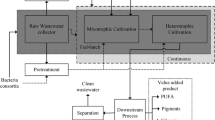
Abstract
Wastewater treatment using microalgae is an eco-friendly process without secondary pollution. During the process, the wastewater produced is reused, which allows efficient nutrient recycling. This review provides constructive information to enable progress of competent technology for microalgae based productions in palm oil mill effluent (POME). The characteristics of POME that will be described in this paper would be a source of pollution in water if discharged directly. Since microalgae have great potential to be isolated and cultivated in POME, previous studies to improve POME based culture media are still limited. Microalgae are highly competent in diminishing CO2 emissions and reducing the organic components in POME. In conclusion, biological treatments by using microalgae discussed in this paper and the lipid production from microalgae biomass can be used as an alternative for energy production. The POME treatment with microalgae may meet the standards or limits before being discharged into the water body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The benefits of microalgae in producing alternative sources of biofuels, animal feed, fertilizer and biopolymer applications seem very promising (Gouveia 2011). Moreover, microalgae can be utilized for wastewater treatment in pilot scales (Abdel-Raouf et al. 2012). The real impact of discharging wastewater rich in natural mixture and inorganic chemicals, for example, is that phosphates and nitrates are for the most part eutrophication (de Godos et al. 2009). Wastewater is utilized for nourishing microalgae that expel excess nutrients and accumulate biomass (Chinnasamy et al. 2010). Great potential has been shown by microalgae to utilize the remaining organic compounds and also to generate microalgae biomass in POME (Lam and Lee 2011).
POME is a major producer of agriculture wastewater. In 2011, there were 426 palm oil mills in Malaysia (Malaysian Palm Oil Board 2012). Table 1 shows the characteristics of POME. The high concentrations of suspended solids and grease are the source of organic compounds in POME.
Around 44 million m3 of POME were produced in year 2013 to yield 19.66 million tonnes of total crude palm oil (Malaysian Palm Oil Board 2014). Around 85 % of palm oil mills have treated raw POME using biological treatment (Tong and Jaafar 2004). The biological treatment of POME is a series of pond systems, including anaerobic, facultative, and aerobic pond systems (Yacob et al. 2009). However, the final treatment by aerobic pond system is struggling to achieve the discharge standards because of inefficient operational design (Parthasarathy et al. 2016). The final effluent of the treated POME must comply with the discharge standards set by the Department of Environment (DOE), Malaysia, as illustrated in Table 2, which remains the standard for POME management. This standard is an action by the Malaysia government to limit the impact of high organic compounds of POME on the environment.
Using microalgae in POME treatment is a known concept (Vairappan and Yen 2008), and the microalgae biomass extraction for lipid can be used as biofuel. However, literature about this topic is still limited. Ponraj and Din (2013) investigated biomass and lipid production of Chlorella pyrenoidosa using POME. Kamyab et al. (2014) studied the effect of POME on lipid productivity of C. pyrenoidosa in hybrid photo bio reactors (HPBR). Ibrahim et al. (2015) reported that optimization of retention time and pH on POME for nutrient removal using microalgae were 15 days and 9.2, respectively. Ding et al. (2016) studied a newly isolate microalgae named Chlamydomonas sp UKM 6 in POME and determined the potential of microalgae production and removal of nutrients.
This review offers constructive information to further progress of competent technology for microalgae-based productions in POME. Hence, the application of microalgae in removing organic components could result in clean and sustainable production of palm oil. This paper will present cultivation operation and also systems for microalgae growth. Furthermore, the biofuel production from microalgae biomass that extracts for lipids will be discussed.
Characteristics of algae for POME treatment
Photosynthetic organisms (including macroalgae and microalgae) need sunlight, water, and carbon dioxide (CO2) to produce biomass (Barber 2009). Macroalgae (algae up to 60 m in length) can grow fast in marine and freshwater environments; such algae are also called “seaweed.” Some macroscopic algae grow on rocks (Graham et al. 2009), while some are photosynthetic microorganisms. Unicellular and multicellular microalgae fall into both general categories of organisms, which are either prokaryotic or eukaryotic (Wang et al. 2015). As primitive plants (thallophytes), microalgae have fewer roots, stems, and leaves and lack a sterile layer of cells around reproductive cells (Cho et al. 2011). Due to the lipid content in microalgae, they can float on the surface of water (phytoplankton). The survival of microalgae in various environmental conditions from aquatic to terrestrial habitats 1000 years ago had made them the Earth’s oldest living microorganism (Mata et al. 2010).
Classification of microalgae
The number of microalgae species in the world is estimated at around 50,000, of which 30,000 have been categorized (Kim et al. 2010). Depending on their pigmentation, life cycle, and basic cell structure, they are classified into several major groups (Mata et al. 2010).
-
Bacillariophyceae class, the diatoms: Marine phytoplanktons are dominated by these microalgae, and some may be present in fresh and brackish water environments. The number of species is around 100,000. Their cell walls contain polymerized silica (Si) and various forms of carbon. Another form of carbon is present in natural oils or carbohydrate polymers (chyrsolaminarin).
-
Chlorophyceae class, the green microalgae: Around 8000 species live in fresh water as single cells or in colonies. Higher plants, which are called “vascular plants,” evolved from these green microalgae. Starch and oil are the main storage compounds that can be generated under certain conditions.
-
Cyanophyceae class, the blue green microalgae: They are not true microalgae, having no nucleus (structure that encloses the DNA) or chloroplast (structure that encloses the photosynthetic membranes) (Avagyan 2011). They play the role of fixing nitrogen in the atmosphere; their structure resembles that of bacteria. Around 8000 species live in various niches.
-
Chrysophyceae class, the golden microalgae: Around 1000 species live in fresh water. They are similar to diatoms but have more complex pigment systems (yellow, brown or orange). Golden microalgae can produce natural oils and starch.
