Abstract
The development of human immunodeficiency virus type 1 (HIV)-associated neurocognitive disorder (HAND) involves the adaptation of viral sequences coding for the V3 loop of the env protein. The plasma and cerebrospinal fluid (CSF) may contain viral populations from various cellular sources and with differing pathogenicity. Combination antiretroviral therapy (cART) may alter the relative abundance of these viral populations, leading to a genetic shift. We characterized plasma and CNS viral populations prior to and during cART and relate the findings to viral elimination kinetics and the clinical phenotype. Longitudinal plasma and CSF samples of five chronically infected HIV patients, four of whom had HAND, and one seroconverter were analyzed for V3 sequences by RT-PCR and sequence analysis. In the chronically infected patients, pre-cART plasma and CSF viral sequences were different irrespective of viral elimination kinetics and clinical phenotype. cART induced replacement of plasma viral populations in all subjects. CSF viral populations underwent a clear genetic shift in some patients but remained stable in others. This was not dependent on the presence of HAND. The genetic shift of CSF V3 sequences was absent in the two subjects whose CSF viral load initially increased during cART. In one patient, pre- and post-treatment CSF sequences were closely related to the post-treatment plasma sequences, suggesting a common cellular source. We found heterogeneous patterns of genetic compartmentalization and genetic shift over time. Although these did not closely match viral elimination kinetics and clinical phenotype, the results imply different patterns of the dynamics and relative contribution of compartment-specific virus populations in chronic HIV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV-1 invariably infects the human brain, but only a subset of infected patients develop HIV-associated neurocognitive disorders (HAND). One possible reason for this is the development of neurovirulent viral strains adapted for infection of cells in the central nervous system (CNS). In principle, combination antiretroviral therapy (cART) blocks the viral life cycle by various means, all of which obstruct the infection of hitherto uninfected cells. Those cells already infected with HIV-1 can, however, continue to produce the virus. As a result, the decay of viral RNA reflects the life span of virus-producing cells. In systemic HIV-1 infection, cART leads to a decay of plasma virus characterized by a rapid first phase and a slower second phase (Perelson et al 1997). The first-phase decay is determined by the elimination of free virus and high-level virus-producing cells, while the second phase decay mainly reflects the elimination of long-lived populations of virus-producing cells such as macrophages. While in most patients the elimination kinetics between the cerebrospinal fluid (CSF) and plasma are comparable (Eggers et al 1999; Harrington et al 2005; Staprans et al 1999), in some individuals viral RNA decays much slower in the CSF (Eggers et al 2003; Pialoux et al 1997). We have previously showed that slow RNA decay is associated with HAND (Eggers et al 2003).
It is now generally assumed that, in the case of rapid virus elimination from the CSF, the virus is produced by shorter-lived lymphocytes (Harrington et al 2005) continuously migrating from the peripheral blood to the CNS: the so-called transitory infection (Staprans et al 1999). The autonomous infection, in contrast, reflects the release of CSF virions from long-lived cells leading to slow CSF virus elimination. Therefore, it is likely that CSF and blood carry virus populations originating from at least two cell types. Thus, depending on the relative abundance of variants within these mixed viral populations, elimination kinetics in a given compartment may be fast or slow. Between compartments (e.g., plasma and CSF) this may result in concordant or discordant elimination kinetics. As the cellular tropism of HIV-1 is governed by the V3 loop of gp120 (Hoffman et al 2002; Korber et al 1995), sequencing the env gene encoding for gp120 might reveal similarities or discrepancies within and across compartments.
