understanding and vision, this work has involved a comprehensive review of the current pretreatment methods for lignocellulosic wastes from agroindustrial activities, for obtaining value-added products. The first section describes specific data on agricultural production and its residues around the world, as well as the problems related to the accumulation of this type of waste. In the second section, we present the chemical composition of the constituents of lignocellulosic material. Consequently, the third section describes a review of the current pretreatment methods for lignocellulosic material that has been carried out. Finally, the last section of this work contains a review of some of the possible products from pretreated lignocellulosic wastes, empathizing the industrial, energetic, and economic importance of this biomass.
2 Agroindustrial production around the world
Worldwide, numerous hectares of land are used for agricultural use (Table 1). In addition, a wide variety of products are generated (Table 2). As a result of the large production of agricultural products around the world, large quantities of agroindustrial wastes are inevitably generated, many of which are lignocellulosic in nature (Table 3).
3 Problems related to agroindustrial activity
Agroindustrial activities have caused a series of problems associated with the environment and human and animal health. Related to the environment, the soil is one of the major sources of pollution and degradation by these activities. Agricultural activities have been linked to soil erosion [29]. This effect can have consequences such as sedimentation, soil fertility loss, and agricultural production decrease, which make agricultural activities unsustainable [30]. Moreover, the most direct effect of soil erosion is sedimentation. Water runoff and excessive sedimentation contribute to the degradation of soil and water bodies [31]. Additionally, components with nitrogen, phosphorus, organic compounds, and heavy metals of pesticides can reach aquifer mantles and bodies of water, causing the intoxication of aquatic life and eutrophication of water [32].
Nowadays, the consequences generated by the accumulation of pesticides and fertilizers used during agroindustrial harvests can contribute to a damaging impact on human health. Therefore, it is necessary to improve the efficiency of agroindustrial processes in order to reduce their environmental impact [33, 34]. Adverse effects may depend on the routes and exposure time, and health conditions of the individual [35]. The most representative damages to human health are dermatological, respiratory, gastrointestinal, carcinogenic, and reproductive and endocrine affections [33, 35]. In European areas, there are taxes denominated as landfill taxes that seek to avoid the accumulation of wastes. This trend is currently expanding to other countries [36]. Moreover, there is a current trend in developed countries to consider by-products of industrial processes as recyclable materials, rather than considering them as wastes. There are currently numerous companies that no longer consider these by-products as wastes, but rather as raw materials that can be used for other processes [27].
4 Agroindustrial waste composition
Agroindustrial wastes of lignocellulosic nature are mostly composed of different structural fractions (Table 4) that can be extracted and applied for the synthesis of different products and materials because of their high energy content [43].
4.1 Cellulose
Plants are responsible for producing many tons of cellulose per year [44]. This makes cellulose the most abundant natural polymer in the world, making it and its derivatives one of the most prolific research topics [45]. Consequently, cellulose-based products and applications are abundant. They are harmless to the environment due to their capacity to easily return to the carbon cycle through a degradation process, in the presence of decomposing organisms [46]. Cellulose is typically differentiated for being a biodegradable compound; having good mechanical properties; being biocompatible, non-toxic, and poorly soluble in water; and having a broad susceptibility to being chemically modified [47]. It is a syndiotactic homopolymer with a molecular formula of (C6H10O5)n, composed of D-anhydroglucopyranose units (glucose units). The glucose units are combined together by means of β-(1-4)-glucosidic bonds forming a dimer called cellobiose, which is the fundamental unit of cellulose [48]. Hydroxy groups (OH) in the structure of D-anhydroglucopyranose, the primary (C6) and the two secondary (C2 and C3), exhibit different polarities, making them able to be involved in intramolecular and intermolecular hydrogen bond interactions [49].
Cellulose can be considered as an isotactic polymer of cellobiose (Fig. 1) [46, 48] Among the most common cellulose applications, there are uses such as an additive for adhesives, paper-based products, drilling fluids, cement-based materials, food coatings, and catalysis support structures, and wide applications in biomedicine [45].
4.2 Lignin
Lignin is one of the three major components of plant organic matter and represents the main natural aromatic source [50]. Lignin has aromatic units instead of the long carbon chains presented by cellulose and hemicellulose. Its chemical characterization depends on the type of vegetal and extraction method [51]. Lignin is a compound with different types of phenols, such as coniferyl, p-comaryl or hydroxyphenyl alcohols, and sinapyl; the first one predominates in softwoods, while the latter ones are present in a greater proportion in hardwoods. These monomers are united by carbon-oxygen bonds between the β-termination of the propenyl group (β-O-4) and half of the p-hydroxy [52] (Fig. 2).
Due to the complexity of its chemical structure, molecular weight is an important parameter. The molecular weight of lignin ranges from 1000 to 20.000 g mol−1, and it is fragmented into subunits that are repeated randomly. This contributes to a more difficult extraction process, making lignin analysis constantly complex [53, 54].
Major applications of lignin include using it as a copolymer in plastics, reinforcement additive in polymeric compounds, adhesives, flocculants, antioxidant coating in the food industry, precursor for the manufacture of carbon fibers, and components in batteries for energy storage, and in biomedical applications [55].
4.3 Hemicellulose
Hemicelluloses are heterogeneous polymers formed by pentoses (xylose and arabinose), hexoses (mannose, glucose, and galactose), and various acidic sugars. The ratio and proportion of these compounds vary between different types of plant species [56]. Hemicellulose polysaccharides can be easily volatilized or hydrolyzed by a dilute acid or base and are highly soluble in alkaline media. Consequently, hemicellulose, unlike cellulose, is removable with alkaline solutions from the plant cell walls [57]. Due to their structural variability, hemicelluloses can be divided into four classes: xylanes, manoproteins, β-glucans with mixed bonds, and xyloglycans (Fig. 3) [58].
Generally, the concept of hemicelluloses refers to all those polysaccharides in the cell wall that cannot be characterized as celluloses or pectins [59]. Among the applications of hemicellulose, there are its uses in biomedical and pharmaceutical β-glucans applications, as reinforcement additive in biomedical polymers, as adhesives, and as flocculants [58, 60,61,62].
5 Pretreatment methods for lignocellulosic agroindustrial wastes
5.1 Chemical methods
5.1.1 Acid pretreatment
Acid pretreatments of lignocellulosic biomass have several advantages. Within them, there are acid solutions that can remove lignin and hemicellulose without any previous treatment, and it is a low-cost method [63, 64]. Various types of mineral acids can be used, with sulfuric acid and hydrochloric acid being the most used. These are usually applied under different conditions of concentration, temperature, and time, depending on the kind of waste. Overall, high temperatures, long reaction periods, and high concentrations usually decrease the yield, as well as the crystallinity and thermal stability of cellulose [65, 66]. In spite of its effectiveness, acid pretreatment has the disadvantage that toxic compounds such as furfural; HMF; and acids, such as acetic, formic, levulinic and fungal, can be released as well as aliphatic and phenolic groups, due to the degradation of sugars and lignin, respectively [67, 68].
In a study conducted by McIntosh et al. [69], the laboratory and pilot capacity of diluted acid as a pretreatment method was evaluated in combination with the steam explosion technique to treat Eucalyptus grandis tree samples for later hydrolysis, in order to produce bioethanol. At the laboratory scale, the digestibility of glucans and glucose yield was 68.0% and 51.3% respectively, for the pretreated biomass at 190 °C for 15 min at 4.8 wt% H2SO4. At the pilot level, the pretreatment is at 180 °C for 15 min at 2.4 wt% H2SO4, followed by steam explosion that led to glucans digestibility yields and glucose yields of 71.8% and 63.6% respectively.
5.1.2 Alkaline pretreatment
Alkaline pretreatments have several advantages, especially the fact that it is a method that uses less aggressive reagents, compared with those used in acid pretreatments such as sulfuric acid [70]. Also, alkaline pretreatments are effective in the delignification of the biomass, without significantly affecting its cellulosic structure [4].
The use of reagents such as sodium hydroxide, sodium carbonate, ammonia, and calcium hydroxide is common for alkaline methods [70]. During the pretreatment process, there are reactions that include the dissolution of lignin and hemicellulose, and the de-esterification of intermolecular ester bonds. This kind of pretreatment removes substitutions of acetyl and uronic acids present in the hemicellulose that would otherwise reduce subsequent accessibility of enzymes to hemicellulose and cellulose [71]. The degree of polymerization of the components is also altered, causing changes in surface area, porosity, and crystallinity of the treated solids [72].
Mittal et al. [73] performed an analysis of the hydrogen peroxide loading during an alkaline pretreatment to separate the lignin from the polysaccharides present in corn stover. The influence of peroxide was examined in a range of 30–500-mg H2O2/g stubble of dried corn at 50 °C for 3 h, obtaining that at 250-mg H2O2/g dry corn stubble, the delignification was approximately 80%.