-
Prymnesiophyceae class: Around 500 species are present in the ocean. Like diatoms, they are brown, and their main components are lipids and chyrsolaminarin.
-
Eustigmatophyceae class: These are the main “picoplankton,” which in diameter is around 2–4 µm. Nannochloropsis sp. is a common genus of this microalgae in the ocean.
Microalgae can be cultivated in open pond systems and in closed photobioreactor systems under controlled environments (Graham et al. 2009), but during cultivation operations, other microorganisms can dominate these systems (Schenk et al. 2008). Moreover, POME contains high nutrient values of BOD and COD, which can cause contamination. Four types of carbon metabolism mechanism are undertaken by microalgae: autotrophic, mixotrophic, heterotrophic, and photoheterotrophic conditions. Some microalgae can use either autotrophic or heterotrophic carbon, such as Chlorella protothecoides, depending on the culture conditions (Lam and Lee 2011). Under environmental flux, they can shift this mechanism, especially due to carbon and light flux (Chen et al. 2011).
Microalgae constituents
The main chemical components in microalgae are C0.48H1.83N0.11P0.01 (Sialve et al. 2009). The major biochemical compounds of microalgae are proteins, carbohydrates, lipids, and nucleic acids. The proportion for each component varies with different species and environments (Markou and Georgakakis 2011). Sialve et al. (2009) reported that the main biochemical compositions in microalgae such as proteins, carbohydrates, and lipids were around 6–52, 5–23, and 7–23 %, respectively.
In the palm oil industry, Yacob et al. (2006) estimated that 1 tonne of POME can produce 12.36 kg of methane, which is emitted from the anaerobic pond. Methane gas can be used as biogas; for example, methanogenesis of rye straw for improving methane yield was done by (MarouĹĄek et al. 2012), and this improvement can be applied to a POME anaerobic pond. The increase in anthropogenic green house gasses (GHGs) discharges and exhausting fossil stores have sparked a lot of research on renewable energy, especially biofuel (Singh et al. 2011). The harvested microalgae can be further processed for lipids. Compared to other crops, the rapid production of microalgae produces higher lipid content (Huang et al. 2009). Microalgae from different taxonomic groups and varied growth environments will contribute different total lipid content and fatty acids (Pratoomyot et al. 2005). The accumulation of lipid content or fatty acid in microalgae biomass is correlated with the growth stage of microalgae (Laurens and Wolfrum 2011). The production of fatty acid is higher in the stationary phase than the exponential phase (Mata et al. 2010). During the stationary phase, the microalgae utilized all the nutrients in the cultivation growth media. This situation creates a stressed environment for microalgae, causing the rate of cell division to decrease. It will lead to the accumulation of lipid content in the microalgae (Mansour et al. 2005).
Many factors contribute to lipid content accumulation in microalgae by extending the available nutrients for microalgae growth (Liu et al. 2011). From the literature reported by (Stephenson et al. 2010), the alteration of nutrients for microalgae growth in cultivation affects lipid content accumulation in microalgae. The nutrients in media correlated with any changes in lipid content from free fatty acid compounds to triacylglyceride (TAG) compounds. Modification of the nutrient profile can be achieved by manipulating the N, Si, Fe content, and CO2 supply during cultivation (Amaro et al. 2011).
A long carbon chain molecule of lipid functions as the structural component of the microalgae membrane. As the lipid content increases, the specific gravity will decrease. This criterion makes algal cell buoy on water surface for solar light source (Shen et al. 2009). Manipulating the specific species and factors affecting the microalgae results in different biomass lipids, proteins, and carbohydrate content (Shen et al. 2009). Examples of cultivation environments are the velocity of process, growth duration, and nutrient content in media (Adams et al. 2009).
Table 3 shows the constituents of various basic microalgae species. Botryococcus braunii have the highest lipid content around 33–86 %. Ibrahim et al. (2015) optimized POME treatment by using Botryococcus braunii, resulting in NH3-N and PO4 3−, removal rates of 92.857 and 56.5 %, respectively. However, they did not focus on microalgae growth and lipid production.
All the constituents shown in Table 3 change by relying on the media and conditions of the environments producing the microalgae biomass (Becker 2007) and will be discussed further in next section in this review. The end product of biofuel can be produced from lipid extraction in microalgae (Shen et al. 2009). The new oil technology that can boost oil yields without surpassing the temperature limit that can degrade oil quality already has been applied to grape seed (Maroušek et al. 2015a). This technology may be applied to lipid production from microalgae, which usually undergoes extraction using chemicals. The remaining compounds such as carbohydrates and proteins are left after lipid extraction. However, the leftover carbohydrates and proteins can be fermented for methane or ethanol, and solid residue after the fermentation process can be pyrolyzed for biogas carbon powder (Maroušek et al. (2015b). These researchers used solid pulp from fermentation of maize silage for biogas carbon powder, which can be an alternative for charcoal or costly wood. Meanwhile, the fermentation of carbohydrates will be producing ethanol (Coppola et al. 2009). Then the ethanol itself can be a key component of the transesterification process for biodiesel production, which serves as a catalyst. The reaction of ethanol with sodium produces sodium ethanolate, which can also be a catalyst. Both these catalysts may produce biodiesel and glycerol after the reaction takes place (Coppola et al. 2009).
Protein is built up by one or more peptides and folded into a solid or fiber form. In several studies on high nutritional quality in microalgae, protein was compared with the protein found in vegetable crops. Humans consume macroalgae or seaweed most often as health food, pharmaceutical and animal feeds produced by using microalgae (Adams et al. 2009). Biogas (hydrogen and methane) for transportation can be produced from microalgae. In industry, domestic, and agriculture, microalgae can assimilate the N and P in effluent or wastewater and sequestrate CO2 derives from industrial flue gas (Clarens et al. 2011).