Many authors sequenced the V3 loop to look for phylogenetic differences of virus from different sources such as brain, CSF and blood (Epstein et al 1991; Keys et al 1993; Korber et al 1994; Steuler et al 1992; Strain et al 2005; Wong et al 1997). The results, however, were inconclusive, as similarities as well as segregation of blood and CSF variants were found. In general, genetic differences across compartments seemed to be more prevalent in subjects with HAND and those with longer duration of HIV-1 infection (Coffin 1995; Ritola et al 2005; Strain et al 2005). Most studies on V3 sequences in the CNS were done before the era of cART or prior to the start of cART in the individual subjects, and these studies were cross-sectional. Therefore, env sequences from lymphocytes causing the transitory infection of brain might have overshadowed possible minority sequences produced by macrophages, thereby obscuring the genetic differences across compartments. Combination ART allows for the study of “pure” populations of virus variants as it eliminates virus produced from short-lived cells while, in the short term, sparing variants produced by long-lived cells.
The current study was undertaken with the widely accepted assumption that the viral elimination kinetics reflect different virus-producing cell types. By starting patients on cART we aimed at separating (in a time-dependent fashion) different viral populations in longitudinal samples of both plasma and CSF. Viral V3 sequences were then determined in these populations and the findings related to the viral elimination characteristics during cART and the clinical phenotype in patients with and without HAND.
We hypothesized that in subjects with slow CSF viral decay (discordant to the plasma), the V3 sequences from before and during cART would remain similar in the CSF while, in the plasma, a genetic shift towards the CSF sequences would be observed.
Methods
Clinical procedures
Lumbar puncture (LP) was performed prior to initiation of therapy and at variable intervals thereafter, with one follow-up puncture during cART. Patients underwent LP for the evaluation of neurological manifestations of HIV infection or as part of an observational study for CNS manifestations of HIV infection approved by the local ethics committee. All subjects declared their informed consent. Peripheral blood samples were obtained in parallel with LP. HAND was quantified by the Memorial Sloan–Kettering (MSK) scale (Price and Brew 1988)
Viral resistance
Sensitivity against antiviral compounds was determined by commercial genotypic and phenotypic tests, as described previously (Hertogs et al 1998). In brief, the genes encoding the reverse transcriptase (RT) and protease (PR) were sequenced from patient material and, for the phenotypic assay, recombined with a defined RT–RT-deleted proviral clone for in vitro susceptibility testing.
Viral load
RNA abundance in plasma and CSF was determined using the Roche Amplicor HIV Monitor quantitative PCR assay (Roche Molecular Systems, Mannheim, Germany) with a lower limit of detection of 20–50 HIV RNA copies/ml according to the manufacturer’s instructions. CSF samples with a red cell count >20/μl were excluded from the analysis.
Sequence analysis
The plasma and CSF samples taken for sequence analysis were from before treatment and the first sample during treatment (Table 1, Fig. 2). Viral RNA was extracted, transcribed and amplified by one step RT-PCR (Roche) using specific primers for env spanning the V3 loop and flanking regions: Sense-primer: 5′-CACAGTACAATG TACACATGGAAT-3′, 6952-6975 outer-antisense: 5′-AGAAAAATTCCCCTCCACA ATTAA-3′ 7373-7350, followed by a hemi-nested PCR with inner-antisense primer: 5′-CAA TTTCTGGGTCCCCTCCTGAGG-3, 7337–7314 (numbering according to HXB2). The PCR products were cloned into pGEM-T-easy (Promega), and a mean of 21 clones were sequenced for each of the patient samples.
Sequence alignment
Sequences were compared to GenBank by HIV-Blast. Sequences were aligned with DNAStar and Clustal X (Thompson et al 1997) from nucleotide 6952 through 7337. All trees were constructed with the MEGA package (Kumar et al 2001). First, an unrooted tree for all sequences of all patients was calculated by the neighbor-joining method (Clustal X) in order to exclude contamination with lab strains and sample mix-ups within patients. Pairwise distances were calculated with the Kimura 2-parameter model. Rooted trees were constructed with a bootstrap resampling (500 replications) to analyze the robustness of the tree.