5.1.3 Ionic liquids pretreatment
Ionic liquids are composed of organic heterocyclic cations and several anions. These are non-volatile, non-flammable, and can exist in liquid state within a wide range of temperatures, the most common being below 100 °C. Imidazole ion salts are currently one of the most popular ionic liquids for pretreatment processes [74]. Its usage is due to its effectiveness in dissolving cellulose. The general mechanism consists of the alteration of the hydrogen bonds that crosslink the lignocellulose, which increases the biomass digestion. Then, using solvents such as water, ethanol, or acetone, it is possible to recover the cellulose fraction; however, it is a method that requires high investment costs [63, 65, 75].
Yamada et al. [76] evaluated the use of an ionic liquid pretreatment in bagasse, for the subsequent use of the biomass in a direct ethanol fermentation by using cellulase-displaying yeast. The results showed that 1-butyl-3-methylimidazolium acetate was the most effective ionic liquid among those studied. After pretreatment, direct fermentation obtained a yield of 73.4% after a 96-h fermentation.
5.1.4 Organic solvent pretreatment
Pretreatment with organic solvents is applied to isolate lignin as a solid material and carbohydrates as a syrup, which is beneficial, since both are raw materials for numerous products [77]. Normally, this type of pretreatment is performed using a strong inorganic acid as a catalyst to hydrolyze the bonds shown in lignin. The main advantage of an organic solvent pretreatment is that it provides easy recovery of the organic solvent by distillation for a recycled use [78].
Salapa et al. [79] evaluated the efficiency of an organic solvent pretreatment over wheat straw at different temperatures and times, using five different organosolvents (ethanol, methanol, butanol, acetone, and diethylene glycol), in the presence of 23 mol/m3 of sulfuric acid as catalytic for a later ethanol production. After the subsequent enzymatic digestion, the highest ethanol production yield was 67%, which was obtained under conditions of 180 °C for 40 min of pretreatment. In addition, pretreatment with diethylene glycol at 160 °C for 40 min also showed good results due to its ethanol production yield of 65%.
5.2 Physical pretreatment methods
5.2.1 Milling
The purpose of this technique is to reduce the particle size and to promote the disruption of the lignocellulosic material crystallinity. The method provides a reduction of the raw material that ranges between 0.2 and 2.0 mm, which increases the specific surface area for a subsequent hydrolysis process [80]. This method affords the advantage of severely reducing the processing time. In addition, it decreases the amount of water consumed; however, it maintains the limitation of requiring high energy consumption [63].
In a study led by Q. Liu et al. [81], the sugar recovery capacity of a two-stage corn stover pretreatment was evaluated. The first stage being a dilute HCl pretreatment, and the second involving a wet grinding technique. The optimum conditions were obtained at a temperature of 120 °C for 40 min for the pretreatment with 0.7% HCl, and 15 min during the wet mill pretreatment. At these conditions, the xylose yield was 81% and 64% for glucose.
5.2.2 Sonication
The sonication technique is based on the application of ultrasonic waves through a fluid to promote pressure variations [82]. Acoustic waves generate microcavities in which gas bubbles grow and then collapse, generating shock waves that cause mechanical effects, such as particle erosion and a consequent particle size reduction [83].
In a study done by Song et al. [84], a sonication pretreatment process was accomplished on roasted wheat straw, in order to obtain biomass pellets for later use as biofuel. The results showed that with the assistance of sonication, pellets of a higher quality than those obtained at the same pressure with no ultrasonic vibrations can be obtained.
5.2.3 Extrusion
The extrusion pretreatment method consists of the operation of generating objects with a defined cross-sectional profile by means of forcing them through a mold with the desired transversal size. The material generally undergoes expansion when it leaves the mold [85]. The process can be affected by variables such as the screw extruder profile, the extrusion speed, and the system temperature [86].
Extrusion methods have different effects on biomass, such as reduction of particle size distribution, increase in specific surface area, changes in crystallinity, and visible changes in biomass structure. It is generally common for the extrusion method to be combined with other chemical methods such as alkaline or acid treatments, aiming to improve the process efficiency [87].
In a study carried out by Oliva et al. [88], an extrusion pretreatment process on barley straw was evaluated. The study combined the steam explosion and extrusion techniques for an optimal fractionation of the lignocellulosic material. The process had a yield of 84%, 91%, and 87% in the recovery of glucans, hemicellulose, and lignin, respectively.
5.3 Physical-chemical pretreatment methods
5.3.1 Steam explosion
Steam explosion is a hydrothermal pretreatment in which the biomass is rapidly heated in a reactor containing saturated steam at high pressures, 0.69–4.83 MPa, in a temperature range between 160 and 260 °C. The time period in which the biomass remains in the reactor varies from seconds to several minutes [89].
During this process, the vapor condenses and penetrates the biomass, initiating an autohydrolysis where the glycosidic hemicellulose bonds are broken due to the organic acids that are generated from the acetyl groups present in this component. This results in subsequent solubilization of the hemicellulose, causing a mechanical disruption in the lignocellulosic matrix [90]. Therefore, this technique is usually used to alter the plant structure, solubilize hemicellulose, and promote rising in the accessible specific surface area of the lignocellulosic material, by enzymes or other compounds. This technique has the advantage of being environmentally friendly; nevertheless, it is still a developing technology, because of the need to improve the design of reactors, the mode of operation, and the mixture of catalysts that can improve the biomass pretreatment effectiveness [91, 92].
Medina et al. [93] studied the effect of steam explosion pretreatment over palm oil empty fruit bunches. Optimal pretreatment results were obtained at a temperature of 195 °C for 6 min. Pretreated biomass showed a 34.69% increase in glycan content and a 68.12% reduction in hemicellulose content. In addition, the increase in the subsequent enzymatic digestion yield was 33%.
5.3.2 Hot water pretreatment
The hot water pretreatment for lignocellulosic biomass keeps liquid water at temperatures between 140 and 240 °C. During the pretreatment, the hemicellulose is depolymerized and the products dissolve in the liquid phase, while the cellulose is completely retained in the solid phase. The temperature of the water causes lignin to suffer from simultaneous depolymerizations and polymerizations, due to its glass transition temperature in aqueous conditions (between 80 and 100 °C) [94]; insoluble lignin is retained in solid waste [95].
This technique has advantages that include the unnecessary use of chemical additives, the non-requirement of using a corrosion-resistant reactor, and the formation of low toxicity compounds [96].
In a study conducted by Mohan et al. [97], the ability to obtain total reducing sugars by means of hot water pretreatment applied over bamboo samples was evaluated. For this, the reaction times were varied between 5 and 40 min, and the temperature between 170 and 220 °C. The results showed that the highest yield of 42.21% was obtained at a temperature of 180 °C for 25 min.
5.3.3 Supercritical CO2 explosion
Supercritical CO2 is characterized for being considered as a green solvent because of its non-flammability and non-emission of organic vapors. Its critical temperature (Tc) is 31 °C, and its critical pressure (Pc) is 1071 psi [98]. After the pretreatment, the solvent can be easily separated. Process variables such as temperature, pressure, extraction bed size, and solvent flow, among others can be modified to maximize yields on specific compounds. In addition, the combined use of this method with others such as sonication and extrusion is common [99]. The explosive release of CO2 after the pretreatment process breaks the biomass cellulose and hemicellulose structures, increasing the surface area for eventual hydrolysis [100].
In a study developed by M. Jiao Zhao et al. [101], agroindustrial wastes including corn stover, corn cob, and sorghum stalk, all with a moisture percentage of around 75%, underwent a long supercritical CO2 pretreatment between 12 and 60 h, at low temperatures (50–80 °C) and pressures between 17.5 and 25.0 MPa. The results showed that in the subsequent enzymatic hydrolysis of the lignocellulosic material, the yield of sugars was 3 to 4 times higher than those obtained for the material that had not received a supercritical CO2 pretreatment.
5.3.4 Ammonia fiber expansion (AFEX™)
The expansion with ammonia fiber is a pretreatment that applies anhydrous or gaseous liquid ammonia in a pressurized container to treat lignocellulosic materials [102]. One of the advantages of this technique is that the ammonia can be reused by a gas recycling system integrated into the main system used [103].
The addition of ammonia under high pressures and temperatures promotes the decrystallization of cellulose, depolymerization of hemicellulose, and deacetylation of acetyl groups. These changes dramatically increase subsequent enzymatic hydrolysis stages, leading to high yields for pretreated biomass by ammonium fiber expansion [104].
In a study carried out by Perez-Pimienta et al. [105], the response of agave bagasse to a pretreatment with ammonia fiber expansion was evaluated. Results showed that the method preserved all the carbohydrates present in the biomass samples, and in subsequent hydrolysis, the yield of sugar production (glucose and xylose) was 42.5%.
5.3.5 Plasma
Pretreatment with plasma uses ozone (O3) for the degradation of the lignin present in lignocellulosic biomass to optimize a subsequent hydrolysis process [106].
