Abstract
Malaysia is one of the world’s largest palm oil producers and exporters, which generates several million tons of palm oil mill effluent (POME). POME is discharged into the water sources and considered as a major environmental pollutants in Malaysia. Alternatively, POME also contains some nutrients and several minerals, thereby POME could prove as a suitable medium for microbial-based applications, such as wastewater treatment and biofuel production. Different techniques have been employed to effectively utilize the POME at point of generation. One of the alternatives is the cultivation of microalgae in the enriched medium of POME. The present study used POME as growth medium for cultivation of Chlorella sp. and determines the effect of light intensity (ranging from 900 to 12,000 lux) and different CO2 concentrations (ranging from 5% (v/v) to 20% (v/v)) by both experimentally and design expert methods. The results revealed that biomass yield was considerably increased by increasing the CO2 concentration and further improved in the photoautotrophic conditions. The optimum value of 10.9% (v/v) CO2 concentration and 9963.8 lux of light intensity was found to capture maximum CO2 and biomass production. The result obtained from optimization of the microalgal growth under various CO2 concentration, light intensity, their interaction effects, and the squared CO2 concentration suitable for microalgal lipid production under suitable light and CO2 supply. This study suggests that the nutrient sources present in POME could be potentially used to capture the carbon or CO2 and reduce the economic impact of carbon emissions. In addition, enhanced biomass yield will increase the yield for biodiesel production by Chlorella sp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The global energy demand is increasing day-by-day and with the increasing population around the world, the total energy demand has been also predicted to increase significantly [1, 2]. On the other hand, the burning of fossil fuels is increasing the emission of greenhouse gases, consequently, leading to air pollution in the surrounding environment. Therefore, it is important to find ecofriendly and renewable energy sources to meet the increasing energy demand and decrease the environmental pollution. In this regard, biomass could be an advantageous feedstock to produce renewable biofuels. Microalgae are considered as promising feedstock for the production of different second- and third-generation biofuels; due to the increase in world population and energy consumption, it has been reported that various species of microalgae were more efficient in utilizing sunlight and higher growth rate than terrestrial plants [3]. Furthermore, careful downstream process planning for commercial biodiesel production from microalgae is also mandatory for establishing a microalgae-based renewable fuel industry. Wastewater for microalgae cultivation and biofuel production has been considered as economical cultivation [4, 5]. Mixotrophic cultivation system has been tested on microalgae species Chlorella and Botryococcus revealed assimilation of organic carbons, nutrients, and CO2 simultaneously, performing both respiration and photosynthesis at the same time [6,7,8,9].
The best method for microalgae to act as a carbon sink is to capture CO2 from the atmosphere for its growth; however, this method is limited by low CO2 concentration in the air which makes it economic infeasible. On the other hand, microalgae showed better adaptability to the high concentration of CO2 up to 20% from flue gases emission [10, 11]. Moreover, only a small number of microalgae strains can tolerate a high level of SOx and NOx present in fuel gases from palm oil mill and that could be used to culture microalgae with POME as nutrients source for CO2 fixation, whereas, it can be easily found in tropical countries like Malaysia [12]. The selection of optimal microalgae species depends on specific strategies based on the capability of microalgae to assimilate N and P in effluent or wastewater and sequestrate CO2 [13].
Microalgae has the ability to consume nutrients for growth and absorb toxins and fix CO2 as it undergoes photosynthesis process where CO2 will be consumed for growth and O2 will be released as the respiration product [14]. Chlorella sp. is a flexible microalgae species that has a great adaption ability in most of wastewater conditions. Most of Chlorella sp. has been reported to have a good performance in nutrient removal from wastewater. In addition, Chlorella sp. is one of the microalgae species that is able to tolerate high concentration of CO2. Moreover, high content of lipid in Chlorella sp. has been obtained by one of the studies was achieved with the cultivation condition of 2% CO2 and 0.26-vvm aeration rate [15, 16]. Microalgae cultivation in POME offers an alternative to conventional forms of tertiary wastewater treatments and spontaneously utilizes organic compounds in POME to generate microalgae biomass for lipid production [17]. Until date, there are only limited literatures have been reported on the effects of supplements addition to wastewater cultivation medium for microalgae biomass and lipid production, especially utilizing POME [18].