Results
Characterization of HAND patients with slow viral load response to cART
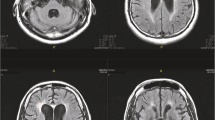
To prove or disprove our hypothesis, we chose four subjects, SB, SP, PI, and TE among patients diagnosed with HAND at our institution, on the basis of the following requirements: (1) naïvety to antiviral medication, (2) a viral load sufficiently high to allow for amplification of genetic material in paired CSF and serum samples from before and during cART, (3) a slow initial viral load response to cART in the CSF (discordant to the plasma compartment), (4) no resistance mutations in the CSF virus, (5) sufficient clinical follow-up to assess possible improvement of HAND under cART. For comparison, two patients with fast initial viral load response to ART in the CSF (concordant to the plasma compartment) without signs of HAND were selected. Patient VC had primary HIV infection with meningo-radiculitis; patient WH had advanced HIV infection and had previously been successfully treated for cryptococcal meningitis with elimination of fungi and improvement of CSF pleocytosis. All six patients were part of a previously published study on the viral elimination kinetics (Eggers et al 2003), and their characteristics are presented in Table 1. Age ranged from 34 to 59 with a mean of 45 years. Three patients were male, three female. All patients with slow initial CSF viral load response and discordant CSF/plasma viral load kinetics were neurologically symptomatic with HAND. Prior to their HAND diagnosis, patients SB, SP, PI, and TE had been in CDC stage B3. Among the patients with fast initial CSF viral load response patient VC was in stage CDC A1 and patient WH in stage C3. In the four patients with initial slow CSF viral load decay (SB, SP, PI, and TE) magnetic resonance imaging (MRI) of the brain was performed to exclude alternative diagnoses of their HAND. They all had different degrees of patchy and sometimes confluent hyperintensities in T2-weighted and FLAIR images in their white matter.
All six patients received three to five antiviral compounds: two nucleoside analogues plus a non-nucleoside reverse transcriptase inhibitor, a protease inhibitor, or both. The CNS penetration effectiveness (CPE) score of the HAND patients regimes varied between 5 and 11 and both controls had a CPE score of 10. The CPE score indicates the likelihood of effective suppression of viral replication in the CNS (Letendre et al 2008). Antiviral treatment remained unchanged during the observation period.
Patient V3 loop sequences are distinct
An unrooted tree was composed of the V3 sequences of all samples of all patients (Fig. 1). This showed readily distinguishable and genetically distant clusters that corresponded to the studied patients and differed from any of the laboratory strains present in the laboratory of the investigators, thereby suggesting patient-specific development of the HIV-1 quasispecies.
Patient V3 loop sequences are distinct. Unrooted tree composed of the V3 sequences of all samples of all patients showing readily distinguishable and genetically distant clusters that correspond to the studied patients and differed from any laboratory strain. The scale bar shows 0.01 sequence divergence
Kinetics of the viral load in plasma and CSF on initiation of cART
After introduction of cART, the initial decrease of viral load in the plasma was rapid in all subjects, and this decrease became slower in the subsequent viral load determinations during cART In contrast, in the four patients with HAND, the initial CSF viral load decrease was either much slower than in the plasma (subjects PI and SP) or the CSF viral load even increased during the first few weeks of treatment (subjects TE and SB) (Fig. 2a). CSF virus decreased rapidly, however, in the two non-HAND patients WH and VC (Fig. 2b). After the first on-cART viral load determination (i.e., several weeks after initiation of cART) the decay kinetics in the two compartments approached each other.