Operation condition for microalgae growth
Generally, abiotic and biotic factors affect microalgae growth. Abiotic factors comprise light, temperature, nutrients, O2 concentration, CO2 concentration, pH, and salinity (Kamarudin et al. 2015). Rapid algal growth can be indicated by the increase of pH (Moheimani 2005). Toxic chemicals influence the growth of microalgae (Yang et al. 2015). Meanwhile, the biotic factor consists of pathogens due to competition with other species of microalgae in cultivation systems (Mata et al. 2010). Operation factors during cultivation such as mixing and aeration go under biotic factors to homogenize microalgae cell distributions, heat and nutrient and to encourage the movement of gas in the culture media (Kamarudin et al. 2015). The optimum condition for microalgae cultivation is summarized in Table 4.
These optimum conditions can be used as basic instructions for microalgae cultivation. If these microalgae optimal conditions are applied in POME treatment, the organic compounds (N and P) available in POME that do not contain heavy metals and radioisotopes can be utilized.
Light intensity and cell density
The important metabolic process for microalgae growth is carried out by the primary energy source, which is light. Light intensity, quality of spectral wavelength, and photoperiod should be taken into account (Perumal et al. 2015). The source of light may be solar or artificial (fluorescent tube). Table 4 shows the optimum intensity. Photo inhibition and overheating may be caused by too high light intensity (Alabi et al. 2009). The most preferred light spectrum, which is blue or red, is suitable for photosynthesis. Turbidity in the culture that reduces photosynthesis will occur if the cell density is high (Chen et al. 2011). Kaewpintong (2004) reported that the increment of cell density and specific growth should be sensible with light intensity up to certain limit. Furthermore, the abundant solar light in Malaysia and other tropical countries is suitable for microalgae cultivation (Rajkumar et al. 2013).
Temperature
The optimal temperature for microalgae cultures may differ depending on the culture media and species. Temperatures of less than 16 °C will decrease microalgae growth, while high temperatures of more than 35 °C are harmful for various species (Perumal et al. 2015). High temperature will cause degradation and inactivation of enzymes involved in the photosynthetic process (Zuppini et al. 2007). Countries in the tropical regions like Malaysia do not have four seasons which are warm throughout the year. Solar radiation is abundant in Malaysia which is closed to equator and the daylight around 6 h per day. Rate of evaporation is affected by cloudiness and temperature. Cloudiness will result in less solar radiation and lower temperature (Singh et al. 2013). During hot days, overheating will occur in closed culture systems, and the temperature in reactors will reach 55 °C. Economical evaporative water cooling systems can be used to reduce the temperature to around 20–26 °C (Moheimani 2005).
Nutrients
Organic carbons (sugars, proteins, and fats), vitamins, ionic salts, and other nutrients (N and P) are essential for the growth of microalgae (Mata et al. 2010). The density of microalgae growth in cultures is greater than that in nature because the cultures are enriched with nutrients. Richmond (2008) describes various specific recipes for algal culture media. Macronutrients needed for microalgae are N, P (in an approximate ratio of 6:1), and Si. Diatoms microalgae need Si for development of protective shells (Perumal et al. 2015). Various trace metals and Vitamins B1, B12, and biotin are called micronutrients. Walne media and Guillard’s F/2 are suitable for the growth of most microalgae. Alternative enrichment media that are suitable for mass production of microalgae in large-scale extensive systems contain only the most essential nutrients and are composed of agriculture grade rather than laboratory grade fertilizers (Perumal et al. 2015).
pH
Failure to maintain the pH will completely break down the culture system. Since POME pH is acidic, there is a need to recheck the pH value after POME dilution for microalgae culture. Moreover, optimum pH can be achieved by using aeration in the culture. In high concentrations of microalgae cell in culture, the pH tends to increase up to pH 9, but the addition of CO2 solves this problem. The CO2 addition acts as a pH buffer in terms of CO2/HCO3 − balance (Perumal et al. 2015). Weissman and Goebel (1987) explained that the assimilation of CO2 into high pH water is accelerated by one of two main reaction paths (without a catalyst), which is direct reaction of CO2 with the hydroxyl ion, H+ to form bicarbonate, HCO3 −. This CO2/HCO3 − balance reaction occurs rapidly at pH values below 8 and above 10.
Mixing
Ambient air contains 0.03 % CO2 for photosynthesis. This condition will limit microalgae growth in culture. Concentrated CO2 may be supplied during cultivation (Alabi et al. 2009). Microalgae sediment can be prevented by mixing, which allows light, gas exchange or nutrients to be uniformly exposed and then can prevent thermal stratification, especially in outdoor cultivation (Kaewpintong 2004). Mixing can be done by stirring manually (test tubes, Erlenmeyer), aerating (bags, tanks), or using paddle wheels and jet pumps (ponds), depending on the culture system (Perumal et al. 2015). Turbulence mixing in certain limit large-scale microalgae productions is vital to promote cell circulation from dark to light regime (Barbosa 2003). Only certain microalgae species can endure rapid mixing (Perumal et al. 2015). The optimum mixing rate should be researched to prevent any decline in microalgae productivity (Barbosa 2003).
Cultivation systems for microalgae growth
Phototrophic cultivation is a condition in which light is used by microalgae as the only source of energy under photosynthesis, the chemical energy being converted (Brennan and Owende 2010). Microalgae can be grown in open pond systems or photobioreactors (PBRs) (Menetrez 2012). Algal cultivation units can be operated in batch or in continuous mode, and the production units can be open or closed systems. This depends on the microalgae species selected, expected environmental conditions, availability of nutrients, and even possibility of combining microalgae growth with a pollution control strategy of another industry, for example, for the removal of CO2 from flue gas emissions or removal of N and P from a wastewater effluent (Mata et al. 2010). Strains of microalgae that produce high lipid yields are usually cultivated in open pond systems (González-Fernández et al. 2011). PBRs are closed, controlled systems with equipment that provides an ideal environment for high productivity (González-Fernández et al. 2011).