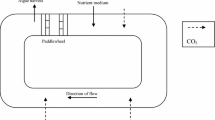
Therefore, in the present study, the microalga Chlorella sp. was cultivated in POME under different concentration of CO2 and light intensity. To further enhance the biomass growth and lipid production of Chlorella sp., varying concentrations of CO2 and light intensities were studied to identify its effects for biomass and subsequently lipid production. The CO2 fixation mediation in POME using statistical analysis was evaluated and discussed (Fig. 1).
2 Materials and methods
2.1 Preparation of inoculum
Chlorella sp. was isolated from the surface of POME by using plankton net made of bolting silk cloth (mesh size 30 μm). The Chlorella sp. was cultivated in 1-L Erlenmeyer’s flask with Bold’s basal medium (BBM) aerated with air and continuously illuminated. The BBM medium was prepared as follows (per liter): KH2PO4, 0.175 g; K2HPO4, 0.075 g; MgSO4·7H2O, 0.075 g; CaCl2·2H2O, 0.025 g; NaNO3, 0.75 g; EDTA ferric sodium salt, 0.3·10–3; H3BO3, 0.061 mg; MnCl2·4H2O, 0.007 mg; ZnSO4·7H2O, 0.287 mg; CuSO4·7H2O, 0.0025 g; NaHCO3 [19, 20]. The pH was adjusted to 6.7 with the NaOH solution. All the material and medium used were autoclaved (PH PM088) at 121 °C for 20 min before use.
2.2 Preparation of medium
POME medium [21] was collected at the last polishing pond from Dominion Square Palm Oil Mill Sdn. Bhd. Gambang, Pahang, Malaysia. Suspended solid in POME was removed by centrifugation (Refrigerated Centrifuge 5810R). After centrifugation, POME was sterilized at 121 °C at 15 lbs pressure for 20 min.
2.3 Microalgae culture and condition
Chlorella sp. was cultivated in 2-L Erlenmeyer’s flask contained 1800 mL of sterilized POME as medium and put in a box completed with fluorescent lamps as a light source. POME incorporates 95–96% of water, 0.6–0.7% of oil, and 2–4% suspended solids [22]. All experiments were carried out at 30 °C and continuously illuminated at light intensities ranging from 900 to 12,000 lux measured by LI-250 Light meter with a LI-190 quantum sensor (LI-COR Biosciences, USA). Agitation of during cultivation of microalgae was provided by bubbling CO2-enriched air through the tube. Different values of CO2 concentration were used in the experiment. CO2 and air flow rate were monitored using flow meters (FR4500, Key Instruments, USA) while the CO2 supply and air were regulated by on/off solenoid valves, switched automatically through the computer control system. The initial algal concentration was the same for all cultivation condition [23].
2.4 Determination of cell dry weight
The biomass in the Erlenmeyer flask has been collected every 24 h for 7-day period. Biomass productivity determined by using cell dry weight of biomass. Biomass sample was harvested by centrifugation (Refrigerated Centrifuge 5810R) at 10000 rpm for 15 min. Then, rinsed twice with distilled water. The dry weights of samples were measured by drying in an oven at 70 °C for 24 h and weighed using analytical balance [24].
2.5 Optimization and experimental design
The experimental optimization design was performed using central composite design. The experimental design was carried out using 22 full-factorial experiments design by using the Design Expert software version 7.1.6. For statistical analysis, the independent variables were coded according to the equation as follows:

where x_i is the coded value of the independent variable; X_i, the real value of independent variable; X_o, real value of an independent variable at center point; and 〖∆X〗_i, the step change value. Table 1 provides a list of independent variables and coded factor level.