Plasma is capable of generating highly reactive compounds such as hydroxyl radical (HO*) and hydrogen peroxide (H2O2), which are capable of degrading cellulose to obtain glucose [78]. Therefore, the pretreatment is capable of breaking the complex structure of lignocellulosic materials, leading to their eventual bleaching and delignification [107].
Ravindran et al. [108] used non-thermal plasma technology to pre-treat brewer spent grains. Variables such as voltage (22, 25, and 28 kV), solvent (acid, basic, and water), and time (5, 10, and 15 min) were taken into account. After a further hydrolysis stage, the best-reducing sugar yield was obtained for the biomass pretreated for 10 min in water at 28 kV. The results showed that 162.9-mg/g brewer spent grain was obtained for the pretreated material, while 75.94 mg/g was obtained for the control.
5.3.6 Microwave pretreatment
This type of pretreatment focuses on irradiating lignocellulosic materials with microwaves [109]. They accelerate chemical, biological, and physical processes due to heat and wide collisions caused by the vibration of polar molecules and movement of ions [110].
The microwave pretreatment performance is highly dependent on the dielectric properties of the lignocellulosic material to be treated. The loss tangent (ratio of the dielectric loss factor to the dielectric constant of the material) is normally calculated to measure the net efficiency of the microwave pretreatment [111, 112].
In a study conducted by Zhu et al. [113], acid (H2SO4) and basic (NaOH) pretreatments assisted by microwave were performed on sugarcane bagasse. The results showed that the microwave-assisted pretreatment was more effective than those pretreatments that used conventional heating methods. Yields for reducing sugars were 4 times higher for the microwave-assisted method and performed in 5.7 times less time.
5.4 Biological pretreatment methods
5.4.1 Microbial consortia
Microbial consortia are complex communities with a high diversity of microorganisms that interact in numerous ways, either cooperatively or competitively. In some cases, these communities often carry out activities that cannot be performed by a single microbe. Microbial consortium applications offer several advantages such as functional robustness, efficient productivity, and high stability when biochemically degrading a substrate [114, 115]. However, this technique has the disadvantage that biomass degradation rates and physicochemical transformation of lignocellulose are easily affected by several factors, such as physical (temperature, aeration, substrate size), chemical (pH, residue composition, enzyme activity), and biological (species of microorganisms and the interaction between them) factors [116]. The microorganisms that are normally used include bacteria Cellulomonas and Cytophaga, and many fungi, such as Humicola, Penicillium, Aspergillus, and Trichoderma, the last being one of the most studied for its ability to secrete large amounts of extracellular protein [117].
Brethauer and Studer [118] applied a microbial consortium that included the fungi Trichoderma reseei, Saccharomyces cerevisiae, and Scheffersomyces stipitis in order to produce ethanol using wheat straw as substrate; the results showed that there was a yield of 67% in the production of ethanol, showing that the technique used has a high potential as a versatile and economical process for the generation of products based on lignocellulosic biomass.
5.4.2 Use of fungal species
Fungi are characterized for being a heterotrophic species and having cell walls composed of chitin. There are fungal species such as white rot, brown rot, and soft rot, which are characterized for their ability to degrade different components present in lignocellulosic biomass. This technique has the advantage that it does not require extensive use of chemical reagents or high energy consumption; however, at an industrial level, it has the disadvantage that it requires long incubation times [119, 120].
White rot fungal are characterized by their ability to degrade lignin through enzymatic complexes that include a wide variety of cellulolytic and ligninolytic enzymes [121, 122]. Brown rot fungi differ by their ability to degrade the cellulose and hemicellulose contained in biomass by means of hydrogen peroxide, endoglucanases, and β-glucosidases [119]. Soft rot fungi have the ability to degrade cellulose due to them possessing a wide range of cellulolytic enzymes such as endo-1,4-glucanase, exo-1,4-β-glucanase, and 1,4-β-glucosidase [119]. In addition, they are capable of degrading lignin to a lesser extent, due to the presence of enzymes such as lacases [119, 123].
Dhiman et al. [124] used a fungal consortium (Pholiota adiposa and Armillaria gemina) in order to produce enzymes to perform a pretreatment over wet rice straw and wet sauce that later underwent enzymatic hydrolysis and conversion to bioethanol. The results obtained showed that the yields obtained in the hydrolysis of the pretreated biomass were 74.2% and 63.6% for the rice straw and the sauce respectively. The good yield was confirmed later, by converting the hydrolyzate into bioethanol with a yield of 72.4%.
5.4.3 Enzymatic pretreatment
Cellulose can be used as a source of carbon for the development of a wide range of living organisms including fungi, protists, bacteria, and plants; these organisms can transform cellulose through cellulases, a synergistic enzyme complex that facilitates the hydrolysis of cellulose and its subsequent biological conversion to a usable source of energy [9, 125]. This system is mainly composed of three enzymes, called endo-β-1,4-glucanase (EC3.2.1.4), cellobiohydrolase (CBH) (EC3.2.1.91), and β-glucosidase (EC3.2.1.21). These act together to hydrolyze the β-1,4-glycosidic bonds of cellulose to release fermentable sugars [126].
The application of enzymes to degrade lignocellulosic materials has several advantages, such as a reduced capital investment, low energy cost, low reagent dependence, and environmental friendliness, yet it keeps the limitation that it is a process with low industrial profitability since the incubation period is prolonged. In many cases, there is low efficiency in the yields obtained [127, 128].
Ziemiński and Kowalska-Wentel [129] analyzed the co-fermentation of a mixture of sugar beet pulp silage and vinasse (3:1, 1:1, and 1:3 w/v) for the production of biogas. It was found that enzymatic pretreatment with Celustar XL and Agropect pomace of sugar beet pulp silage, before methane fermentation, generated a partial degradation of the biomass polysaccharides, significantly increasing biogas production. The results showed that the highest biogas yield (765.5 mL/g) was obtained from the 3:1 mixtures of sugar beet pulp silage and pretreated vinasse. This had a 27.9% better performance than its non-pretreated counterpart.
6 Generated products and their application in various areas
6.1 Products in the bioenergy area
Currently, there is a growing demand on investigating different lignocellulosic sources of low-cost pretreatment methods and inoculum to produce environmentally friendly fuels (Table 5). This is due to different factors such as the rising cost of conventional fuels, global warming problems related to CO2 emissions, and production rentability, among others [140]. Usually, the concept of bioenergy refers to any gas, liquid, or solid that was processed by biorefinery technologies; these have a tendency to be used as a renewable energy source. One of the main advantages of biofuels is that their source crop can sequester CO2 from the atmosphere due to the increased biomass productivity. In total, 17.97 million tons (carbon dioxide-equivalent) accumulative greenhouse gas emission could be mitigated [141]. Crops can be used to produce energy through combustion methods without increasing net CO2 emissions, due to the CO2 removed from the atmosphere by the crops themselves during their growth process [13].
Bioethanol and biodiesel are classic examples of this type of energies [140]. However, in the last few years, the conversion of lignocellulosic wastes into biohydrogen is being considered as a promising technology for the replacement of fossil fuels [142]. This is principally because hydrogen production by fermentation is a plausible alternative when compared with other feedstocks, due to lignocellulose abundance and different available pretreatment methods [143]. Biohydrogen could easily be produced from agricultural wastes via fermentation processes [144, 145]. Soltan et al. produced biohydrogen by a multi-fermentation of a complex mix of agroindustrial wastes and achieved a net energy gain of 1.82-kJ/g feedstock. Similarly, Soltan et al. achieved a net energy gain of 32.2 ± 4.2-kJ/kg feedstock using an integrated fermentation of a mixture of 30:70 metals in mixed fruit peels (MFPS):paper mill sludge (PMS). Besides the production of bioethanol, biodiesel, and biohydrogen, biogases like methane and propane have been generated from lignocellulosic wastes [146, 147]. Dahunsi et al. used an aerobic digestion of peanut hull to produce biomethane and obtained a net energy gain of 303 kWh tons−1 [148]. It has been reported that by 2025, biodiesel production and power generation could amount to 72.11 thousand tons and 1.59 billion kW h respectively. Due to its higher energy recovery, the organic wastewater biogas industry has the highest output and net profit, followed by the waste incineration power generation industry [141]. Panigrahi et al. produced biogas using an electrochemical pretreatment on yard waste followed by a anaerobic digestion and obtained a net energy gain of 4.75-kJ/g volatile solid (VS) [149]. These results suggest that the conversion of lignocellulosic wastes into biofuels can be a promising technology due to the high net energy gain obtained by several studies [15, 16, 149].
6.2 Chemical products and their applications beyond bioenergy
It is possible to obtain a variety of value-added chemical products using the lignocellulosic biomass from agroindustrial wastes as a raw material due to their chemical composition [150] (Table 6). Cellulose degradation generally entails glucose production; meanwhile, hemicellulose depolymerization can be used to produce pentoses like xylose; arabinose; and hexoses, like mannose, glucose, and galactose [49, 56]. A wide variety of aromatic compounds can be obtained from lignin due to its molecular structure being abundant in phenolic structures [162].