Phototrophic cultivation in open system
Phototrophic cultivation in open ponds has been used since the 1950s and can be categorized as stagnant water (lagoons, lakes, and small ponds) and tanks or containers. Shallow circular ponds and tanks (Fig. 1) or parallel raceway ponds (PRPs) are also open systems (González-Fernández et al. 2011).
Plan view of a raceway open pond adapted from Chisti (2007)
The main problem of open systems is contamination by other microorganisms (González-Fernández et al. 2011). The nature of open ponds and their weakness in handling contamination have restricted cultivation of Spirulina sp., Dunaliella sp., and Chlorella sp. as reported by Huntley and Redalje (2007). Dealing with contamination involves genetic modification of microalgae (Spolaore et al. 2006). Processes using genetically modified microalgae are currently being developed by many biofuel industry participants (Raja et al. 2008).
Phototrophic cultivation in closed/photobioreactors system
Many studies have attempted to increase microalgal biomass production rates by developing closed photobioreactors (PBRs) system (Chisti 2007). Indeed, this system may cost ten times more than open systems (Mata et al. 2010). Table 5 summarizes the advantages and disadvantages of both systems. It seems that open systems are more suitable for POME treatment. The advantage of microalgae approach in POME treatment is readily available facilities; for example, ponds and tanks could reduce the cost of coupling microalgae treatment with conventional treatment (Kamarudin et al. 2015).
PBRs can facilitate good control for culture environment and meet the optimal growth requirements like the concentration of carbon dioxide, temperature, light intensity, mixing, density of culture, and pH (Mussgnug et al. 2007). The requirements for specific growth are internally maintained in a closed system. The PBRs illustration is seen in Fig. 2.
Basic design of a horizontal tubular PBRs adapted from Becker (1994)
High density cultures of microalgae contained within bioreactors may limit light intensity (Mussgnug et al. 2007). The exposed 7.6–10 cm of light penetration because of high turbidity is caused by microalgae growth and culture media (Mussgnug et al. 2007). The helical tubular shape PBRs designed by Briassoulis et al. (2010) have been applied to limit growth optimization for nonstop production. The following are conditions optimizations in this PBRs design:
-
the size of volume to surface area ratio;
-
inhibition for temperature fluctuation and contamination;
-
spatial distribution of fresh ambient air and CO2;
-
CO2 exchange rate; and
-
automated flow through sensors.
Generally, PRPs (open pond) are cheaper than PBRs because of low costs for construction materials and operation, but low biomass productivity will result (Hu et al. 2008). PBRs are expensive, but they can produce more microalgae biomass for lipid contents (40–55 %) (Chisti 2007). The production of biodiesel from microalgae in an open system costs around US $9–25 per gallon, whereas in a closed system, it will cost US $15–40 per gallon (Amaro et al. 2011). The pros and cons of both systems associated with a promised hybrid culture system made as the best practice (Hu et al. 2008). Since POME utilization using microalgae is still in the research phase and not commercialized, there is a need to study the potential of microalgae selection, type of treatment reactor, bioprocess optimization, carbon dioxide sequestration by microalgae, and potential of microalgae biomass recovery as a biofuel resource.
Heterotrophic cultivation
Heterotrophic cultivation is a condition in which organic carbon sources are used by microalgae as carbon and energy sources in dark conditions (Chojnacka and Marquez-Rocha 2004). Limited light in high density microalgae usually occurs in PBRs under phototrophic cultivation, which can be solved under heterotrophic cultivation (Huang et al. 2010). High microalgae biomass and high lipid content (40 % increase) under heterotrophic cultivation using Chlorella protothecoides was investigated by Xu et al. (2006). Heterotrophic microalgae can utilize various organic carbon sources (i.e., glucose, acetate, glycerol, fructose, sucrose, lactose, galactose, and mannose) for growth (Liang et al. 2009). However, organic carbon sources used in heterotrophic cultivation face the contamination problem (Ogbonna et al. 1997).
Mixotrophic cultivation
The microalgae that can grow in mixotrophic cultivation uses light as the main source of energy for photosynthesis. Essential energy sources from organic carbon and CO2 are necessary for microalgae. The organisms can live either autotrophically or heterotrophically (Graham and Wilcox 2000). This ability is called amphitrophy (subtype of mixotrophy), and it depends on the availability of either organic carbon or light (Chojnacka and Marquez-Rocha 2004). Compared with phototrophic and heterotrophic cultivations, mixotrophic cultivation is rarely used in microalgae lipid production because only a few microalgae species are mixotrophic (Liang et al. 2009).
Photoheterotrophic cultivation
To use organic carbon source to produce energy, a light source is required for photoheterotrophic cultivation (Mata et al. 2010). This mechanism is known as photoorganitrophy, photo assimilation, or photo metabolism. The photoheterotrophic and mixotrophic metabolisms have no differences according to different sources of carbon used to produce energy for growth and specific metabolite production. This type of cultivation requires organic carbon like sugar and light simultaneously (Chojnacka and Marquez-Rocha 2004). Although the production of some light regulated useful metabolites can be enhanced using photoheterotrophic cultivation, this approach to producing biofuel is very rarely used, as in the case with mixotrophic cultivation (Ogbonna et al. 2002).