A total of 14 runs with four replicates at the central point were used as provided in Table 1 to optimize the range and levels of the chosen variables. Each run was completed in 7 days. Final cell dry weight was taken as response of the experiment. The quality of developed model was determined by the value of correlation (R2) while analysis of variance (ANOVA) was used to evaluate the statistical significance of the model by using the Fisher statistical test (F test). The experimental data obtained from CCD model experiments can be represented in the form of the following equation:
where y is the predicted response; n is the number of factors; X_ii and X_j are the independent variables; b_0 is the interception coefficient; b_i is the linear coefficient; b_ij is the interaction coefficient; and b_ii is the quadratic coefficient.
2.6 Validation of model
The mathematical model generated by the CCD was validated by conducting various checkpoint studies. The experimentally obtained data were compared with the predicted one [25,26,27].
2.7 Lipid contents analysis
Nile red method was followed for lipid content analysis. 0.1 mg of Nile red (9-diethylamino-5H-benzo [α] phenoxazine-5-one) was dissolved in 1 mL of acetone. To 4.0 mL of algal culture, 0.04 mL of the Nile red solution was added. Then, mixed well and incubated for 5 min in darkness. Fluorescence measurements were made from the top using 530-nm excitation and 570-nm emission wavelengths [28].
2.8 Lipid extraction from microalgae
The algae oil extraction procedure was adapted from the protocol described [29]. 0.2 g of algae powder was washed with 5 mL of deionized water. Methanol, chloroform, and deionized water 2:2:1 (v/v) respectively were added to the washed algae in the series and the tubes were vortexed after the addition of chemical. Then, the samples were centrifuged for 10 min at 3000 rpm to separate the layers. The bottom layer containing lyophilic material was drawn off. 12.5 mL of chloroform was added to the content left in the test tube and then vortexed. The bottom layer containing the chloroform was again drawn off until only the top layer which contained methanol and water was left in the tubes. The vials with lipid residue were collected [30]. The total oil yield was calculated using the following equation:
2.9 Biodiesel production using trans esterification method
The microalgae oil was heated at 75 °C for 5 min to evaporate water content. A mixture of methanol, sodium hydroxide (NaOH), and n-hexane was added to the microalgae oil and the reaction mixture was blended for 35 min at a temperature of 75 °C. After the reaction was completed, the samples were cooled down to room temperature; the crude ester layer (the upper phase) was separated from glycerol layer. The FAME layer was dried by using anhydrous sodium sulfate. The upper phase containing FAMEs was collected and transferred to a GC vial [31]. The solvent was removed by keeping in the fume hood for overnight.
2.10 Gas-chromatography analysis
A high-speed gas chromatographic method has been developed to determine the FAME distribution of biodiesel, including the analysis of fatty acid methyl esters (FAME) in the reaction products and final biodiesel; the determination of mono-, di-, and tri-glycerides; the contents and distribution of free fatty acids; and the determination of trace methanol in biodiesel. For the quantification of total lipids, the methodology proposed by Folch et al. [32] was used with a preliminary stage of breaking up the cell wall using an ultrasonication. The lipid fraction was esterified to obtain methyl esters of fatty acids, finally distillated by soxhlet device according to the adapted methodology of Prartono et al. [33]. The crude extract was analysed by gas-chromatography mass spectrometer (GC-MS), equipped with an HP Innowax capillary column (30 m × 0.25 mm). Analysis times are typically on the order of 4–5 min depending upon the composition. Nitrogen was used as the carrier gas at a constant flow rate of 20 mL/min. The column oven temperature was programmed from 50 to 130 °C (at the rate of 20 °C/min) and held at 130 °C for 5 min, then raised to 260 °C at 2.5 °C/min and maintained at this temperature for 10 min [31].