Some of the precursor compounds with six carbons in their structure that can be obtained from biomass degradation processes are citric acid, 5-hydroxymethyl-furfural, lysine, gluconic acid, glucaric acid, and sorbitol. Similarly, for compounds with five carbons, there are itaconic acid, furfural, levulinic acid, glutamine acid, xylonic acid, and arabito. For compounds with four carbons, there are succinic acid, fumaric acid, malic acid, aspartic acid, acetoin, threonine, etc. Among the obtainable precursor compounds with three carbons in their structure, there are glycerol, 3-hydroxypropionate, propionic acid, and malonic acid [163].
The precursor compounds have an inherent possibility of generating a wide range of organic compounds [164]. Organic acids are some of the most relevant value-added products that can be synthesized from the previously mentioned precursor compounds. Some commonly produced organic acids from agroindustrial wastes are acetic acid, oxalic acid, lactic acid, itaconic acid, succinic acid, butyric acid, and propionic acid [163,164,165].
The production of biochemical compounds such as enzymes, vitamins, hormones, amino acids, and antibiotics from agroindustrial wastes are commonly obtained from microorganisms such as Aspergillus niger, Trichoderma reesei, Halomonas sp, Bacillus subtilis, and Aspergillus fumigatus [165, 166]. The industrial enzyme market is one of the most prolific between the different chemical and biochemical compounds that are marketed worldwide. It is projected that by 2023, the market for industrial enzymes should reach $ 7.0 billion from the value of $ 5.5 billion reached in 2018 [14].
There is also an uprising interest in the production of carotenoids using different lignocellulosic wastes as feedstock. This is due to the high production cost of these through biotechnological methods. Carotenoids have wide applications as pigments in the food industry and in the pharmaceutical and nutraceutical industries, and as additives in animal feed [167].
Among other compounds that are produced from agroindustrial wastes, there are the chelating agents. These work through bioabsorption mechanisms which are based on a combination of electrostatic attraction mechanisms, complexing processes, ion exchanges, and covalent bond forming [168]. Chelating agents can found application in water cleaning, since they can remove heavy metals and dyes through bioabsorption mechanisms [169].
6.3 Material and material additive production
In recent years, lignocellulosic biomass has been attributed with a high potential for obtained value-added materials with a wide range of applications (Table 7), this due to their qualities of being renewable, recyclable, and sustainable; however, they are accused of having an important impact on environmental contamination [140, 183]. Currently, biotechnological advances together with nanotechnology and engineering have generated interest in doing research focused on the use of lignocellulosic biomass for the design of materials that are multifunctional for the bio- and non-bio-sectors of the modern world. This new direction is socially acceptable and commercially viable, and moreover, it possesses an important impact on industrial societies for the development of a sustainable global economy [7]. One of the most representative types of materials obtained from lignocellulosic sources lies in the field of biodegradable polymers. These kinds of materials can reduce oil consumption and help reduce problems caused by plastic contamination, since they are easily degradable in soils and marine environments [164]. When cellulose, hemicellulose, and lignin have been isolated, they can be incorporated into a wide variety of materials. There are three main routes through which polymeric materials can be obtained from lignocellulosic materials. The first route involves the use of biorefinery waste streams or paper industry wastes. The second route refers to direct use of the biomass cell walls; therefore, cellulose, hemicellulose, and lignin must be isolated to subsequently convert them into final products. The third route is based on hexoses and pentoses obtainment from lignocellulosic materials for their further processing into a wide variety of compounds, additives, and biopolymers [12, 184, 185]. Depending on the methodology, a wide range of compounds can be developed. They include materials such as bacterial cellulose, keratin, polyhydroxyalkanoate (PHA), chitosan, and polylactic acid (PLA), among other synthetic counterparts [164, 186] (Table 7).
7 Conclusions and perspectives
Worldwide, there is currently a problem related to agroindustrial waste generation. This is due to it having a negative impact on the environment. Therefore, the continuous search for alternatives that help mitigate this problem is essential. As discussed above, the main pretreatment methods for lignocellulosic wastes, i.e., chemical, physical, physicochemical, and biological methods, are looking for an efficient transformation of lignocellulosic biomass into value-added products. Several agricultural residues such as rice, sugarcane, soybean, corn, wheat, cotton, pineapple, sorghum, cassava, barley, banana, oil palm, and bean, among other residues from the wood industry, as well as municipal cellulosic solid wastes, could be used as raw materials to produce many different products. However, each technology has its own associated advantages and disadvantages, depending on the source of biomass, the methods used, and the desired final product. The advantages of the lignocellulosic wastes are that such materials have a great potential to produce biomolecules, chemicals products, and biofuels to address the current energy crisis. Within the bioenergy area, a great potential is suggested for energy production from pretreated biomass, due to the obtainable high net energy gain values. There are substantial prospects for economic growth due to the wide range of chemical and biochemical compounds that are possible to produce after a pretreatment process of lignocellulosic feedstock. The enormous amount of obtainable compounds could directly benefit several different industries (textile, materials, biomedical, pharmaceutical, etc.), in as much as many of those use some of the obtainable value-added products as raw materials. Among the main limitations distinguished from the use of lignocellulosic wastes, there are the excessive use of water, energy consumption, and toxic reagents. In addition, it is important to take into consideration the collection, transport, and handling of lignocellulosic wastes in a cost-effective manner. Despite the multiple possibilities of using lignocellulosic biomass, more research is still required, specially to improve the efficiency of the existent pretreatment methods, reduce the costs associated with the processes, and reduce the production of toxic compounds. Moreover, depending on the geographic locations and season, changes in agroindustrial wastes price and its supply could emerge. This could affect production costs and efficiency of the overall process. Moreover, many literature and preliminary studies are made in a small scale but there is still an enormous gap between the laboratory preliminary results, the pilot scale, and finally, the industrial scale.
References
Nagendran R (2011) Agricultural waste and pollution. Waste Streams 2:341–355. https://doi.org/10.1016/B978-0-12-381475-3.10024-5
Food and Agriculture Organization of the United Nations (2019) Crop residues. FAOSTAT
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Rabemanolontsoa H, Saka S (2016) Various pretreatments of lignocellulosics. Bioresour Technol 199:83–91. https://doi.org/10.1016/j.biortech.2015.08.029
Kumari D, Singh R (2018) Pretreatment of lignocellulosic wastes for biofuel production: a critical review. Renew Sust Energ Rev 90:877–891. https://doi.org/10.1016/j.rser.2018.03.111
Pérez J, Muñoz-Dorado J, De La Rubia T, Martínez J (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol 5:53–63. https://doi.org/10.1007/s10123-002-0062-3
Iqbal HMN, Kyazze G, Keshavarz T (2013) Advances in the valorization of lignocellulosic materials by biotechnology: an overview. BioResources 8:3157–3176. https://doi.org/10.15376/biores.8.2.3157-3176
Gatt E, Rigal L, Vandenbossche V (2018) Biomass pretreatment with reactive extrusion using enzymes: a review. Ind Crop Prod 122:329–339. https://doi.org/10.1016/j.indcrop.2018.05.069