POME as alternative media for microalgae cultivation
Microalgae cultivation in POME offers an alternative to conventional forms of tertiary wastewater treatments and spontaneously utilizes organic compounds in POME to generate microalgae biomass for lipid production (Lam and Lee 2011). The next section will review the current microalgae growth infrastructure and the potential of using POME as alternative media for microalgae biomass production.
POME concentration for microalgae growth and infrastructure
Various environmental and operational factors affect microalgae growth to make cultivation fruitful. The natural effluent discarded from POME might be colloidal, dark, and viscous, which should be considered prior to media preparation for microalgae culture. Microalgae cannot tolerate the high ammonium concentration in POME (Bello et al. 2013). Vairappan and Yen (2008) found that for the marine Isochrysis sp., the concentration of POME at 5 % dilution is the best concentration for culture media, due to properties of POME shown in Table 1. This dilution procedure will enhance light penetration into media (Olguín et al. 2003).
Table 6 shows that limited growth infrastructures were done on palm oil mill effluent, whereas Table 7 shows recent and evolved growth of infrastructures using various industrial wastewaters. Microalgae biomass productivity and biomass composition will be affected by these factors (Markou and Georgakakis 2011).
Most of the microalgae species used in POME treatment as shown in Table 6 were Chlorella sp., Spirulina sp., and Arthrospira sp., which seems limited in the literature compared to other microalgae species. Most studies use PBRs systems and focus on lab scale microalgae cultivation for POME, which could be a challenge in real POME treatment aimed at commercialization.
Various types of industrial wastewater used in the studies mentioned in Table 7 can successfully produce different types of microalgae. However, most of the studies were done on a laboratory scale. Osundeko and Pittman (2014) conducted an experiment in an open system using a 150-L tank (depth 10 cm) without artificial lighting and a flow rate around 2000 L h−1 which are possible if applied in POME treatment.
Microalgae performance cultivate in POME
The concentrated nutrients (i.e., C, N, P, carbohydrates, lipids, proteins, and minerals) in POME are highly applied in biotechnology studies (Kamyab et al. 2014). As mentioned earlier, the concentration range of POME in different receiving water bodies may have a high impact on the aquatic environments if the discharge exceeds the limit of standards of the Malaysia Environmental Quality Act. Various species of microalgae present in freshwater, seawater, or brackish water make it possible to grow them in large scale reactors on unfertile lands. Hence, microalgae cultivation would not compete with other food crops (Brennan and Owende 2010). The use of microalgae in the utilization or remediation of excess nutrients and CO2 present in natural water resources, lagoons, and ponds is phycoremediation (Olguí 2003). The biological treatment was introduced about 40 years ago in places that usually used tertiary wastewater treatment (Rawat et al. 2011). Previously, it was found that microalgae could be cultivated very well in metal concentrated water environment (Parameswari et al. 2010).
The harvested microalgae can be further processed for various applications, including biofuel production and energy production. Microalgae can be extracted for lipid, and this lipid can be used as biofuel (Pokoo-Aikins et al. 2010). Many autotrophic microalgae such as Chlorella vulgaris, Isochrysis galbana, Botryococcus braunii, Navicula pell iculosa, Scenedsmus acutus, Crypthecodinium cohnii, Dunaliella primolecta, Monallanthus salina, Neochloris oleoabundans, Phaeodactylum tricornutum, Chlamydomonas reinhardtii, and Tetraselmis sueica can produce lipid (Picardo et al. 2013). Species of microalgae used for biofuel production must meet these criteria:
-
high microalgae specific growth rate;
-
high lipid content;
-
can survive in stressful environments caused by temperature, nutrient input, light intensity and contamination from bacteria;
-
easy harvesting process;
-
further lipid extraction method; and
-
potential to be applied in food formulation, cosmetics, and pharmaceutical production.
The specific growth rate (μ) and biomass productivity for microalgae adapted from Converti et al. (2009) are calculated by Eq. (1).
where X m is the concentration of biomass at the end of the batch run, X 0 is the concentration of biomass at the beginning of the batch run, and t is the duration of the batch run (h, d). In addition, lipid productivity is achieved through Eq. 2:
where C l is the concentration of lipids at the end of the batch run and t is the duration of the run (h, d).
The production process consists of four stages: microalgae cultivation, dry weight biomass (DWB) separation, lipid extraction, and transesterification. The performance of many microalgae cultivated in POME is reported in works cited in Table 8.
Kamyab et al. (2014) focussed on nutrients reduction in POME, lipid production, and microalgae growth. Other researchers were not focussed on nutrients reduction. Nutrients reduction in POME is important to relate with microalgae growth. However, excess nutrients also can inhibit microalgae growth, and not all nutrients can be utilized by microalgae. Then excess nutrients will be converted to toxic compounds that can inhibit microalgae growth (Munn et al. 1989). Optimum POME concentration mixed with synthetic nutrient for Spirulina sp. cultivation was done by Azimatun and Hadiyanto (2014) to obtain optimum microalgae biomass.
Conclusion
In this review, integration of POME with microalgae cultivation has provided a sustainable way to reduce pollutants in wastewater or final effluents from POME. There is a need to study this potential to contribute to alternative culture medium for microalgae growth. The open system is relevant for pilot scale POME treatment using high lipid content microalgae such as Botryococcus braunii, Nannocloropsis sp., and Chlamydomonas sp. Various aspects need to be studied for microalgae cultivation in POME, especially to control contamination. Moreover, the location of Malaysia on the Earth’s equator makes microalgae cultivation potentially productive. Other aspects that should be considered include the concentration of POME used as media, isolation/selection of microalgae strains, reactor/system selection and optimization of growth operating conditions for microalgae biomass to be extracted for lipids as an alternative biofuel resource.