Kinetics of the viral load in plasma and CSF on introduction of cART and V3 loop sequences of the corresponding baseline and first on-cART samples. Viral load in plasma and CSF was quantitated at the indicated time points by quantitative RT-PCR. Unrooted trees showing clustering of V3 loop sequences of plasma and CSF from before cART and the first sample during cART (right part of each figure). Open upright triangle CSFbefore (CSF taken before cART); filled upright triangle CSFduring (CSF taken during cART); open circle Pl before (Plasma taken before cART); filled circle Plduring (Plasma taken during cART). The scale bar shows 0.01 sequence divergence. a patients with HIV-associated neurocognitive disorder (HAND). b non-HAND patients
Rapid plasma virus decay suggests good medication adherence. Low CNS drug concentrations are an unlikely explanation for the slow CSF viral decay because the CPE scores of the patients’ antiviral regimes were relatively high and the CSF virus load eventually decreased to levels similar to those in the plasma. Furthermore, we found no isolated resistance mutations in CSF virus, and the measured CSF drug levels and ratios of the CSF drug levels to the IC50 values were high enough to suggest efficacy of the antiviral regimen (data not shown) (Eggers et al 2003).
V3 sequences in plasma and CSF of individual patients before and after initiation of cART
Next, sequence alignments were carried out for each individual patient to detect differences between V3 loop sequences in the plasma and CSF samples according to their anatomical and time-associated origin before and during cART (Fig. 2, Table 2). Clear between-sample genetic differences were found in all patients except for the non-HAND subject VC, who had primary HIV-1 infection with rapid cART-induced viral decay in the CSF. Here, the genetic distances of the clones of the different samples were low and no segregation or clustering was observed. The considerations below therefore apply only to the remaining five patients who had chronic HIV-1 infection and were in the CDC stage C3.
The plasma sequences from before and during cART (Plasmabefore, Plasmaduring) segregated themselves in almost all patients into clusters according to the sampling time point, as is most evident in subject SB (Fig. 2a). Only in one subject, TE, was there a population with an intermingling of sequences from before and during cART. In subject PI, we unfortunately failed to amplify the target RNA in the on-treatment plasma sample. The CSF sequences from before and during cART (CSFbefore, CSFduring) were closely related in the two subjects (SB and TE) who were conspicuous for an initial increase of CSF viral load on cART (Fig. 2a). They were, however, unrelated in the two other CSF-slow patients with HAND (SP and PI) in whom the CSF viral load decreased, albeit discordantly to the plasma viral load. In the non-HAND subject WH who had chronic HIV infection and rapid CSF viral decay, the CSFbefore and CSFduring sequences showed intermingling. The majority of plasma and CSF sequences prior to cART (Plasmabefore, CSFbefore) segregated themselves into different clusters in all patients, including the subject without HAND (WH) (Fig. 2). Clustering occurred according to compartment, but there was to a lesser degree some segregation into different clusters within a sample of a given compartment (e.g., CSFbefore sequences in subject WH, Fig. 2b). Some of the plasma and CSF sequences showed intermingling in subjects WH, TE, and SP.
Comparing the sequences obtained in the course of antiviral therapy (Plasmaduring, CSFduring), there was a considerable across-compartment segregation in subjects WH, TE, and SP. In subject SB, the two virus populations seemed closely related. In subject PI, no Plasmaduring sequences could be detected.
Discussion
In this work of sequencing of the V3 loop of HIV-1 in plasma and concomitant CSF of patients with and without HAND we used longitudinal sampling before and during antiviral therapy. This approach allowed us to differentiate between viral sequences from fast- and slow-replicating cells, the relative abundance of which govern the viral elimination kinetics during cART. With cART, virus from fast replicating cells decays first, thereby uncovering possible minority viral populations from slow-replicating cells such as macrophages and microglia that are important factors in the pathogenesis of HAND (Gonzalez-Scarano and Martin-Garcia 2005).
In the patient with meningo-radiculitis in the context of primary HIV infection (VC), the sequences from both compartments and both sampling time points showed close clustering. As the development of sequence heterogeneity is known to depend on the duration of viral infection (Coffin 1995; Fulcher et al 2004; Ritola et al 2004), the short time period since seroconversion in this subject might have precluded the evolution of diverging viral populations. Although the virus population infecting this patient was obviously “neurotropic”, the virus probably already had that property at the time of infestation.