3 Results and discussion
3.1 Effect of light intensity on CO2 sequestration
Light intensity is an important factor for the maximal conversion of incident light energy to algal biomass [34]. Three light intensities of 900, 6450, and 12,000 lux were used in this study and the results are shown in Fig. 2. It can be observed from the results that light intensity had significant effect on the growth of Chlorella sp. and its growth increased with increase in incubation time, producing the maximum cell dry weight at the 7th day of incubation time. On the other hand, it was noticed that the highest light intensity of 12,000 lux showed maximum growth of Chlorella sp. compared with other light intensities of 6450 and 900 lux. For instance, the total cell dry weight of Chlorella sp. obtained at 12,000 lux light intensity after 7 days was 0.0653 g, while the light intensities of 6450 and 900 lux produced the dry cell weight of 0.0582 g and 0.0574 g, respectively. It is well known that the growth of microalgae is directly proportional to the CO2 fixation rate [35, 36]. Therefore, it could be suggested that the higher light intensity of 12,000 lux was more favorable for CO2 fixation and consequently for growth of Chlorella sp. The results of the study are consistent with previous studies that also achieved higher growth of microalgae under higher light intensities.
3.2 Effect of CO2 concentration on growth of Chlorella sp.
Carbon dioxide is the main carbon source required by microalgae for photosynthetic process. Therefore, the concentration of CO2 plays a significant role in microalgae growth. In the present study, the effect of three different CO2 concentrations on growth of Chlorella sp. was examined and the results are shown in Fig. 3. The results showed that CO2 concentration of 12.5% was suitable for enhanced growth of Chlorella sp. Noticeably, the cell dry weight obtained at 12.5% concentration was 0.0793 g, which was 27.61% higher than 5% and 55.61% higher than 20% CO2 concentration. Previous studies revealed that high CO2 concentration results in low pH of the culture medium, which could decrease the photosynthesis activity and consequently inhibit the microalgal growth [37, 38] (Table 2).
A slight increase on the growth rate from 0.23 to 0.34 g per day was observed when CO2 concentration increased from 5 to 12.5% (Fig. 3). High cell metabolism during cell growth activity could increase the pH of the medium. The cellular metabolism which is carried out inside the microalgae cell produces an alkaline condition, based on the intercellular metabolism reaction.
CO2 from bubbling gas enter the medium in the form of bicarbonate substances [39]. This bicarbonate substance [HCO3] is absorbed by the cells of Chlorella sp. Subsequently, the cell metabolism process will produce organic substances such as glucose and OH− ion as written in the equation above. The amount of calculated carbonate ion reflects the amount of carbonate ion attained in medium during cell culture cultivation. The dissolved carbonate ions are consumed by microalgae to maintain cellular metabolism or CO2 pools in cell vacuole, inside microalgae cell structure [40].
3.3 Optimization of lipid production
In order to maximize the lipid production, Chlorella sp. was cultivated under different light intensities and carbon dioxide concentrations. Process optimization using statistical analysis of central composite design (CCD) with the aids of ANOVA Design Expert Version 11 software was performed. A total of 14 experiments were necessary to estimate the coefficient of each model using linear regression analysis. The cell dry weight (R1) has been calculated from the independent input variables X1 (CO2 concentration) and X2 (light intensity) based on CCD experiments are presented in Table 3.
The quadratic model was selected by the software for both the responses. Multiple regression analysis was used to correlate the responses of cell dry weight with the two variables studied using a second-order polynomial. Apparently, the quadratic regression models for cell dry weight (R1) can be represented by the following equations:
Here in Eq. (1), X1 and X2 represent CO2 concentration and light intensity, respectively. The coefficients with one factor of light intensity or CO2 concentration represent the effect of that particular factor on the CO2 sequestration into cell dry weight. The coefficients with two factors and others with second-order terms show the interaction between the two factors and quadratic effect respectively. The sign of the effect marks the performance of the response. In this way, when a factor has a positive effect, the response is higher at the high level and when a factor has a negative effect, the response is lower at high level. The results show that the increase in light intensity led to an increase in CO2 sequestration in term of cell dry weight as it had a positive coefficient. The same is observed with the X1X2 interaction. The negative sign of the factor X1 and squared variables X12 and X22 revealed a reduction in cell dry weight productivity when their values were increased in the system. According to the equation, the largest value of the estimated regression coefficient for cell dry weight was X2. Thus, it is worth to note that the light intensity was the prime factor which had the greatest effect on the cell dry weight.