Shahzadi T, Mehmood S, Irshad M et al (2014) Advances in lignocellulosic biotechnology: a brief review on lignocellulosic biomass and cellulases. Adv Biosci Biotechnol 05:246–251. https://doi.org/10.4236/abb.2014.53031
Abraham A, Mathew AK, Sindhu R, Pandey A, Binod P (2016) Potential of rice straw for bio-refining: an overview. Bioresour Technol 215:29–36
Zheng Y, Shi J, Tu M, Cheng Y-S (2017) Principles and development of lignocellulosic biomass pretreatment for biofuels. In: Advances in Bioenergy. Elsevier Ltd, pp 1–68
Isikgor FH, Becer RC (2015) Lignocellulosic biomass: a sustainable platform for production of bio-based chemicals and polymers. Polym Chem. https://doi.org/10.1039/C5PY00263J
Lemus R, Lal R (2005) Bioenergy crops and carbon sequestration. CRC Crit Rev Plant Sci 24:1–21. https://doi.org/10.1080/07352680590910393
BCC Publishing (2018) Global markets for enzymes in industrial applications. Wellesley, MA
Rajagopal D, Liu B (2020) The United States can generate up to 3.2 EJ of energy annually from waste. Nat Energy 5:18–19. https://doi.org/10.1038/s41560-019-0532-x
Liu B, Rajagopal D (2019) Life-cycle energy and climate benefits of energy recovery from wastes and biomass residues in the United States. Nat Energy 4:700–708. https://doi.org/10.1038/s41560-019-0430-2
Fedorova, Caló, Pongrácz (2019) Balancing socio-efficiency and resilience of energy provisioning on a regional level, case Oulun Energia in Finland. Clean Technol 1:273–293. https://doi.org/10.3390/cleantechnol1010019
Food and Agriculture Organization of the United Nations (2019) Agricultural land. FAOSTAT
Li W, Zhao W, Liu H et al (2018) Supercritical ethanolysis of wheat stalk over calcium oxide. Renew Energy. https://doi.org/10.1016/j.renene.2017.12.078
Engindeniz S, Bolatova Z (2018) 29 th International conference of agriculture
Ramos RC, Nachiluk K (2017) Geração de Bioenergia de Biomassa da Cana-de-açúcar nas Usinas Signatárias ao Protocolo Agroambiental Paulista, Safra 2015/2016. Análises e Indicadores do Agronegócio 12:7
Reinprecht Y, Arif M, Simon LC, Pauls KP (2015) Genome regions associated with functional performance of soybean stem fibers in polypropylene thermoplastic composites. PLoS One 10:1–33. https://doi.org/10.1371/journal.pone.0130371
United States Departament of Agriculture (2018) International: World soybean production
Howe J, Bowyer J, Pepke E, et al (2014) Municipal solid waste (msw) and construction and demolition (c&d) wood waste generation and recovery in the United States. Minneapolis
Patel VR (2017) Cost-effective sequential biogas and bioethanol production from the cotton stem waste. Process Saf Environ Prot 111:335–345. https://doi.org/10.1016/j.psep.2017.07.019
Wikandari R, Nguyen H, Millati R et al (2015) Improvement of biogas production from orange peel waste by leaching of limonene. Biomed Res Int 2015:6. https://doi.org/10.1155/2015/494182
Nigam PS (2017) An overview: recycling of solid barley waste generated as a by-product in distillery and brewery. Waste Manag 62:255–261
The Brewers of Europe (2017) Beer statistics. Brussels
Montgomery DR (2007) Soil erosion and agricultural sustainability. Proc Natl Acad Sci U S A 104:13268–13272. https://doi.org/10.1073/pnas.0611508104
Keesstra S, Pereira P, Novara A, Brevik EC, Azorin-Molina C, Parras-Alcántara L, Jordán A, Cerdà A (2016) Effects of soil management techniques on soil water erosion in apricot orchards. Sci Total Environ 551–552:357–366. https://doi.org/10.1016/j.scitotenv.2016.01.182
Streeter MT, Schilling KE, St. Clair M, Demanett Z (2019) Soil sedimentation and quality within the roadside ditches of an agricultural watershed. Sci Total Environ 657:1432–1440. https://doi.org/10.1016/j.scitotenv.2018.12.113
Moss B (2008) Water pollution by agriculture. Philos Trans R Soc B Biol Sci 363:659–666. https://doi.org/10.1098/rstb.2007.2176
World Health Organization (1990) Public health impact of pesticides used in agriculture. England
Ritchie H, Roser M (2017) Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ Res Lett 12:064016. https://doi.org/10.1088/1748-9326/aa6cd5
Nicolopoulou-Stamati P, Maipas S, Kotampasi C et al (2016) Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health 4:1–8. https://doi.org/10.3389/fpubh.2016.00148
Väisänen T, Haapala A, Lappalainen R, Tomppo L (2016) Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: a review. Waste Manag 54:62–73. https://doi.org/10.1016/j.wasman.2016.04.037
Yang J, Ching YC, Chuah CH (2019) Applications of lignocellulosic fibers and lignin in bioplastics: a review. Polymers (Basel) 11:1–26. https://doi.org/10.3390/polym11050751
Rabemanolontsoa H, Saka S (2013) Comparative study on chemical composition of various biomass species. RSC Adv 3:3946–3956. https://doi.org/10.1039/c3ra22958k
Toegepast Natuurwetenschappelijk Onderzoek (2019) ECN Phyllis Classification. https://phyllis.nl/. Accessed 30 Aug 2019
Lv G, Wu S (2012) Analytical pyrolysis studies of corn stalk and its three main components by TG-MS and Py-GC/MS. J Anal Appl Pyrolysis 97:11–18. https://doi.org/10.1016/j.jaap.2012.04.010
Rivas B, Torrado A, Torre P, Converti A, Domínguez JM (2008) Submerged citric acid fermentation on orange peel autohydrolysate. J Agric Food Chem 56:2380–2387. https://doi.org/10.1021/jf073388r
Tibolla H, Pelissari FM, Martins JT et al (2018) Cellulose nanofibers produced from banana peel by chemical and mechanical treatments: characterization and cytotoxicity assessment. Food Hydrocoll 75:192–201. https://doi.org/10.1016/j.foodhyd.2017.08.027
Yang H, Yan R, Chen H et al (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Chen H, Wang L (2017) Microbial fermentation strategies for biomass conversion. Technol Biochem Convers Biomass:165–196. https://doi.org/10.1016/b978-0-12-802417-1.00007-7
Moon RJ, Schueneman GT, Simonsen J (2016) Overview of cellulose nanomaterials, their capabilities and applications. Jom 68:2383–2394. https://doi.org/10.1007/s11837-016-2018-7
Suhas, Gupta VK, Carrott PJM et al (2016) Cellulose: a review as natural, modified and activated carbon adsorbent. Bioresour Technol 216:1066–1076. https://doi.org/10.1016/j.biortech.2016.05.106
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crop Prod 93:2–25. https://doi.org/10.1016/j.indcrop.2016.02.016
Klemm D, Philpp B, Heinze T, Wagenknecht W (1998) Comprehensive celullose chemistry. Volume 1: fundamentals and analytical methods. Wiley-VHC Verlag GmbH, Winheim
Ciolacu DE (2018) Biochemical modification of lignocellulosic biomass. Elsevier B.V
Holmgren A, Brunow G, Henriksson G, Zhang L, Ralph J (2006) Non-enzymatic reduction of quinone methides during oxidative coupling of monolignols: implications for the origin of benzyl structures in lignins. Org Biomol Chem 4:3456–3461. https://doi.org/10.1039/b606369a
Duval A, Molina-Boisseau S, Chirat C (2013) Comparison of kraft lignin and lignosulfonates addition to wheat gluten-based materials: mechanical and thermal properties. Ind Crop Prod 49:66–74. https://doi.org/10.1016/j.indcrop.2013.04.027
Agrawal A, Kaushik N, Biswas S (2014) Derivatives and applications of lignin–an insight. SciTech J 1:30–36
Thakur VK, Thakur MK, Raghavan P, Kessler MR (2014) Progress in green polymer composites from lignin for multifunctional applications: a review. ACS Sustain Chem Eng 2:1072–1092. https://doi.org/10.1021/sc500087z
Tolbert A, Akinosho H, Khunsupat R (2014) Characterization and analysis of the molecular weight of lignin for biorefi ning studies. Biofuels Bioprod Biorefin. https://doi.org/10.1002/bbb
Ten E, Vermerris W (2015) Recent developments in polymers derived from industrial lignin. J Appl Polym Sci 132:1–13. https://doi.org/10.1002/app.42069
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291. https://doi.org/10.1007/s10295-003-0049-x
Rose JK (2003) The plant cell wall. Blackwell, Oxford
Ebringerová A, Thomas H (2005) Hemicellulose. Adv Polym Sci 186:1–67
Zhou X, Li W, Mabon R, Broadbelt LJ (2017) A critical review on hemicellulose pyrolysis. Energy Technol 5:52–79. https://doi.org/10.1002/ente.201600327
Liu J, Chinga-Carrasco G, Cheng F et al (2016) Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 23:3129–3143. https://doi.org/10.1007/s10570-016-1038-3