The comparison of V3 sequences in the plasma and CSF before cART revealed clustering according to compartment in the four HAND subjects with slow initial CSF viral elimination kinetics but also in the patient with a non-HAND-manifestation and rapid initial CSF virus elimination during cART. Assuming that V3 loop-based viral adaptation to CNS host cells plays a role in the pathogenesis of HAND, such a cross-compartment segregation might be expected in HAND patients but not necessarily in subjects without HAND. However, in addition to symptomatic brain disease, a longer duration of HIV infection and a higher degree of immunosuppression have been identified as predisposing to different viral characteristics in plasma and peripheral tissues (Wolinsky et al 1996), and this may have caused the segregation in this chronically infected and severely immunosuppressed non-HAND patient
From the existence of a fast and slow phase of viral elimination in plasma, it was inferred that both fast- and slow-replicating cells produce the plasma virus (Perelson et al 1997). As the cellular tropism of HIV is largely governed by the V3 loop of gp120, one might expect the V3 sequences of virus from these different cell types to also be different. We indeed found clearly distinct plasma viral populations from before and during cART in all subjects with chronic HIV infection in our study. We interpret this shift of predominant plasma V3 sequences after initiation of cART as the removal of (fast replicating) lymphocytes from the pool of HIV-producing cells that thereby uncover the minority population of V3 sequences contributed by slow-replicating cells such as macrophages. Accordingly, in a longitudinal study, Fulcher et al. found compartmentalization of env sequences in purified blood macrophages and CD4-lymphocytes that evolved over time through the course of infection but was absent in the early stages (Fulcher et al 2004).
Applying the observations on viral elimination kinetics and HIV-producing cells from the plasma to the CSF (Eggers et al 2003; Harrington et al 2005), a cART-induced shift of V3 sequences might again reflect the removal of fast replicating virus-producing cells. Here, our results were heterogeneous. The CSF V3 sequences from before and during cART were strikingly similar in subjects SB and TE. Interestingly, these two patients responded to cART with an initial increase of viral load in the CSF while the plasma viral decay followed the usual kinetics. This initial viral load increase combined with the lack of V3-genetic shift and the lack of resistance mutations suggests that the identical cell population continuously produced the majority of virus pre- and post-cART. The opposite pattern was evident in subjects SP, PI, and WH. Here, the clear V3-genetic shift in the CSF suggests replacement of one HIV-producing cell population by another. While in all HAND patients the initial viral decay kinetics were slower in the CSF than in the plasma, a closer matching of the viral-genetic pattern (decay kinetics, V3 sequences) with the clinical pattern (degree of immunosuppression, neurological findings) could not be established.
After several weeks of cART, and in the absence of resistance to antivirals, the remaining virus population is produced by slow-replicating cells. Sequencing of virus from the plasma and CSF during cART revealed striking similarity in patient SB but clear-cut divergence in all other subjects with chronic infection, including the non-HAND subject. With the assumption that “neuroadaptation”, achieved by the evolution of distinct env sequences in the CNS compartment, is a prerequisite for the development of symptomatic HIV brain disease, the divergence of plasma and CSF sequences during cART in most of our patients was as expected. The close relatedness of the plasma and CSF V3 sequences during cART in patient SB with HAND, however, does not fit with this notion. Here, an origin of the CSF viruses from the hematolymphatic system might instead be considered. Indeed, this concept was suggested by Liu et al., who reported an autopsied patient with HAND where, on extensive sequencing of the env gene, virus recovered from the brain was closely related to virus from the spleen and bone marrow (Liu et al 2000). Accordingly, while slow virus elimination from the CSF means production of virus in slow-replicating cells, these cells may well be monocytes/macrophages continuously invading the CSF spaces, thus carrying virus from the hematolymphatic system into the CSF.