In order to show the fitness of the model, the coefficient of determination (R2) was used. However, the adjusted determination coefficient (R2 adj.) and the prediction determination coefficient (R2 pred.) are better criteria to be considered than the absolute regression coefficient [26]. Since the R always decreases when a regression variable is eliminated from model, in statistical modeling, the R adj. which takes the number of regression variables into account is usually selected. R2 indicates the ratio between the sum of the squares (SSR) with total sum of the square (SST) and it describes up to what extent perfectly the model estimated experimental data points. In our study, the R2 adjusted was found to be 0.9584 which corresponding to cell dry weight which was apparently indicated that 95.8% of the total variation in the cell dry weight can be explained by the fitted model (Table 4).
Table 5 depicts the results of analysis of variance (ANOVA) for CO2 sequestration. The model’s p value was less than 0.05 reflects that the model terms were significant at the 95% confidence level; the null hypothesis is rejected at the α-level of significance and infers that the variation accounted for the model is significantly greater than the unexplained variation. In this case, the null hypothesis is rejected at < 0.0001 level of significance for cell dry weight. This p value (< 0.0001) indicated that the model terms were significant. Furthermore, according to Table 4, X1, X2, and X1X2 interaction term and squared variables X12 except X22 were a significant model. The lack-of-fit F value of 0.154 implied that the lack-of-fit was not significant.
After analysis of data, the response surface of the full quadratic model between the response and the variables were depicted and demonstrated the effects of CO2 concentration and light intensity on cell dry weight. The optimized ranges for each factor that leads to the best response (the highest cell dry weight) were extracted from these surfaces. The cell dry weight was linearly increased when the process variables were increased up to certain limit, further increase in CO2 concentration and light intensity resulted in decrease in cell dry weight. Thus, the optimum point is located within the range of study for the selected algae Chlorella sp. This experiment has provided the optimum requirement of essential factors such as light intensity and CO2 for the growth of algae for lipid production (Fig. 4).
Based on the model predicted above, the optimized parameters predicted by the software were 10.91% CO2 concentration and light intensity of 9963.85 lux. This combination of parameters setting was predicted to give 0.076 g cell dry weight. This optimum experimental condition was run to validate the model by comparing the experimental result and predicted value of the software. The average cell dry weight obtained from the validation runs was 0.075 g and the percentage error of only 5%. Thus, the model was successfully validated as the experimental value is very close to predicted value
3.4 Lipid contents analysis
Nile red (NR) staining was used to analyse [41] the lipid contents of Chlorella sp. has shown the highest NR fluorescence reading at the highest light intensity of 6450 umol m−2 s−1 which 2781.17 fsu and lowest value of 2071.00 fsu at 900 umol m−2 s−1 (Table 6).
3.5 Lipid extraction from microalgae
Chlorella sp. was harvested at 8th day and freeze-dried into dry algae powder. The algae oil extraction procedure was adapted from the protocol described by Bligh and Dyer in 1959 [42]. The two layers appeared after the lipid extraction process. Mixture was centrifuged at low speed (2000 rpm) in order to separate the two phases [43]. Then, the upper phase was removed by siphoning. Then, the interface was rinse one or two times with methanol/water (1/1) without mixing the whole preparation. After centrifugation and siphoning of the upper phase, the lower chloroform phases that containing lipid and chloroform was collected. The lipid content extracted from Chlorella sp. was 13.858 g and the total yield was 66 % represented in Table 7. It has been revealed that was the maximum yield of lipid was observed in the present study compared with the previously reported studies [44].
3.6 Gas-chromatography mass spectrometer analysis
The fatty acid profile of biodiesel was analysed by GC-MS [37]. Table 8 shows the fatty acid compositions of Chlorella sp. It was observed that unsaturated fatty acids for Chlorella sp.