Farhat W, Venditti R, Quick A et al (2017) Hemicellulose extraction and characterization for applications in paper coatings and adhesives. Ind Crop Prod 107:370–377. https://doi.org/10.1016/j.indcrop.2017.05.055
Segura N, Orozco F, Mora L, et al (2017) Synthesis and reinforcement of thermostable polymers from Costarican renewable resources. J Renew Mater
Seidl PR, Goulart AK (2016) Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr Opin Green Sustain Chem 2:48–53. https://doi.org/10.1016/j.cogsc.2016.09.003
Verardi A, De Bari I, Ricca E, Calabro V (2012) Hydrolysis of lignocellulosic biomass: current status of processes and technologies and future perspectives. Bioethanol. https://doi.org/10.5772/23987
Kargarzadeh H, Ioelovich M, Ahmad I et al (2017) Methods for extraction of nanocellulose from various sources. Handbook of Nanocellulose and Cellulose Nanocomposites:1–49. https://doi.org/10.1002/9783527689972.ch1
Liu H, Zhang YX, Hou T et al (2018) Mechanical deconstruction of corn stover as an entry process to facilitate the microwave-assisted production of ethyl levulinate. Fuel Process Technol 174:53–60. https://doi.org/10.1016/j.fuproc.2018.02.011
Rasmussen H, Sørensen HR, Meyer AS (2014) Formation of degradation compounds from lignocellulosic biomass in the biorefinery: sugar reaction mechanisms. Carbohydr Res 385:45–57. https://doi.org/10.1016/j.carres.2013.08.029
Zeng Y, Zhao S, Yang S, Ding SY (2014) Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr Opin Biotechnol 27:98–45. https://doi.org/10.1016/j.copbio.2013.09.008
McIntosh S, Zhang Z, Palmer J, et al (2016) Pilot-scale cellulosic ethanol production using eucalyptus biomass pretreated by dilute acid and steam explosion. Biofuels, Bioprod Biorefining 246–256. https://doi.org/10.1002/bbb.1651
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48. https://doi.org/10.1016/j.biortech.2015.08.085
Singh DP, Trivedi RK (1999) Acid and alkaline pretreatment of lignocellulosic biomass to produce ethanol as biofuel. Int J ChemTech 5:727–734
Kim S, Holtzapple MT (2006) Delignification kinetics of corn stover in lime pretreatment. Bioresour Technol 97:778–785. https://doi.org/10.1016/j.biortech.2005.04.002
Mittal A, Katahira R, Donohoe BS et al (2017) Alkaline peroxide delignification of corn stover. ACS Sustain Chem Eng 5:6310–6321. https://doi.org/10.1021/acssuschemeng.7b01424
Adeleye AT, Louis H, Temitope HA, et al (2019) Ionic liquids ( ILs ): advances in biorefinery for the efficient conversion of lignocellulosic biomass. Asian J Green Chem 3:391–417. https://doi.org/10.22034/ajgc.2018.146881.1100
Menezes DB, Brazil OAV, Romanholo-Ferreira LF, Lourdes T. M. Polizeli M, Ruzene DS, Silva DP, Costa LP, Hernández-Macedo ML (2017) Prospecting fungal ligninases using corncob lignocellulosic fractions. Cellulose 24:4355–4365. https://doi.org/10.1007/s10570-017-1427-2
Yamada R, Nakashima K, Asai-Nakashima N, Tokuhara W, Ishida N, Katahira S, Kamiya N, Ogino C, Kondo A (2017) Direct ethanol production from ionic liquid-pretreated lignocellulosic biomass by cellulase-displaying yeasts. Appl Biochem Biotechnol 182:229–237. https://doi.org/10.1007/s12010-016-2322-2
Zhao X, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827. https://doi.org/10.1007/s00253-009-1883-1
Ravindran R, Jaiswal AK (2016) A comprehensive review on pretreatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102. https://doi.org/10.1016/j.biortech.2015.07.106
Salapa I, Katsimpouras C, Topakas E, Sidiras D (2017) Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 100:10–16. https://doi.org/10.1016/j.biombioe.2017.03.011
Monika DS, Goyal S (2016) Pretreatment of lignocellulosic biomass for bioethanol production: a brief review. J Agric Sci Technol 5:1–7
Liu Q, Li W, Ma Q, An S, Li M, Jameel H, Chang HM (2016) Pretreatment of corn stover for sugar production using a two-stage dilute acid followed by wet-milling pretreatment process. Bioresour Technol 211:435–442. https://doi.org/10.1016/j.biortech.2016.03.131
Shah Y, Pandit A, Moholkar V (1999) Cavitation reaction engineering. Springer Science+ Business Media LCC, New York
Madison MJ, Coward-Kelly G, Liang C et al (2017) Mechanical pretreatment of biomass–part I: acoustic and hydrodynamic cavitation. Biomass Bioenergy 98:135–141. https://doi.org/10.1016/j.biombioe.2017.01.007
Song X, Yang Y, Zhang M et al (2018) Ultrasonic pelleting of torrefied lignocellulosic biomass for bioenergy production. Renew Energy 129:56–62. https://doi.org/10.1016/j.renene.2018.05.084
Zheng J, Rehmann L (2014) Extrusion pretreatment of lignocellulosic biomass: a review. Int J Mol Sci 15:18967–18984. https://doi.org/10.3390/ijms151018967
Karunanithy C, Muthukumarappan K (2013) Thermo-mechanical pretreatment of feedstocks, in green biomass pretreatment for biofuels production. Berlin Heindelberg
Duque A, Manzanares P, Ballesteros M (2017) Extrusion as a pretreatment for lignocellulosic biomass: fundamentals and applications. Elsevier Ltd
Oliva JM, Negro MJ, Manzanares P et al (2017) A sequential steam explosion and reactive extrusion pretreatment for lignocellulosic biomass conversion within a fermentation-based biorefinery perspective. Fermentation 3:1–15. https://doi.org/10.3390/fermentation3020015
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11. https://doi.org/10.1016/S0960-8524(01)00212-7
Carvalheiro F, Duarte LC, Gírio FM (2008) Hemicellulose biorefineries: a review on biomass pretreatments. J Sci Ind Res (India) 67:849–864
Duque A, Manzanares P, Ballesteros I, Ballesteros M (2016) Steam explosion as lignocellulosic biomass pretreatment. Elsevier Inc.
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sust Energ Rev 32:713–728. https://doi.org/10.1016/j.rser.2014.01.051
Medina JDC, Woiciechowski A, Filho AZ, Nigam PS, Ramos LP, Soccol CR (2016) Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: a biorefinery approach. Bioresour Technol 199:173–180. https://doi.org/10.1016/j.biortech.2015.08.126
Salmén L (1984) Viscoelastic properties of in situ lignin under water-saturated conditions. J Mater Sci 19:3090–3096. https://doi.org/10.1007/BF01026988
Zhuang X, Wang W, Yu Q, Qi W, Wang Q, Tan X, Zhou G, Yuan Z (2016) Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour Technol 199:68–75. https://doi.org/10.1016/j.biortech.2015.08.051
Jiang W, Chang S, Li H, Oleskowicz-Popiel P, Xu J (2015) Liquid hot water pretreatment on different parts of cotton stalk to facilitate ethanol production. Bioresour Technol 176:175–180. https://doi.org/10.1016/j.biortech.2014.11.023
Mohan M, Banerjee T, Goud VV (2015) Hydrolysis of bamboo biomass by subcritical water treatment. Bioresour Technol 191:244–252. https://doi.org/10.1016/j.biortech.2015.05.010
Gu T, Held MA, Faik A (2013) Supercritical CO2 and ionic liquids for the pretreatment of lignocellulosic biomass in bioethanol production. Environ Technol (United Kingdom) 34:1735–1749. https://doi.org/10.1080/09593330.2013.809777
Duarte SH, dos Santos P, Michelon M et al (2017) Recovery of yeast lipids using different cell disruption techniques and supercritical CO 2 extraction. Biochem Eng J 125:230–237. https://doi.org/10.1016/j.bej.2017.06.014
Bhutto AW, Qureshi K, Harijan K et al (2017) Insight into progress in pretreatment of lignocellulosic biomass. Energy 122:724–745. https://doi.org/10.1016/j.energy.2017.01.005
Zhao MJ, Xu QQ, Li GM et al (2019) Pretreatment of agricultural residues by supercritical CO 2 at 50–80 °C to enhance enzymatic hydrolysis. J Energy Chem 31:39–45. https://doi.org/10.1016/j.jechem.2018.05.003
Chundawat SPS, Donohoe BS, Da Costa SL et al (2011) Multi-scale visualization and characterization of lignocellulosic plant cell wall deconstruction during thermochemical pretreatment. Energy Environ Sci 4:973–984. https://doi.org/10.1039/c0ee00574f
Bonner IJ, Thompson DN, Plummer M et al (2016) Impact of ammonia fiber expansion (AFEX) pretreatment on energy consumption during drying, grinding, and pelletization of corn stover. Dry Technol 34:1319–1329. https://doi.org/10.1080/07373937.2015.1112809
Abdul PM, Jahim JM, Harun S, Markom M, Lutpi NA, Hassan O, Balan V, Dale BE, Mohd Nor MT (2016) Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour Technol 211:200–208. https://doi.org/10.1016/j.biortech.2016.02.135
Perez-Pimienta JA, Flores-Gómez CA, Ruiz HA, Sathitsuksanoh N, Balan V, da Costa Sousa L, Dale BE, Singh S, Simmons BA (2016) Evaluation of agave bagasse recalcitrance using AFEX™, autohydrolysis, and ionic liquid pretreatments. Bioresour Technol 211:216–223. https://doi.org/10.1016/j.biortech.2016.03.103