References
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. doi:10.1016/j.sjbs.2012.04.005
Adams JM, Gallagher JA, Donnison IS (2009) Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol 21:569–574
Ahmad AL, Ismail S, Bhatia S (2003) Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 157:87–95
Ahmad AD, Salihon J, Tao DG (2015) Evaluation of CO2 Sequestration by microalgae culture in palm oil mill effluent (POME) medium. In: Advanced materials research. Trans Tech Publ, pp 311–316
Alabi AO, Bibeau E, Tampier M, Council BCI (2009) Microalgae technologies & processes for biofuels–bioenergy production in British Columbia: current technology, suitability & barriers to implementation. The British Columbia Innovation Council, University of Manitoba, Manitoba
Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energy 88:3402–3410
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Avagyan AB (2011) Water global recourse management through the use of microalgae addressed to sustainable development. Clean Technol Environ Policy 13:431–445
Azimatun NMM, Hadiyanto GD (2014) Evaluation of carbon, nitrogen and phosphorus ratio of palm oil mill effluent digested (POMED) wastewater as replacement synthetic medium for Spirulina sp. growth. Am-Eurasian J Agric & Environ Sci 14:536–540
Barber J (2009) Photosynthetic energy conversion: natural and artificial. Chem Soc Rev 38:185–196
Barbosa MJGV (2003) Microalgal photobioreactors: scale-up and optimisation. Wageningen University, Wageningen
Becker EW (1994) Microalgae: biotechnology and microbiology, vol 10. Cambridge University Press, Melbourne
Becker E (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Bello M, Nourouzi M, Abdullah LC, Choong TS, Koay Y, Keshani S (2013) POME is treated for removal of color from biologically treated POME in fixed bed column: applying wavelet neural network (WNN). J Hazard Mater 262:106–113
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Briassoulis D, Panagakis P, Chionidis M, Tzenos D, Lalos A, Tsinos C (2010) An experimental helical-tubular photobioreactor for continuous production of Nannochloropsis sp. Bioresour Technol 101:6768–6777
Chen C-Y, Yeh K-L, Aisyah R, Lee D-J, Chang J-S (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Cheng J, Huang R, Yu T, Li T, Zhou J, Cen K (2014) Biodiesel production from lipids in wet microalgae with microwave irradiation and bio-crude production from algal residue through hydrothermal liquefaction. Bioresour Technol 151:415–418
Chinnasamy S, Bhatnagar A, Hunt RW, Das K (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101:3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cho S, Luong TT, Lee D, Oh Y-K, Lee T (2011) Reuse of effluent water from a municipal wastewater treatment plant in microalgae cultivation for biofuel production. Bioresour Technol 102:8639–8645
Chojnacka K, Marquez-Rocha F-J (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
Clarens AF, Nassau H, Resurreccion EP, White MA, Colosi LM (2011) Environmental impacts of algae-derived biodiesel and bioelectricity for transportation. Environ Sci Technol 45:7554–7560
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151
Coppola F, Simoncini E, Pulselli R, Brebbia C, Tiezzi E (2009) Bioethanol potentials from marine residual biomass: an emergy evaluation. In: Brebbia C, Tiezzi E (eds) 7th international conference on ecosystems and sustainable development, 08–10 Jul 2009. WIT transactions on ecology and the environment. Ecosystems and sustainable development VII, Chianciano Terme, pp 379–387
Damayanti A, Ujang Z, Salim M, Olsson G, Sulaiman A (2010) Respirometric analysis of activated sludge models from palm oil mill effluent. Bioresour Technol 101:144–149
de Godos I, Blanco S, García-Encina PA, Becares E, Muñoz R (2009) Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour Technol 100:4332–4339
Ding GT, Yaakob Z, Takriff MS, Salihon J, Rahaman MSA (2016) Biomass production and nutrients removal by a newly-isolated microalgal strain Chlamydomonas sp in palm oil mill effluent (POME) International Journal of Hydrogen Energy
Du Z, Mohr M, Ma X, Cheng Y, Lin X, Liu Y (2012) Hydrothermal pretreatment of microalgae for production of pyrolytic bio-oil with a low nitrogen content. Bioresour Technol 120:13–18
Gobi K, Vadivelu V (2013) By-products of palm oil mill effluent treatment plant—a step towards sustainability. Renew Sustain Energy Rev 28:788–803
Gobi K, Mashitah M, Vadivelu V (2011) Development and utilization of aerobic granules for the palm oil mill (POM) wastewater treatment. Chem Eng J 174:213–220
González-Fernández C, Molinuevo-Salces B, García-González MC (2011) Nitrogen transformations under different conditions in open ponds by means of microalgae–bacteria consortium treating pig slurry. Bioresour Technol 102:960–966
Gouveia L (2011) Microalgae as a feedstock for biofuels. Springer, New York
Graham L, Wilcox L (2000) Algae. Prentice Hall, Upper Saddler River
Graham LE, Graham JM, Wilcox LW (2009) Algae. Benjamin Cummings, University of California, San Francisco
Guo Z, Liu Y, Guo H, Yan S, Mu J (2013) Microalgae cultivation using an aquaculture wastewater as growth medium for biomass and biofuel production. J Environ Sci 25:S85–S88
Hadiyanto M, Hartanto G (2012) Enhancement of biomass production from Spirulina sp. cultivated in POME medium. In: Proceedings of the international conference on chemical and material engineering, pp 1–6
Hadiyanto H, Nur MMA, Hartanto GD (2012) Cultivation of Chlorella sp. as biofuel sources in palm oil mill effluent (POME). Int J Renew Energy Dev (IJRED) 1:45–49