In the attempt to uncover differences in HIV-1 species as the cause of HAND, other authors often found inconclusive results in terms of cross-compartment segregation (Epstein et al 1991; Harrington et al 2009; Keys et al 1993; Korber et al 1994; Steuler et al 1992; Wong et al 1997; Zhang et al 2001). We think that one factor contributing to this failure might be the co-existence, before cART, of different populations of HIV-1 species. These are likely to be produced by long- and short-lived cells in the CNS, and they may differ in their relevance to the pathogenesis of brain disease. As exemplified by three of our patients, cART confers a shift of viral populations in the CSF, where the replaced population might have been the majority population but might have lacked a pathogenetic role. Thus, using cART for the “separation” of co-existing viral populations might be a means to study relatively homogeneous viral populations with a putative pathogenic role.
The rapid elimination of CSF virus with cART has led to the hypothesis of “transient” infection of the CSF spaces, where lymphocytes capable of producing HIV continuously enter the brain (Staprans et al 1999). Our finding that the pre-treatment plasma and CSF env species were highly divergent also in the non-HAND patient with rapid CSF virus elimination during cART indicates production of compartmentalized virus in short-lived cells within the CSF spaces. This cell type might well be CD4-lymphocytes crossing the blood–brain barrier and becoming infected only in the CNS or, alternatively, lymphocytes carrying minority populations from the blood into the CNS.
Limitations to our study include the low patient number and a selection bias, both in part due to the complex eligibility criteria for the patients. Another limitation is inherent to human in vivo studies of the CNS, where brain tissue is inaccessible. We think, however, that virus from the CSF may serve as a surrogate for virus from the brain parenchyma, as these two are closely related (Wong et al 1997).
To our knowledge, this is the first study to sequence the V3 loop of HIV-1 in longitudinal samples from both before and during antiviral therapy. Although we were unable to match the clinical phenotype of HAND to certain patterns of genetic compartmentalization and genetic shift over time, our approach demonstrates different patterns of the dynamics and relative contribution of compartment-specific virus populations in chronic HIV-1 infection. Further studies might include sequencing of more comprehensive segments of the env gene from defined cell types in a larger sample of well-characterized patients pre- and post-cART.
Abbreviations
- CNS:
-
Central nervous system
- CPE:
-
CNS penetration effectiveness score
- CSF:
-
Cerebrospinal fluid
- cART:
-
Combination antiretroviral therapy
- PCR:
-
Polymerase chain reaction
- HAND:
-
HIV-associated neurocognitive disorder
References
Coffin JM (1995) HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483–489
Eggers C, van Lunzen J, Buhk T, Stellbrink HJ (1999) HIV infection of the central nervous system is characterized by rapid turnover of viral RNA in the cerebrospinal fluid. J Acquir Immune Defic Syndr Hum Retrovirol 20:259–264
Eggers C, Hertogs K, Stuerenburg HJ, van Lunzen J, Stellbrink HJ (2003) Delayed CNS virus suppression during HAART is associated with HIV encephalopathy, but not with viral drug resistance or poor CNS drug penetration. AIDS 17:1897–1906
Epstein LG, Kuiken C, Blumberg BM, Hartman S, Sharer LR, Clement M, Goudsmit J (1991) HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology 180:583–590
Fulcher JA, Hwangbo Y, Zioni R, Nickle D, Lin X, Heath L, Mullins JI, Corey L, Zhu T (2004) Compartmentalization of HIV-1 between blood monocytes and CD4+ T cells during infection. J Virol 78:7883–7893
Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81
Harrington PR, Haas DW, Ritola K, Swanstrom R (2005) Compartmentalized HIV-1 present in cerebrospinal fluid is produced by short-lived cells. J Virol 79:7959–7966
Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, Price RW, Swanstrom R (2009) Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS 23:907–915
Hertogs K, de Bethune MP, Miller V, Ivens T, Schel P, Van Cauwenberge A, Van Den Eynde C, Van Gerwen V, Azijn H, Van Houtte M, Peeters F, Staszewski S, Conant M, Bloor S, Kemp S, Larder B, Pauwels R (1998) A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother 42:269–276
Hoffman NG, Seillier-Moiseiwitsch F, Ahn J, Walker JM, Swanstrom R (2002) Variability in the HIV-1 gp120 Env protein linked to phenotype-associated changes in the V3 loop. J Virol 76:3852–3864
Keys B, Karis J, Fadeel B, Valentin A, Norkrans G, Hagberg L, Chiodi F (1993) V3 sequences of paired HIV-1 isolates from blood and cerebrospinal fluid cluster according to host and show variation related to the clinical stage of disease. Virology 196:475–483
Korber BT, Allen EE, Farmer AD, Myers GL (1995) Heterogeneity of HIV-1 and HIV-2. AIDS 9(Suppl A):S5–S18
Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM (1994) Genetic differences between blood- and brain-derived viral sequences from HIV-1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol 68:7467–7481
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ (2008) Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the CNS. Arch Neurol 65:65–70
Liu Y, Tang XP, McArthur JC, Scott J, Gartner S (2000) Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol 6(Suppl 1):S70–S81
Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD (1997) Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191
Pialoux G, Fournier S, Moulignier A, Poveda JD, Clavel F, Dupont B (1997) Central nervous system as a sanctuary for HIV-1 infection despite treatment with zidovudine, lamivudine and indinavir (letter). AIDS 11:1302–1303
Price RW, Brew BJ (1988) The AIDS dementia complex. Journal of Infectious Diseases 158:1079–1083
Ritola K, Pilcher CD, Fiscus SA, Hoffman NG, Nelson JA, Kitrinos KM, Hicks CB, Eron JJ Jr, Swanstrom R (2004) Multiple V1/V2 env variants are frequently present during primary infection with HIV-1. J Virol 78:11208–11218
Ritola K, Robertson K, Fiscus SA, Hall C, Swanstrom R (2005) Increased HIV-1 env compartmentalization in the presence of HIV-1-associated dementia. J Virol 79:10830–10834
Staprans S, Marlowe N, Glidden D, Novakovic-Agopian T, Grant RM, Heyes M, Aweeka F, Deeks S, Price RW (1999) Time course of CSF response to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS 13:1051–1061
Steuler H, Storch-Hagenlocher B, Wildemann B (1992) Distinct populations of HIV type 1 in blood and CSF. AIDS Res Hum Retroviruses 8:53–59
Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, Marquie-Beck J, Durelle J, Grant I, Richman DD, Marcotte T, McCutchan JA, Ellis RJ, Wong JK (2005) Genetic composition of HIV-1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol 79:1772–1788
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Wolinsky SM, Korber BT, Neumann AU, Daniels M, Kunstman KJ, Whetsell AJ, Furtado MR, Cao Y, Ho DD, Safrit JT (1996) Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science 272:537–542
Wong JK, Ignacio CC, Torriani F, Havlir D, Fitch NJS, Richman DD (1997) In vivo compartmentalization of HIV: evidence from the examination of pol sequences from autopy tissues. J Virol 71:2059–2071
Zhang K, Hawken M, Rana F, Welte FJ, Gartner S, Goldsmith MA, Power C (2001) Human immunodeficiency virus type 1 clade a and d neurotropism: molecular evolution, recombination, and coreceptor use. Virology 283:19–30
Acknowledgments
This study was supported by a grant of the Joachim-Kuhlmann-Stiftung, Essen, Germany, to CE. We thank Christie Dietz for proofreading the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eggers, C., Müller, O., Thordsen, I. et al. Genetic shift of env V3 loop viral sequences in patients with HIV-associated neurocognitive disorder during antiretroviral therapy. J. Neurovirol. 19, 523–530 (2013). https://doi.org/10.1007/s13365-013-0207-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0207-5