Kádár Z, Schultz-Jensen N, Jensen JS et al (2015) Enhanced ethanol production by removal of cutin and epicuticular waxes of wheat straw by plasma assisted pretreatment. Biomass Bioenergy 81:26–30. https://doi.org/10.1016/j.biombioe.2015.05.012
Vanneste J, Ennaert T, Vanhulsel A, Sels B (2017) Unconventional pretreatment of lignocellulose with low-temperature plasma. ChemSusChem 10:14–31. https://doi.org/10.1002/cssc.201601381
Ravindran R, Sarangapani C, Jaiswal S, Lu P, Cullen PJ, Bourke P, Jaiswal AK (2019) Improving enzymatic hydrolysis of brewer spent grain with nonthermal plasma. Bioresour Technol 282:520–524. https://doi.org/10.1016/j.biortech.2019.03.071
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318. https://doi.org/10.1016/j.biortech.2018.04.099
De La Hoz A, Díaz-Ortiz Á, Moreno A (2005) Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev 34:164–178. https://doi.org/10.1039/b411438h
Amin FR, Khalid H, Zhang H et al (2017) Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 7. https://doi.org/10.1186/s13568-017-0375-4
Jiménez-Bonilla P, Salas-Arias J, Esquivel M, Vega-Baudrit JR (2014) Optimization of microwave-assisted and conventional heating comparative synthesis of poly(lactic acid) by direct melt polycondensation from agroindustrial banana (Musa AAA Cavendish) and pineapple (Ananas comosus) fermented wastes. J Polym Environ 22:393–397. https://doi.org/10.1007/s10924-014-0667-6
Zhu Z, Rezende CA, Simister R et al (2016) Efficient sugar production from sugarcane bagasse by microwave assisted acid and alkali pretreatment. Biomass Bioenergy 93:269–278. https://doi.org/10.1016/j.biombioe.2016.06.017
Jia X, Liu C, Song H, Ding M, du J, Ma Q, Yuan Y (2016) Design, analysis and application of synthetic microbial consortia. Synth Syst Biotechnol 1:109–117. https://doi.org/10.1016/j.synbio.2016.02.001
Liu F, Monroe E, Davis RW (2019) Engineering microbial consortia for bioconversion of multisubstrate biomass streams to biofuels. In: Qubeissi AM (ed) Biofuels Challengues and Oportunities. IntechOpen, p 21
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz 10:235–251. https://doi.org/10.1007/s12649-017-0059-y
Darabzadeh N, Hamidi-Esfahani Z, Hejazi P (2019) Optimization of cellulase production under solid-state fermentation by a new mutant strain of Trichoderma reesei. Food Sci Nutr 7:572–578. https://doi.org/10.1002/fsn3.852
Brethauer S, Studer MH (2014) Consolidated bioprocessing of lignocellulose by a microbial consortium. Energy Environ Sci 7:1446–1453. https://doi.org/10.1039/c3ee41753k
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2016) Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment-a review. Renew Sust Energ Rev 54:217–234. https://doi.org/10.1016/j.rser.2015.10.003
Van Kuijk SJA, Sonnenberg ASM, Baars JJP et al (2015) Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnol Adv 33:191–202. https://doi.org/10.1016/j.biotechadv.2014.10.014
Moya R, Berrocal A, Rodriguez-Zuñiga A et al (2014) Effect of silver nanoparticles on white-rot wood decay and some physical properties of three tropical wood species. Wood Fiber Sci 46:527–538
Sánchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 27:185–194. https://doi.org/10.1016/j.biotechadv.2008.11.001
Hildén K, Mäkelä MR (2018) Role of fungi in wood decay. Ref Modul Life Sci 1–9. https://doi.org/10.1016/b978-0-12-809633-8.12424-0
Dhiman SS, Haw JR, Kalyani D, Kalia VC, Kang YC, Lee JK (2015) Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresour Technol 179:50–57. https://doi.org/10.1016/j.biortech.2014.11.059
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases-production, applications and challenges. J Sci Ind Res (India) 64:832–844
Srivastava N, Srivastava M, Ramteke PW, Mishra PK (2019) Synthetic biology strategy for microbial cellulases. Elsevier B.V
Jayasekara S, Ratnayake R (2019) Microbial cellulases : an overview and applications. In: Rodríguez A, Eugenio ME (eds) Cellulose. IntechOpen
Mohanan K, Ratnayake RR, Mathaniga K et al (2014) Effect of co-culturing of cellulolytic fungal isolates for degradation of lignocellulosic material. J Yeast Fungal Res 5:31–38. https://doi.org/10.5897/jyfr2014.0134
Ziemiński K, Kowalska-Wentel M (2015) Effect of enzymatic pretreatment on anaerobic co-digestion of sugar beet pulp silage and vinasse. Bioresour Technol 180:274–280. https://doi.org/10.1016/j.biortech.2014.12.035
Alayoubi R, Mehmood N, Husson E et al (2019) Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew Energy 145:1808–1816. https://doi.org/10.1016/j.renene.2019.07.091
Liu Y, Xu J, Zhang Y et al (2015) Sequential bioethanol and biogas production from sugarcane bagasse based on high solids fed-batch SSF. Energy 90:1199–1205. https://doi.org/10.1016/j.energy.2015.06.066
Terán Hilares R, Kamoei DV, Ahmed MA, da Silva SS, Han JI, Santos JCD (2018) A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason Sonochem 43:219–226. https://doi.org/10.1016/j.ultsonch.2018.01.016
Zhang Y, Chen X, Gu Y, Zhou X (2015) A physicochemical method for increasing methane production from rice straw: extrusion combined with alkali pretreatment. Appl Energy 160:39–48. https://doi.org/10.1016/j.apenergy.2015.09.011
Sheng T, Zhao L, Gao L et al (2018) Enhanced biohydrogen production from nutrient-free anaerobic fermentation medium with edible fungal pretreated rice straw. RSC Adv 8:22924–22930. https://doi.org/10.1039/C8RA03361G
Bhatia SK, Gurav R, Choi TR, Han YH, Park YL, Park JY, Jung HR, Yang SY, Song HS, Kim SH, Choi KY, Yang YH (2019) Bioconversion of barley straw lignin into biodiesel using Rhodococcus sp. YHY01. Bioresour Technol 289:121704. https://doi.org/10.1016/j.biortech.2019.121704
Zhang K, Xu R, Abomohra AE-F et al (2019) A sustainable approach for efficient conversion of lignin into biodiesel accompanied by biological pretreatment of corn straw. Energy Convers Manag 199:111928. https://doi.org/10.1016/j.enconman.2019.111928
Mancini G, Papirio S, Lens PNL, Esposito G (2018) Increased biogas production from wheat straw by chemical pretreatments. Renew Energy 119:608–614. https://doi.org/10.1016/j.renene.2017.12.045
Pérez-Rangel M, Quiroz-Figueroa FR, González-Castañeda J, Valdez-Vazquez I (2015) Microscopic analysis of wheat straw cell wall degradation by microbial consortia for hydrogen production. Int J Hydrog Energy 40:151–160. https://doi.org/10.1016/j.ijhydene.2014.10.050
Tsafrakidou P, Bekatorou A, Koutinas AA et al (2018) Αcidogenic fermentation of wheat straw after chemical and microbial pretreatment for biofuel applications. Energy Convers Manag 160:509–517. https://doi.org/10.1016/j.enconman.2018.01.046
Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HMN (2017) Lignocellulose: a sustainable material to produce value-added products with a zero waste approach—a review. Int J Biol Macromol 99:308–318. https://doi.org/10.1016/j.ijbiomac.2017.02.097
Song J, Yang W, Li Z et al (2016) Discovering the energy, economic and environmental potentials of urban wastes: an input-output model for a metropolis case. Energy Convers Manag 114:168–179. https://doi.org/10.1016/j.enconman.2016.02.014
Kumar G, Bakonyi P, Periyasamy S et al (2015) Lignocellulose biohydrogen: practical challenges and recent progress. Renew Sust Energ Rev 44:728–737. https://doi.org/10.1016/j.rser.2015.01.042
Sivagurunathan P, Kumar G, Mudhoo A et al (2017) Fermentative hydrogen production using lignocellulose biomass: an overview of pretreatment methods, inhibitor effects and detoxification experiences. Renew Sust Energ Rev 77:28–42. https://doi.org/10.1016/j.rser.2017.03.091
Soltan M, Elsamadony M, Tawfik A (2017) Biological hydrogen promotion via integrated fermentation of complex agro-industrial wastes. Appl Energy 185:929–938. https://doi.org/10.1016/j.apenergy.2016.10.002
Soltan M, Elsamadony M, Mostafa A, Awad H, Tawfik A (2019) Nutrients balance for hydrogen potential upgrading from fruit and vegetable peels via fermentation process. J Environ Manag 242:384–393. https://doi.org/10.1016/j.jenvman.2019.04.066
Tagne TFR, Anagho GS, Ionel I et al (2019) Experimental biogas production from Cameroon lignocellulosic waste biomass. J Environ Prot Ecol 20:1335–1344
Buitrón G, Hernández-Juárez A, Hernández-Ramírez MD, Sánchez A (2019) Biochemical methane potential from lignocellulosic wastes hydrothermally pretreated. Ind Crop Prod 139:111555. https://doi.org/10.1016/j.indcrop.2019.111555