Han F, Huang J, Li Y, Wang W, Wang J, Fan J, Shen G (2012) Enhancement of microalgal biomass and lipid productivities by a model of photoautotrophic culture with heterotrophic cells as seed. Bioresour Technol 118:431–437
Hernández D, Riaño B, Coca M, Solana M, Bertucco A, García-González M (2016) Microalgae cultivation in high rate algal ponds using slaughterhouse wastewater for biofuel applications. Chem Eng J 285:449–458
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huang GH, Chen G, Chen F (2009) Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenergy 33:1386–1392
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Huntley ME, Redalje DG (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strat Glob Change 12:573–608
Ibrahim RI, Wong Z, Mohammad A (2015) Optimization and performance evaluation for nutrient removal from palm oil mill effluent wastewater using microalgaze. In: IOP conference series: materials science and engineering, vol 1. IOP Publishing, p 012006
Jena U, Das K (2011) Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 25:5472–5482
Kaewpintong K (2004) Cultivation of Haematococcus pluvialis in airlift bioreactor. Chulalongkorn University, Chulalongkorn
Kamarudin KF, Tao DG, Yaakob Z, Takriff MS, Rahaman MSA, Salihon J (2015) A review on wastewater treatment and microalgal by-product production with a prospect of palm oil mill effluent (POME) utilization for algae. Der Pharma Chemica 7:73–89
Kamyab H, Md Din MF, Lee CT, Keyvanfar A, Shafaghat A, Majid MZA (2014) Lipid production by microalgae Chlorella pyrenoidosa cultivated in palm oil mill effluent (POME) using hybrid photo bioreactor (HPBR). Desalination and water treatment:1-13
Kamyab H, Din MFM, Keyvanfar A, Majid MZA, Talaiekhozani A, Shafaghat A (2015) Efficiency of microalgae chlamydomonas on the removal of pollutants from palm oil mill effluent (POME). Energy Procedia 75:2400–2408
Kebelmann K, Hornung A, Karsten U, Griffiths G (2013) Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components. Biomass Bioenergy 49:38–48
Kim J, Lingaraju BP, Rheaume R, Lee J-Y, Siddiqui KF (2010) Removal of ammonia from wastewater effluent by Chlorella vulgaris. Tsinghua Sci Technol 15:391–396
Lam MK, Lee KT (2011) Renewable and sustainable bioenergies production from palm oil mill effluent (POME): win–win strategies toward better environmental protection. Biotechnol Adv 29:124–141
Laurens LM, Wolfrum EJ (2011) Feasibility of spectroscopic characterization of algal lipids: chemometric correlation of NIR and FTIR spectra with exogenous lipids in algal biomass. BioEnergy Res 4:22–35
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liu J, Huang J, Sun Z, Zhong Y, Jiang Y, Chen F (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresour Technol 102:106–110
Malaysian Palm Oil Board (2012) Malaysian oil palm statistics, 2011. Malaysian Palm Oil Board (MPOB), Malaysia
Malaysian Palm Oil Board (2014) Malaysian oil palm statistics, 2013. Malaysian Palm Oil Board (MPOB), Malaysia
Mansour MP, Frampton DM, Nichols PD, Volkman JK, Blackburn SI (2005) Lipid and fatty acid yield of nine stationary-phase microalgae: applications and unusual C24–C28 polyunsaturated fatty acids. J Appl Phycol 17:287–300
Markou G, Georgakakis D (2011) Cultivation of filamentous cyanobacteria (blue–green algae) in agro-industrial wastes and wastewaters: a review. Appl Energy 88:3389–3401
MarouĹĄek J, Kawamitsu Y, Ueno M, Kondo Y, Kolar L (2012) Methods for improving methane yield from rye straw. Appl Eng Agric 28:747–755
Maroušek J, Hašková S, Maroušková A, Myšková K, Vaníčková R, Váchal J (2015a) Financial and biotechnological assessment of new oil extraction technology. Energy Sources 37:1723–1728
Maroušek J, Hašková S, Zeman R, Váchal J, Vaníčková R (2015b) Processing of residues from biogas plants for energy purposes. Clean Technol Environ Policy 17:797–801
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Menetrez MY (2012) An overview of algae biofuel production and potential environmental impact. Environ Sci Technol 46:7073–7085
Moheimani NR (2005) The culture of coccolithophorid algae for carbon dioxide bioremediation. Murdoch University, Perth
Munn M, Osborne L, Wiley M (1989) Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia 174:89–97
Mussgnug JH, Thomas-Hall S, Rupprecht J, Foo A, Klassen V, McDowall A (2007) Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol J 5:802–814
Ogbonna JC, Masui H, Tanaka H (1997) Sequential heterotrophic/autotrophic cultivation—an efficient method of producing Chlorella biomass for health food and animal feed. J Appl Phycol 9:359–366
Ogbonna JC, Ichige E, Tanaka H (2002) Regulating the ratio of photoautotrophic to heterotrophic metabolic activities in photoheterotrophic culture of Euglena gracilis and its application to α-tocopherol production. Biotechnol Lett 24:953–958
Olguí EJ (2003) Phycoremediation: key issues for cost-effective nutrient removal processes. Biotechnol Adv 22:81–91
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15:249–257
Osundeko O, Pittman JK (2014) Implications of sludge liquor addition for wastewater-based open pond cultivation of microalgae for biofuel generation and pollutant remediation. Bioresour Technol 152:355–363
Parameswari E, Lakshmanan A, Thilagavathi T (2010) Phycoremediation of heavy metals in polluted water bodies. Electron J Environ Agric Food Chem 9:808–814
Park S, Kim J, Yoon Y, Park Y, Lee T (2015) Blending water-and nutrient-source wastewaters for cost-effective cultivation of high lipid content microalgal species Micractinium inermum NLP-F014. Bioresour Technol 198:388–394
Parthasarathy S, Gomes RL, Manickam S (2016) Process intensification of anaerobically digested palm oil mill effluent (AAD-POME) treatment using combined chitosan coagulation, hydrogen peroxide (H2O2) and Fenton’s oxidation. Clean Technol Environ Policy 18:219–230