The FAs profile of microalgae was dominated by palmitic acids, citric acids, palmitoleic acids, linoleic acids, and linolenic acids [24, 45]. It also stated that palmitic acid was a predominant FA in most microalgae culture. Skeletonema costatum and Nitzschia sp. were identified as having high-palmitic acids content which was 43.34 and 38.87 respectively. In this study, Chlorella sp. had most of the FAs profile but the percentage content of FAs was different due to different strains used and culture conditions. Complementary to this, Chlorella sp. had higher palmitic acids content than Skeletonema costatum and Nitzschia sp. provided that the palmitic acid content of Chlorella sp. was 46.382 mg. This proposed that Chlorella sp. could be a new potential source for biodiesel production [43]. More importantly, the percentage of FAs obtained from microalgae is depending on the period of harvesting. According to Pratoomyot et al. [33], the percentages of FAs were found higher at stationary phase than at log phase. This is due to the limitation of nutrition at stationary phase, the cell division began to decrease and store products. It is suggested that the microalgae should be harvested where it reached in the stationary phase.
4 Conclusions
The present study compared various growth conditions for the production of microalgae biomass for the production of biodiesel. It has been confirmed that POME could be utilized as growth medium for the biomass production of diverse microalgae species. Also, the potential applicability of using microalgae as a biological method to sequester carbon dioxide has been studied extensively by the design expert optimization method. The optimum value of 10.91% CO2 concentration and 9963.85 lux of light intensity on growth of the algae for lipid production maximized CO2 sequestration was obtained through full-factorial design which was highly significant. The extracted lipid contents were increased from the low light intensity with high light intensity. In addition, extraction of total lipid content from Chlorella sp. was maximum in the present study than the previously reported studies. The fatty acid components of microalgae obtained were considerably suitable for the production of good-quality biodiesel. Overall, the present study potentially utilized POME as nutrient source for microalgal biomass production and bioremediation of POME simultaneously to reduce the pollution load to the environment.
References
Bhuyar P, Yusoff M, et al (2019) Antimicrobial study of algal enzymes extracted from microalgae by ultrasonication. https://doi.org/10.2139/ssrn.3356472
Ben Yahmed N, Jmel MA, Ben Alaya M et al (2016) A biorefinery concept using the green macroalgae Chaetomorpha linum for the coproduction of bioethanol and biogas. Energy Convers Manag 119:257–265. https://doi.org/10.1016/j.enconman.2016.04.046
Gouveia L, Oliveira AC, Congestri R et al (2017) Biodiesel from microalgae. Elsevier Ltd.
Islam MA, Brown RJ, O’Hara I et al (2014) Effect of temperature and moisture on high pressure lipid/oil extraction from microalgae. Energy Convers Manag 88:307–316. https://doi.org/10.1016/j.enconman.2014.08.038
Jayakumar S, Yusoff MM, Rahim MHA et al (2017) The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia. Renew Sust Energ Rev 72:33–47. https://doi.org/10.1016/j.rser.2017.01.002
Gupta PL, Choi HJ, Pawar RR et al (2016) Enhanced biomass production through optimization of carbon source and utilization of wastewater as a nutrient source. J Environ Manag 184:585–595. https://doi.org/10.1016/j.jenvman.2016.10.018
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135. https://doi.org/10.1016/j.biortech.2014.03.064
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516. https://doi.org/10.1016/j.biortech.2012.01.125