Dahunsi SO, Oranusi S, Efeovbokhan VE (2017) Optimization of pretreatment, process performance, mass and energy balance in the anaerobic digestion of Arachis hypogaea (peanut) hull. Energy Convers Manag 139:260–275. https://doi.org/10.1016/j.enconman.2017.02.063
Panigrahi S, Dubey BK (2019) Electrochemical pretreatment of yard waste to improve biogas production: understanding the mechanism of delignification, and energy balance. Bioresour Technol 292:121958. https://doi.org/10.1016/j.biortech.2019.121958
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173. https://doi.org/10.1016/j.jrras.2014.02.003
Patthawaro S, Lomthaisong K, Saejung C (2019) Bioconversion of agro-industrial waste to value-added product lycopene by photosynthetic bacterium Rhodopseudomonas faecalis and its carotenoid composition. Waste Biomass Valoriz:1–12. https://doi.org/10.1007/s12649-018-00571-z
Lee JJL, Cooray ST, Mark R, Chen WN (2019) Effect of sequential twin screw extrusion and fungal pretreatment to release soluble nutrients from soybean residue for carotenoid production. J Sci Food Agric 99:2646–2650. https://doi.org/10.1002/jsfa.9476
Sandmann G (2001) Carotenoid biosynthesis and biotechnological application. Arch Biochem Biophys 385:4–12. https://doi.org/10.1006/abbi.2000.2170
Jiménez-Quero A, Pollet E, Zhao M, Marchioni E, Averous L, Phalip V (2017) Fungal fermentation of lignocellulosic biomass for itaconic and fumaric acid production. J Microbiol Biotechnol 27:1–8. https://doi.org/10.4014/jmb.1607.07057
Zhang L, Xi G, Yu K et al (2017) Furfural production from biomass–derived carbohydrates and lignocellulosic residues via heterogeneous acid catalysts. Ind Crop Prod 98:68–75. https://doi.org/10.1016/j.indcrop.2017.01.014
Asgher M, Wahab A, Bilal M, Nasir Iqbal HM (2016) Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal Agric Biotechnol 6:195–201. https://doi.org/10.1016/j.bcab.2016.04.003
Xu X, Lin M, Zang Q, Shi S (2018) Solid state bioconversion of lignocellulosic residues by Inonotus obliquus for production of cellulolytic enzymes and saccharification. Bioresour Technol 247:88–95. https://doi.org/10.1016/j.biortech.2017.08.192
Chen Z, Liu G, Zhang J, Bao J (2019) A preliminary study on L-lysine fermentation from lignocellulose feedstock and techno-economic evaluation. Bioresour Technol 271:196–201. https://doi.org/10.1016/j.biortech.2018.09.098
Zhou PP, Meng J, Bao J (2017) Fermentative production of high titer citric acid from corn stover feedstock after dry dilute acid pretreatment and biodetoxification. Bioresour Technol 224:563–572. https://doi.org/10.1016/j.biortech.2016.11.046
Bai Z, Gao Z, Sun J, Wu B, He B (2016) D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresour Technol 207:346–352. https://doi.org/10.1016/j.biortech.2016.02.007
Salvachúa D, Smith H, St. John PC et al (2016) Succinic acid production from lignocellulosic hydrolysate by Basfia succiniciproducens. Bioresour Technol 214:558–566. https://doi.org/10.1016/j.biortech.2016.05.018
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. https://doi.org/10.1021/cr900354u
PNNL (2004) Top value added chemicals from biomass volume I—results of screening for potential candidates from sugars and synthesis gas energy efficiency and renewable energy. National Renewable Energy Laboratory, Oak Ridge
Liguori R, Amore A, Faraco V (2013) Waste valorization by biotechnological conversion into added value products. Appl Microbiol Biotechnol 97:6129–6147. https://doi.org/10.1007/s00253-013-5014-7
Sindhu R, Gnansounou E, Binod P, Pandey A (2016) Bioconversion of sugarcane crop residue for value added products–an overview. Renew Energy 98:203–215. https://doi.org/10.1016/j.renene.2016.02.057
El-Bakry M, Abraham J, Cerda A et al (2015) From wastes to high value added products: novel aspects of SSF in the production of enzymes. Crit Rev Environ Sci Technol 45:1999–2042. https://doi.org/10.1080/10643389.2015.1010423
Das A, Yoon SH, Lee SH, Kim JY, Oh DK, Kim SW (2007) An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl Microbiol Biotechnol 77:505–512. https://doi.org/10.1007/s00253-007-1206-3
Montazer-Rahmati MM, Rabbani P, Abdolali A, Keshtkar AR (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J Hazard Mater 185:401–407. https://doi.org/10.1016/j.jhazmat.2010.09.047
Abdolali A, Guo WS, Ngo HH, Chen SS, Nguyen NC, Tung KL (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160:57–66. https://doi.org/10.1016/j.biortech.2013.12.037
Awal A, Rana M, Sain M (2015) Thermorheological and mechanical properties of cellulose reinforced PLA bio-composites. Mech Mater 80:87–95. https://doi.org/10.1016/j.mechmat.2014.09.009
Castro-Aguirre E, Iñiguez-Franco F, Samsudin H, Fang X, Auras R (2016) Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev 107:333–366. https://doi.org/10.1016/j.addr.2016.03.010
Camargo LA, Pereira SC, Correa AC, Farinas CS, Marconcini JM, Mattoso LHC (2016) Feasibility of manufacturing cellulose nanocrystals from the solid residues of second-generation ethanol production from sugarcane bagasse. Bioenergy Res 9:894–906. https://doi.org/10.1007/s12155-016-9744-0
Bondancia TJ, Mattoso LHC, Marconcini JM, Farinas CS (2017) A new approach to obtain cellulose nanocrystals and ethanol from eucalyptus cellulose pulp via the biochemical pathway. Biotechnol Prog 28. https://doi.org/10.1002/btpr.2486
Camacho M, Ureña YRC, Lopretti M et al (2017) Synthesis and characterization of nanocrystalline cellulose derived from pineapple peel residues. J Renew Mater 5:271–279. https://doi.org/10.7569/JRM.2017.634117
Abdo HS, Elzatahry AA, Alharbi HF, Khalil KA (2016) Electrical conductivity behavior of biopolymer composites. Elsevier Inc.
Herzele S, Veigel S, Liebner F et al (2016) Reinforcement of polycaprolactone with microfibrillated lignocellulose. Ind Crop Prod 93:302–308. https://doi.org/10.1016/j.indcrop.2015.12.051
Kataria R, Woods T, Casey W et al (2018) Surfactant-mediated hydrothermal pretreatment of ryegrass followed by enzymatic saccharification for polyhydroxyalkanoate production. Ind Crop Prod 111:625–632. https://doi.org/10.1016/j.indcrop.2017.11.029
Yin F, Li D, Ma X, Zhang C (2019) Pretreatment of lignocellulosic feedstock to produce fermentable sugars for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production using activated sludge. Bioresour Technol 290:121773. https://doi.org/10.1016/j.biortech.2019.121773
Sawant SS, Salunke BK, Kim BS (2015) Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp. LL1. Bioresour Technol 194:247–255. https://doi.org/10.1016/j.biortech.2015.07.019
Satari B, Karimi K, Zamani A (2016) Oil, chitosan, and ethanol production by dimorphic fungus Mucor indicus from different lignocelluloses. J Chem Technol Biotechnol 91:1835–1843. https://doi.org/10.1002/jctb.4776
Moreno G, Ramirez K, Esquivel M, Jimenez G (2019) Biocomposite films of polylactic acid reinforced with microcrystalline cellulose from pineapple leaf fibers. J Renew Mater 7:9–20. https://doi.org/10.32604/jrm.2019.00017
Kellersztein I, Amir E, Dotan A (2016) Grafting of wheat straw fibers with poly (ε-caprolactone) via ring-opening polymerization for poly(lactic acid) reinforcement. Polym Adv Technol 27:657–664. https://doi.org/10.1002/pat.3736
Bilal M, Asgher M, Iqbal HMN, Hu H, Zhang X (2017) Biotransformation of lignocellulosic materials into value-added products—a review. Int J Biol Macromol 98:447–458. https://doi.org/10.1016/j.ijbiomac.2017.01.133
Vega-Baudrit J, González-Paz R, Miranda M, Corrales Y (2016) Biorefinery by the hand of the nanotechnology: biodegradable polymers from industrial biomass waste. J Nanosci Technol
Ten E, Vermerris W (2013) Functionalized polymers from lignocellulosic biomass: state of the art. Polymers (Basel) 5:600–642. https://doi.org/10.3390/polym5020600
Iqbal H (2015) Development of bio-composites with novel characteristics through enzymatic grafting. University of Westminster
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Batista Meneses, D., Montes de Oca-Vásquez, G., Vega-Baudrit, J.R. et al. Pretreatment methods of lignocellulosic wastes into value-added products: recent advances and possibilities. Biomass Conv. Bioref. 12, 547–564 (2022). https://doi.org/10.1007/s13399-020-00722-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00722-0