Perumal S, Thirunavukkarasu A, Pachiappan P (2015) Advances in marine and brackishwater aquaculture. Springer, India
Picardo MC, de Medeiros JL, Monteiro JGM, Chaloub RM, Giordano M, Araújo OdF (2013) A methodology for screening of microalgae as a decision making tool for energy and green chemical process applications. Clean Technol Environ Policy 15:275–291
Pokoo-Aikins G, Nadim A, El-Halwagi MM, Mahalec V (2010) Design and analysis of biodiesel production from algae grown through carbon sequestration. Clean Technol Environ Policy 12:239–254
Ponraj M, Din MFM (2013) Effect of light/dark cycle on biomass and lipid productivity by Chlorella pyrenoidosa using palm oil mill effluent (POME). J Sci Ind Res 72:703–706
Pratoomyot J, Srivilas P, Noiraksar T (2005) Fatty acids composition of 10 microalgal species. Songklanakarin J Sci Technol 27:1179–1187
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Putri EV, Din MFM, Ahmed Z, Jamaluddin H, Chelliapan S (2011) Investigation of microalgae for high lipid content using palm oil mill effluent (Pome) as carbon source. In: International conference on environment and industrial innovation, IPCBEE
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34:77–88
Rajkumar R, Yaakob Z, Takriff MS (2013) Potential of micro and macro algae for biofuel production: a brief review. Bioresources 9:1606–1633
Rawat I, Kumar RR, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88:3411–3424
Ren H-Y, Liu B-F, Kong F, Zhao L, Ren N (2015) Hydrogen and lipid production from starch wastewater by co-culture of anaerobic sludge and oleaginous microalgae with simultaneous COD, nitrogen and phosphorus removal. Water Res 85:404–412
Richmond A (2008) Handbook of microalgal culture: biotechnology and applied phycology. John Wiley & Sons, Cornwall
Rizkytata BT, Gumelar MT, Abdullah TH (2014) Industrial tofu wastewater as a cultivation medium of microalgae Chlorella vulgaris. Energy Procedia 47:56–61
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43
Shen Y, Pei Z, Yuan W, Mao E (2009) Effect of nitrogen and extraction method on algae lipid yield. Int J Agric Biol Eng 2:51–57
Shuping Z, Yulong W, Mingde Y, Chun L, Junmao T (2010) Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour Technol 101:359–365
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27:409–416
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Singh GKS, Kuppan P, Goto M, Sugiura N, Noor MJMM, Ujang Z (2013) Physical water quality and algal density for remediation of algal blooms in tropical shallow eutrophic reservoir. J Novel Carbon Resour Sci 7:33–41
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Sukumaran P, Nulit R, Zulkifly S, Halimoon N, Omar H, Ismail A (2014) Potential of fresh POME as a growth medium in mass production of Arthrospira platensis. Int J Curr Microbiol App Sci 3:235–250
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
Tong S, Jaafar AB (2004) Waste to energy: methane recovery from anaerobic digestion of palm oil mill effluent. Energy Smart 4:1–8
Vairappan CS, Yen AM (2008) Palm oil mill effluent (POME) cultured marine microalgae as supplementary diet for rotifer culture. J Appl Phycol 20:603–608
Wagner J et al (2016) Co-production of bio-oil and propylene through the hydrothermal liquefaction of polyhydroxybutyrate producing cyanobacteria. Bioresour Technol 207:166–174
Wang R, Peng B, Huang K (2015) The research progress of CO2 sequestration by algal bio-fertilizer in China. J CO2 Util 11:67–70. doi:10.1016/j.jcou.2015.01.007
Weissman JC, Goebel R (1987) Design and analysis of microalgal open pond systems for the purpose of producing fuels: a subcontract report. Solar Energy Res Inst, Golden
Wong YS, Kadir MOA, Teng TT (2009) Biological kinetics evaluation of anaerobic stabilization pond treatment of palm oil mill effluent. Bioresour Technol 100:4969–4975
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Xu L, Brilman DWW, Withag JA, Brem G, Kersten S (2011) Assessment of a dry and a wet route for the production of biofuels from microalgae: energy balance analysis. Bioresour Technol 102:5113–5122
Yacob S, Hassan MA, Shirai Y, Wakisaka M, Subash S (2006) Baseline study of methane emission from anaerobic ponds of palm oil mill effluent treatment. Sci Total Environ 366:187–196
Yacob S, Shirai Y, Ali Hassan M (2009) Treatment of palm oil wastewaters. Waste treatment in the food processing industry. CRC Press, Boca Raton
Yadavalli R, Rao C, Rao RS, Potumarthi R (2014) Dairy effluent treatment and lipids production by Chlorella pyrenoidosa and Euglena gracilis: study on open and closed systems. Asia-Pac J Chem Eng 9:368–373
Yang J, Cao J, Xing G, Yuan H (2015) Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour Technol 175:537–544
Zhang Y, Li Y, Xiangli Q, Lina C, Xiangjun N, Zhijian M, Zhang Z (2008) Integration of biological method and membrane technology in treating palm oil mill effluent. J Environ Sci 20:558–564
Zuppini A, Andreoli C, Baldan B (2007) Heat stress: an inducer of programmed cell death in Chlorella saccharophila. Plant Cell Physiol 48:1000–1009
Acknowledgments
The authors would like to acknowledge the funding provided for this work by Universiti Teknologi Malaysia (UTM) under research Grant R.J130000.7809.4F618.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Resdi, R., Lim, J.S., Kamyab, H. et al. Review of microalgae growth in palm oil mill effluent for lipid production. Clean Techn Environ Policy 18, 2347–2361 (2016). https://doi.org/10.1007/s10098-016-1204-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1204-1