Leite GB, Paranjape K, Abdelaziz AEM, Hallenbeck PC (2015) Utilization of biodiesel-derived glycerol or xylose for increased growth and lipid production by indigenous microalgae. Bioresour Technol 184:123–130. https://doi.org/10.1016/j.biortech.2014.10.117
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577. https://doi.org/10.1016/j.rser.2009.10.009
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Gayen K, Mandal MK, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3. Biotech 7:1–21. https://doi.org/10.1007/s13205-017-0727-4
Rajkumar R, Sobri Takriff M (2016) Prospects of algae and their environmental applications in Malaysia: a case study. J Bioremediation Biodegrad 07:1–12. https://doi.org/10.4172/2155-6199.1000321
Clarens AF, Nassau H, Resurreccion EP, White MA, Colosi LM (2011) Environmental impacts of algae-derived biodiesel and bioelectricity for transportation. Environ Sci Technol 45:7554–7560. https://doi.org/10.1021/es200760n
Hariz HB, Takriff MS (2017) Palm oil mill effluent treatment and CO2 sequestration by using microalgae—sustainable strategies for environmental protection. Environ Sci Pollut Res 24:20209–20240. https://doi.org/10.1007/s11356-017-9742-6
Wang L, Min M, Li Y et al (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186. https://doi.org/10.1007/s12010-009-8866-7
Chiu SY, Kao CY, Chen CH, Kuan TC, Ong SC, Lin CS (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396. https://doi.org/10.1016/j.biortech.2007.08.013
Lam MK, Lee KT (2011) Renewable and sustainable bioenergies production from palm oil mill effluent (POME): win-win strategies toward better environmental protection. Biotechnol Adv 29:124–141. https://doi.org/10.1016/j.biotechadv.2010.10.001
Ramanan R, Kannan K, Deshkar A, Yadav R, Chakrabarti T (2010) Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour Technol 101:2616–2622. https://doi.org/10.1016/j.biortech.2009.10.061
Connon R (2007) Culturing of Chlorella vulgaris—standard operating procedure. Daphnia Res Gr 7:S4. https://doi.org/10.1002/biot.201400594
Bhuyar P, Yusoff MM, Rahim MHA, Sundararaju S, Gaanty Pragas Maniam NG (2020) Effect of plant hormones on the production of biomass and lipid extraction for biodiesel production from microalgae Chlorella Sp. J Microbiol Biotechnol Food Sci 9:4
Idris NA, Loh SK, Lau HLN et al (2018) Palm oil mill effluent as algae cultivation medium for biodiesel production. J Oil Palm Res 30:141–149. https://doi.org/10.21894/jopr.2018.0011
Foo KY, Hameed BH (2010) Insight into the applications of palm oil mill effluent: a renewable utilization of the industrial agricultural waste. Renew Sust Energ Rev 14:1445–1452. https://doi.org/10.1016/j.rser.2010.01.015
Santos TD, Martin JLM, Molina E, et al (2016) Optimization of carbon dioxide supply in raceway reactors: influence of carbon dioxide molar fraction and gas flow rate.
Hartati R, Endrawati H, Mamuaja J (2013) Fatty acid composition of marine microalgae in Indonesia. J Trop Biol Conserv 10:75–82
Ahmad S, Kothari R, Pathania D, Tyagi VV (2019) Optimization of nutrients from wastewater using RSMfor augmentation of Chlorella pyrenoidosa with enhanced lipid productivity, FAME content, and its quality assessment using fuel quality index. Biomass Convers Biorefinery:1–18. https://doi.org/10.1007/s13399-019-00443-z
Ahmad S, Pathak V V., Kothari R, Kumar A, Naidu Krishna S B (2018) Optimization of nutrient stress using C. pyrenoidosa for lipid and biodiesel production in integration with remediation in dairy industry wastewater using response surface methodology. 3 Biotech 8:0. https://doi.org/10.1007/s13205-018-1342-8, 1, 13
Chauhan DS, Goswami G, Dineshbabu G et al (2019) Evaluation and optimization of feedstock quality for direct conversion of microalga Chlorella sp. FC2 IITG into biodiesel via supercritical methanol transesterification. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00432-2
Held P, Raymond K (2011) Determination of algal cell lipids using Nile Red-using microplates to monitor neutral lipids in Chlorella vulgaris. Biofuel Res 8:1–5
Breil C, Abert Vian M, Zemb T et al (2017) “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. Int J Mol Sci 18:1–21. https://doi.org/10.3390/ijms18040708
Martinez-Guerra E, Gude VG, Mondala A, Holmes W, Hernandez R (2014) Extractive-transesterification of algal lipids under microwave irradiation with hexane as solvent. Bioresour Technol 156:240–247. https://doi.org/10.1016/j.biortech.2014.01.026
Phuong Thao NT, Thanh Tin N, Xuan Thanh B (2013) Biodiesel production from microalgae by extraction—transesterification method. Waste Technol 1:6–9. https://doi.org/10.12777/wastech.1.1.2013.6-9
Folch J, Lees M, Sloane Stanley G. (1987) a Simple. 55:999–1033
PRARTONO TRI, KAWAROE M, SARI DW, AUGUSTINE DINA (2010) Fatty acid content of Indonesian aquatic microalgae. HAYATI J Biosci 17:196–200. https://doi.org/10.4308/hjb.17.4.196
Edwards KF, Pfister CA, Van Alstyne KL (2006) Nitrogen content in the brown alga Fucus gardneri and its relation to light, herbivory and wave exposure. J Exp Mar Biol Ecol 336:99–109. https://doi.org/10.1016/j.jembe.2006.04.013
Bhola V, Swalaha F, Ranjith Kumar R et al (2014) Overview of the potential of microalgae for CO2 sequestration. Int J Environ Sci Technol 11:2103–2118. https://doi.org/10.1007/s13762-013-0487-6
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, van Langenhove H (2010) Enhanced CO 2 fixation and biofuel production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380. https://doi.org/10.1016/j.tibtech.2010.04.004
Tang Y, Zhang Y, Rosenberg JN et al (2016) Efficient lipid extraction and quantification of fatty acids from algal biomass using accelerated solvent extraction (ASE). RSC Adv 6:29127–29134. https://doi.org/10.1039/C5RA23519G
Jensen RG, Bahr JT (1977) Ribulose 1,5-bisphosphate .7636 carboxylase-oxygenase. 379–400
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Yang ZZ, Zhao YN, He LN (2011) CO<inf>2</inf> chemistry: Task-specific ionic liquids for CO<inf>2</inf> capture/activation and subsequent conversion. RSC Adv 1:545–567. https://doi.org/10.1039/c1ra00307k
Natunen K, Seppälä J, Schwenk D, Rischer H, Spilling K, Tamminen T (2015) Nile Red staining of phytoplankton neutral lipids: species-specific fluorescence kinetics in various solvents. J Appl Phycol 27:1161–1168. https://doi.org/10.1007/s10811-014-0404-5
Blich EG, Dyer WJ (1959) Canadian Journal of Biochemistry and Physiology. Can J Biochem Physiol 37:911–917
Bhuyar P, Rahim MHA, Yusoff MM, Gaanty Pragas Maniam NG (2019) A selective microalgae strain for biodiesel production in relation to higher lipid profile. Maejo Int J Energy Environ Commun 1:8–14
Dvoretsky D, Dvoretsky S, Temnov M, Akulinin E (2016) Enhanced lipid extraction from microalgae Chlorella vulgaris biomass: Experiments , Modelling , Optimization. https://doi.org/10.3303/CET1649030
Ferreira SP, Holz JCP, Lisboa CR, Costa JAV (2017) Fatty acid profile of Chlorella biomass obtained by fed batch heterotrophic cultivation. Int Food Res J 24:284–291
Funding
The authors gratefully acknowledged Universiti Malaysia Pahang for the financial assistance through the Internal Research Grant No. RDU190337 and Flagship Grant No. RDU182205. Author [Prakash Bhuyar] is thankful to UMP for providing Doctoral Research Scholarship (DRS) and Grant No. PGRS190351as a financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhuyar, P., Sundararaju, S., Rahim, M.H.A. et al. Microalgae cultivation using palm oil mill effluent as growth medium for lipid production with the effect of CO2 supply and light intensity. Biomass Conv. Bioref. 11, 1555–1563 (2021). https://doi.org/10.1007/s13399-019-00548-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00548-5